Abstract
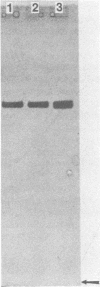
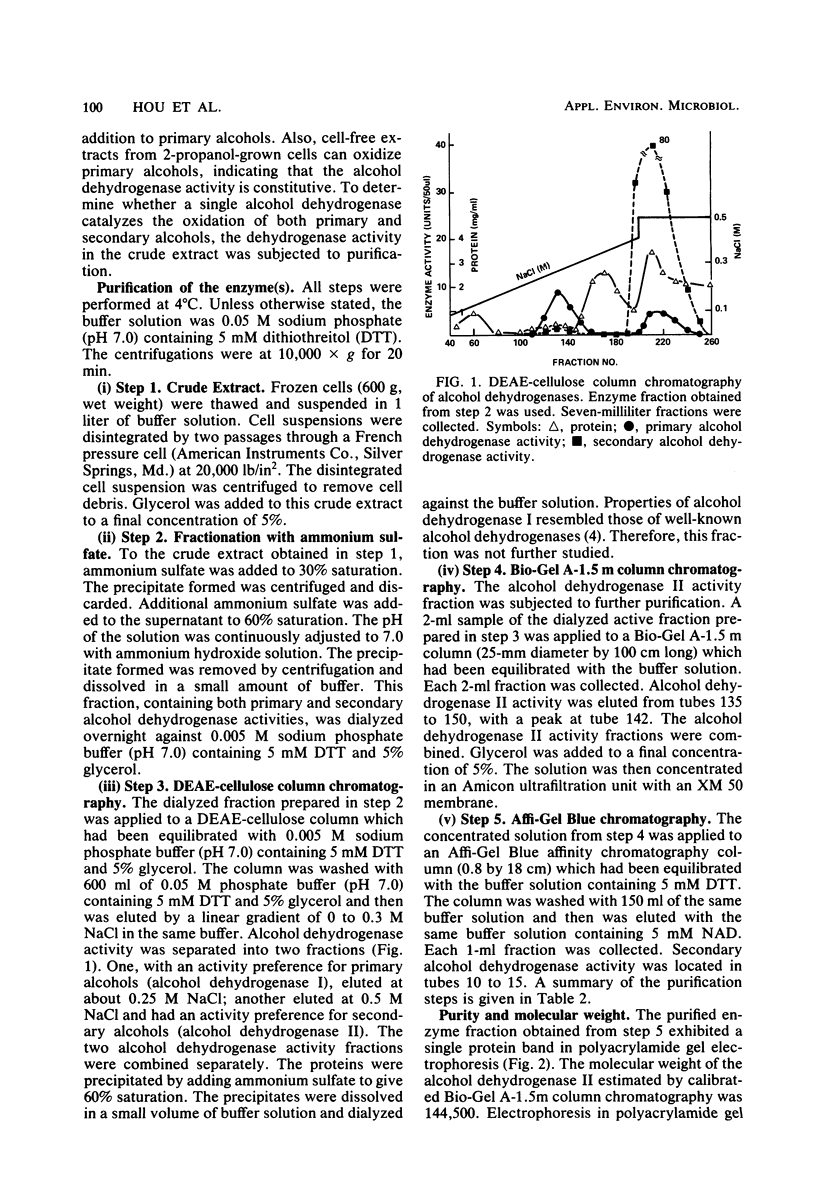
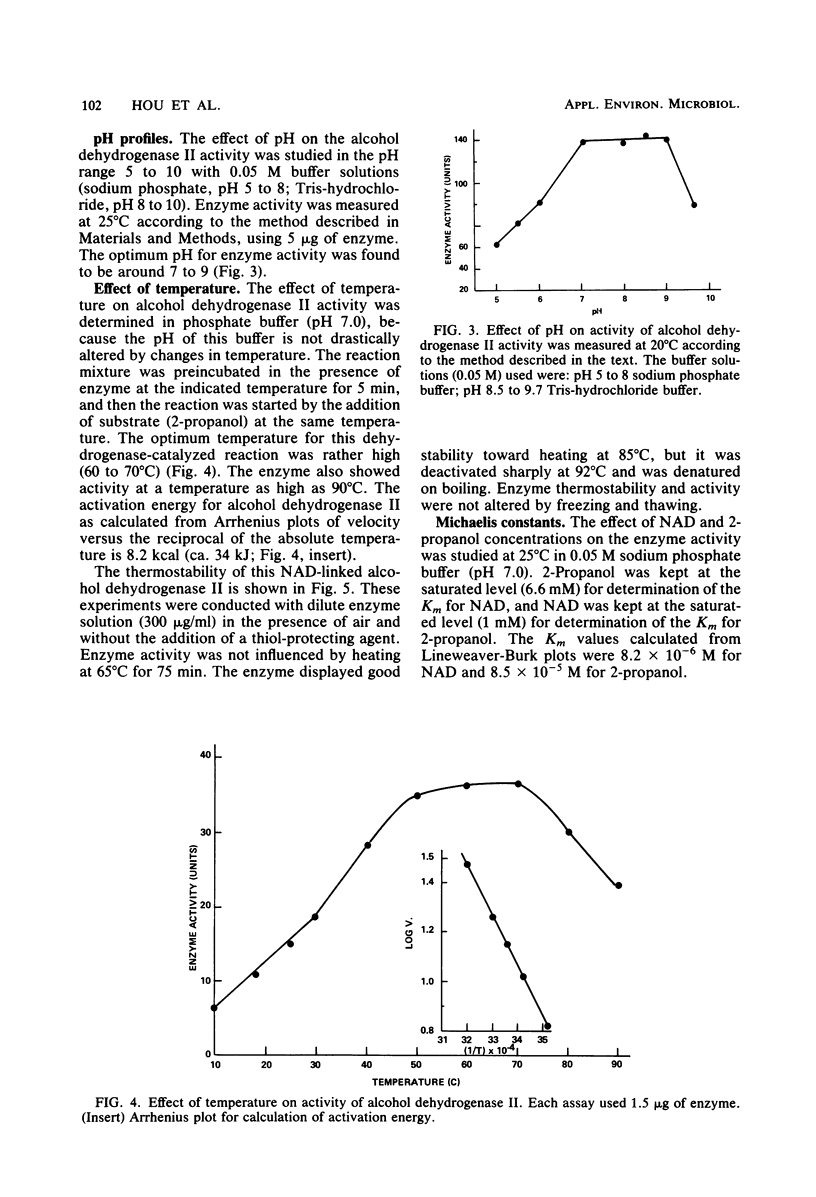
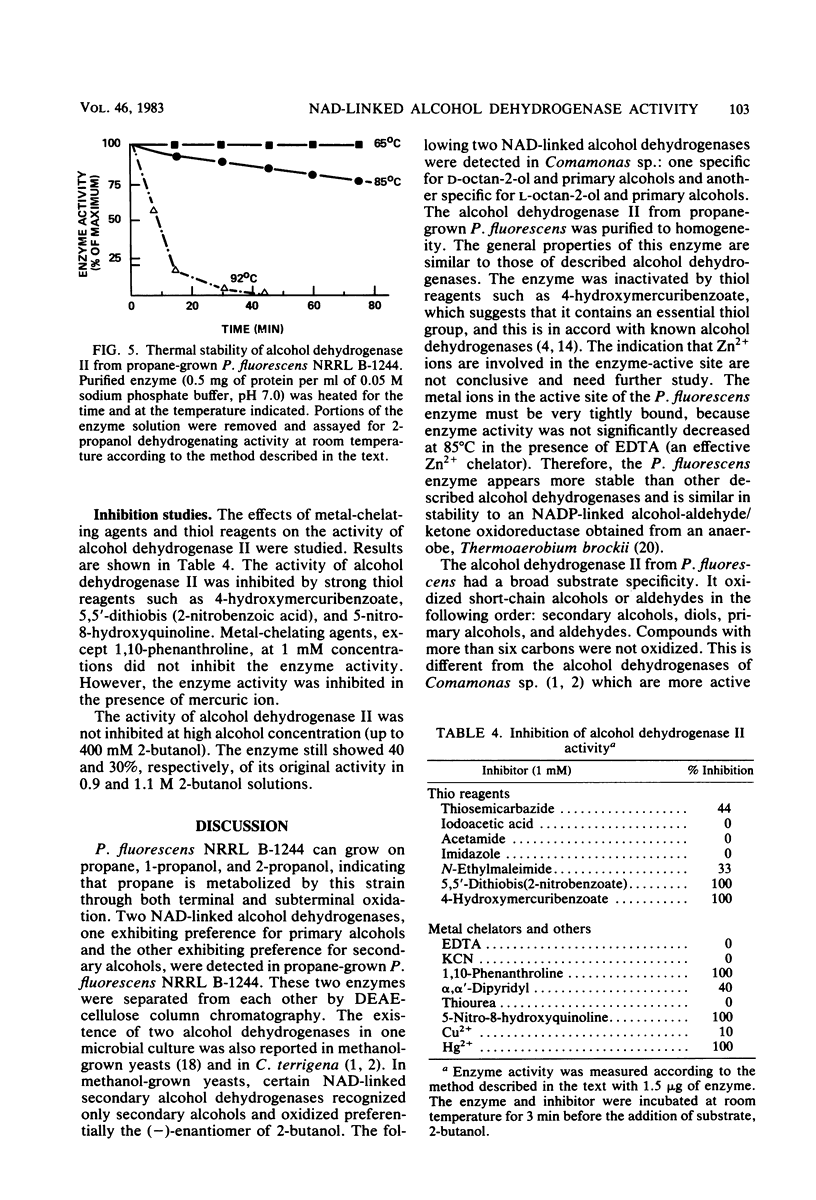
NAD-linked alcohol dehydrogenase activity was detected in cell-free crude extracts from various propane-grown bacteria. Two NAD-linked alcohol dehydrogenases, one which preferred primary alcohols (alcohol dehydrogenase I) and another which preferred secondary alcohols (alcohol dehydrogenase II), were found in propane-grown Pseudomonas fluorescens NRRL B-1244 and were separated from each other by DEAE-cellulose column chromatography. The properties of alcohol dehydrogenase I resembled those of well-known primary alcohol dehydrogenases. Alcohol dehydrogenase II was purified 46-fold; it was homogeneous as judged by acrylamide gel electrophoresis. The molecular weight of this secondary alcohol dehydrogenase is 144,500; it consisted of four subunits per molecule of enzyme protein. It oxidized secondary alcohols, notably, 2-propanol, 2-butanol, and 2-pentanol. Primary alcohols and diols were also oxidized, but at a lower rate. Alcohols with more than six carbon atoms were not oxidized. The pH and temperature optima for secondary alcohol dehydrogenase activity were 8 to 9 and 60 to 70 degrees C, respectively. The activation energy calculated from an Arrhenius plot was 8.2 kcal (ca. 34 kJ). The Km values at 25 degrees C, pH 7.0, were 8.2 X 10(-6) M for NAD and 8.5 X 10(-5) M for 2-propanol. The secondary alcohol dehydrogenase activity was inhibited by strong thiol reagents and strong metal-chelating