Abstract
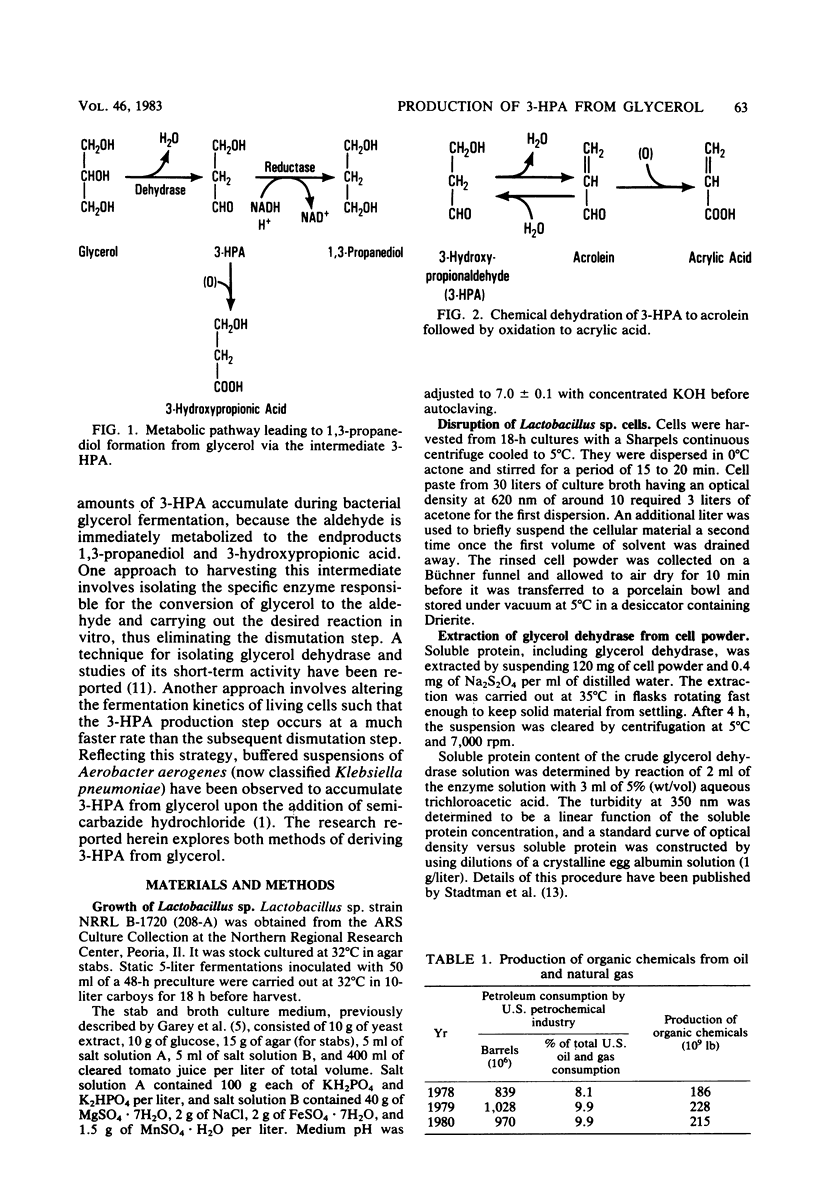
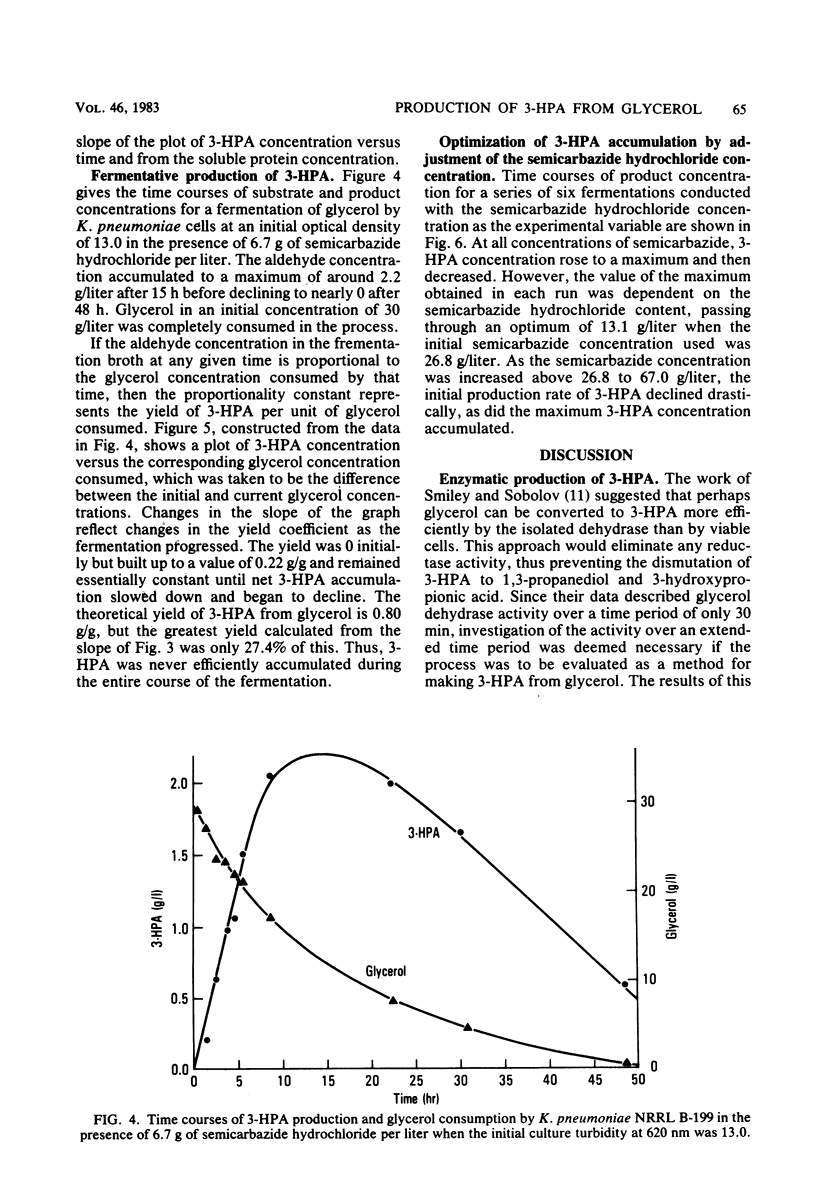
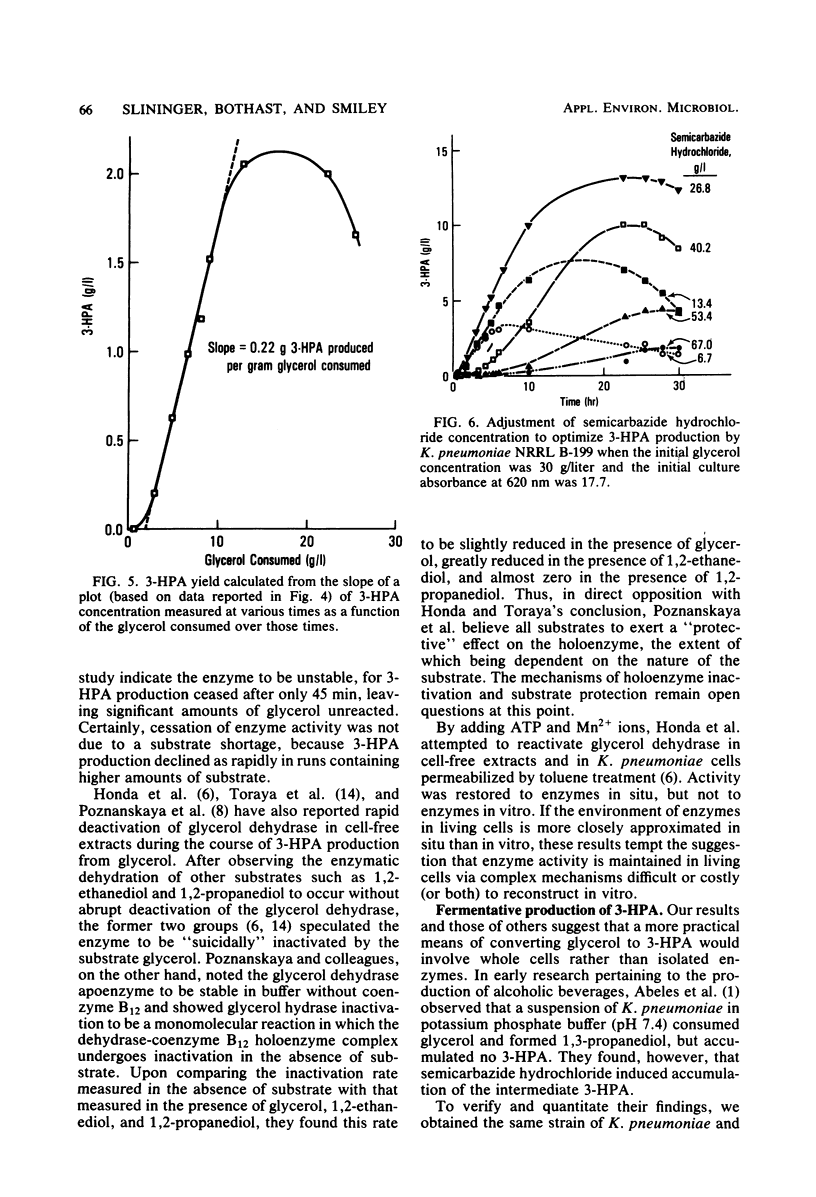
3-Hydroxypropionaldehyde is a precursor to acrolein, which can be used as an intermediate for making acrylic acid and a variety of other useful industrial chemicals. Conversion of glycerol, a renewable resource, to 3-hydroxypropionaldehyde was attempted via action of glycerol dehydrase isolated from Lactobacillus sp. strain NRRL B-1720. This method, however, was unsatisfactory because enzyme activity was lost within 60 to 90 min after the reaction initiation. Fermentation of glycerol by whole cells of Klebsiella pneumoniae NRRL B-199 in the presence of optimal semicarbazide hydrochloride proved more effective. Using this technique, glycerol solutions of 30 g/liter yielded 3-hydroxypropionaldehyde solutions of 13.1 g/liter. Thus, a conversion efficiency equal to 55% of the theoretical maximum was realized.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABELES R. H., BROWNSTEIN A. M., RANDLES C. H. beta-Hydroxypropionaldehyde, an intermediate in the formation of 1,3-propanediol by Aerobacter aerogenes. Biochim Biophys Acta. 1960 Jul 15;41:530–531. doi: 10.1016/0006-3002(60)90054-8. [DOI] [PubMed] [Google Scholar]