agents such as 4-hydroxymercuribenzoate, 5,5'-dithiobis(2-nitrobenzoic acid), 5-nitro-8-hydroxyquinoline, and 1,10-phenanthroline. The enzyme oxidized the stereoisomers of 2-butanol at an equal rate. Alcohol dehydrogenase II had good thermal stability and the ability to catalyze reactions at high temperature (85 degrees C). It appears to have properties distinct from those of previously described primary and secondary alcohol dehydrogenases.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett C. H., Dodgson K. S., White G. F., Payne W. J. Preliminary observations on alcohol dehydrogenases in Comamonas terrigena that exhibit stereospecificity towards secondary alcohols. Biochem J. 1980 Jun 1;187(3):703–709. doi: 10.1042/bj1870703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellion E., Wu G. T. Alcohol dehydrogenases from a facultative methylotrophic bacterium. J Bacteriol. 1978 Jul;135(1):251–258. doi: 10.1128/jb.135.1.251-258.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant F., Ljungdahl L. G. Characterization of an alcohol, dehydrogenase from Thermoanaerobacter ethanolicus active with ethanol and secondary alcohols. Biochem Biophys Res Commun. 1981 May 29;100(2):793–799. doi: 10.1016/s0006-291x(81)80244-6. [DOI] [PubMed] [Google Scholar]
- Dickinson F. M., Dalziel K. Substrate specificity and stereospecificity of alcohol dehydrogenases. Nature. 1967 Apr 1;214(5083):31–33. doi: 10.1038/214031a0. [DOI] [PubMed] [Google Scholar]
- Dickinson F. M., Dalziel K. The specificities and configurations of ternary complexes of yeast and liver alcohol dehydrogenases. Biochem J. 1967 Jul;104(1):165–172. doi: 10.1042/bj1040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. T., Patel R. N., Laskin A. I., Barnabe N., Marczak I. Identification and purification of a nicotinamide adenine dinucleotide-dependent secondary alcohol dehydrogenase from C1-utilizing microbes. FEBS Lett. 1979 May 1;101(1):179–183. doi: 10.1016/0014-5793(79)81321-6. [DOI] [PubMed] [Google Scholar]
- Hou C. T., Patel R., Barnabe N., Marczak I. Stereospecificity and other properties of a novel secondary-alcohol-specific alcohol dehydrogenase. Eur J Biochem. 1981 Oct;119(2):359–364. doi: 10.1111/j.1432-1033.1981.tb05616.x. [DOI] [PubMed] [Google Scholar]
- Hou C. T., Patel R., Laskin A. I., Barnabe N., Barist I. Production of Methyl Ketones from Secondary Alcohols by Cell Suspensions of C(2) to C(4)n-Alkane-Grown Bacteria. Appl Environ Microbiol. 1983 Jul;46(1):178–184. doi: 10.1128/aem.46.1.178-184.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. T., Patel R., Laskin A. I., Barnabe N., Marczak I. Microbial oxidation of gaseous hydrocarbons: production of methyl ketones from their corresponding secondary alcohols by methane- and methanol-grown microbes. Appl Environ Microbiol. 1979 Jul;38(1):135–142. doi: 10.1128/aem.38.1.135-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. T., Patel R., Laskin A. I., Barnabe N., Marczak I. Substrate specificity and stereospecificity of nicotinamide adenine dinucleotide-linked alcohol dehydrogenases from methanol-grown yeasts. Appl Environ Microbiol. 1981 Mar;41(3):829–832. doi: 10.1128/aem.41.3.829-832.