- Garey J. C., Rittschof L. A., Stone L., Boruff C. S. A Study of Cultural Methods for the Quantitative Determination of Bacterial Populations of Distillery Mashes. J Bacteriol. 1945 Mar;49(3):307–310. doi: 10.1128/jb.49.3.307-310.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S., Toraya T., Fukui S. In situ reactivation of glycerol-inactivated coenzyme B12-dependent enzymes, glycerol dehydratase and diol dehydratase. J Bacteriol. 1980 Sep;143(3):1458–1465. doi: 10.1128/jb.143.3.1458-1465.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLS D. E., BAUGH W. D., CONNER H. A. Studies on the formation of acrolein in distillery mashes. Appl Microbiol. 1954 Jan;2(1):9–13. doi: 10.1128/am.2.1.9-13.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanskaya A. A., Yakusheva M. I., Yakovlev V. A. Study of the mechanism of action of adenosylcobalamindependent glycerol dehydratase from Aerobacter aerogenes. II. The inactivation kinetics of glycerol dehydratase complexes with adenosylobalamin and its analogs. Biochim Biophys Acta. 1977 Sep 15;484(1):236–243. doi: 10.1016/0005-2744(77)90128-0. [DOI] [PubMed] [Google Scholar]
- SERJAK W. C., DAY W. H., VAN LANEN J. M., BORUFF C. S. Acrolein production by bacteria found in distillery grain mashes. Appl Microbiol. 1954 Jan;2(1):14–20. doi: 10.1128/am.2.1.14-20.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMILEY K. L., SOBOLOV M. A cobamide-requiring glycerol dehydrase from an acrolein-forming Lactobacillus. Arch Biochem Biophys. 1962 Jun;97:538–543. doi: 10.1016/0003-9861(62)90118-2. [DOI] [PubMed] [Google Scholar]
- SOBOLOV M., SMILEY K. L. Metabolism of glycerol by an acrolein-forming lactobacillus. J Bacteriol. 1960 Feb;79:261–266. doi: 10.1128/jb.79.2.261-266.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STADTMAN E. R., NOVELLI G. D., LIPMANN F. Coenzyme A function in and acetyl transfer by the phosphotransacetylase system. J Biol Chem. 1951 Jul;191(1):365–376. [PubMed] [Google Scholar]
- Toraya T., Shirakashi T., Kosuga T., Fukui S. Substrate specificity of coenzyme B12-dependent diol dehydrase: glycerol as both a good substrate and a potent inactivator. Biochem Biophys Res Commun. 1976 Mar 22;69(2):475–480. doi: 10.1016/0006-291x(76)90546-5. [DOI] [PubMed] [Google Scholar]