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUKINS H. B., FOSTER J. W. METHYL KETONE METABOLISM IN HYDROCARBON-UTILIZING MYCOBACTERIA. J Bacteriol. 1963 May;85:1074–1087. doi: 10.1128/jb.85.5.1074-1087.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamed R. J., Zeikus J. G. Novel NADP-linked alcohol--aldehyde/ketone oxidoreductase in thermophilic ethanologenic bacteria. Biochem J. 1981 Apr 1;195(1):183–190. doi: 10.1042/bj1950183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehaus W. G., Jr, Frielle T., Kingsley E. A., Jr Purification and characterization of a secondary alcohol dehydrogenase from a pseudomonad. J Bacteriol. 1978 Apr;134(1):177–183. doi: 10.1128/jb.134.1.177-183.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Hou C. T., Laskin A. I., Derelanko P., Felix A. Microbial production of methyl ketones. Purification and properties of a secondary alcohol dehydrogenase from yeast. Eur J Biochem. 1979 Nov;101(2):401–406. doi: 10.1111/j.1432-1033.1979.tb19732.x. [DOI] [PubMed] [Google Scholar]
- RAYMOND S. A convenient apparatus for vertical gel electrophoresis. Clin Chem. 1962 Sep-Oct;8:455–470. [PubMed] [Google Scholar]
- Takemori S., Furuya E., Suzuki H., Katagiri M. Stabilization of enzyme activity by an organic solvent. Nature. 1967 Jul 22;215(5099):417–419. doi: 10.1038/215417a0. [DOI] [PubMed] [Google Scholar]
- Yoneya T., Sato Y. Alcohol dehydrogenase from Rhizopus javanicus. Appl Environ Microbiol. 1979 Jun;37(6):1073–1078. doi: 10.1128/aem.37.6.1073-1078.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]