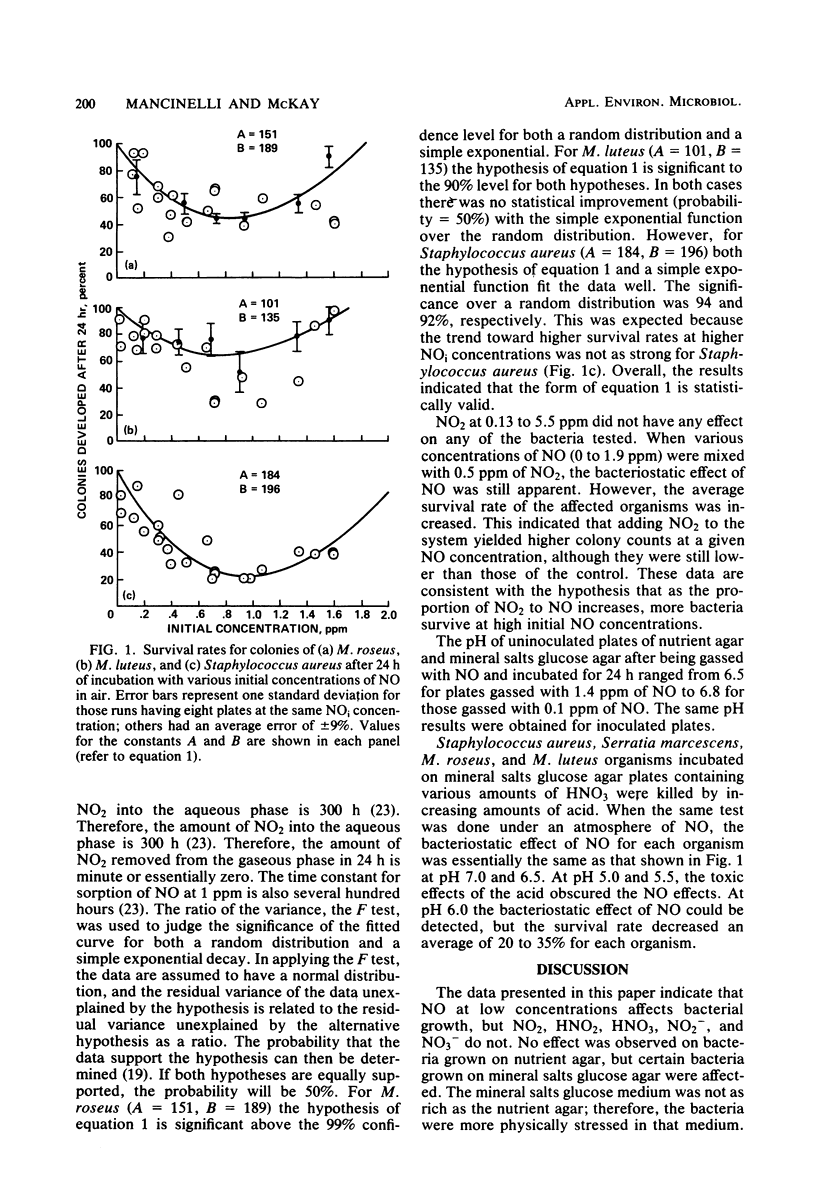
Abstract
The effects of low concentrations of nitric oxide (NO) and nitrogen dioxide (NO2) on actively dividing cultures of Staphylococcus aureus, Micrococcus luteus, Micrococcus roseus, Serratia marcescens, Bacillus subtilis, Bacillus circulans, Bacillus megaterium, and Bacillus cereus were studied. Fresh cultures of each organism were incubated for 24 h at 25 degrees C on both nutrient agar and mineral salts glucose agar plates under atmospheres containing various low concentrations of NO in air (0 to 1.9 ppm [0 to 2.0 micrograms/g of air]), NO2 in air (0 to 5.5 ppm [0 to 8.8 micrograms/g of air]), or NO and NO2 in air. Bacteria grown under air only were used as controls. After incubation, the colonies that developed on the plates were counted. None of the bacteria tested was affected by NO or NO2 at the indicated concentrations while growing on nutrient agar. Serratia marcescens, B. circulans, B. subtilis, B. megaterium, and B. cereus grown on mineral salts glucose agar were not significantly affected by NO or NO2. Low concentrations (0 to 1.9 ppm) of NO were bacteriostatic to log-phase cultures of M. roseus, M. luteus, and Staphylococcus aureus grown on mineral salts glucose agar. Bacteriostatic activity over a 24-h interval was maximal at an initial NO concentration of 1 ppm. Appreciable amounts of NO2 were produced in 24 h at initial NO concentrations greater than 1 ppm. These results suggest that NO2 may reduce the bacteriostatic activity of NO. Low concentrations (0 to 5.5 ppm) of NO2 in air did not affect any of the bacteria tested. At these low concentrations, NO affected bacterial growth, although NO2, NO2-, and NO3- did not. In addition, it was determined that the bacteriostatic activity observed in this study was not due to an increase in the acidity of the medium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft K., Grant I. F., Alexander M. Toxicity of NO(2): Effect of Nitrite on Microbial Activity in an Acid Soil. Appl Environ Microbiol. 1979 Nov;38(5):940–944. doi: 10.1128/aem.38.5.940-944.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbough J. E. Death mechanisms in airborne Escherichia coli. J Gen Microbiol. 1967 Jun;47(3):325–333. doi: 10.1099/00221287-47-3-325. [DOI] [PubMed] [Google Scholar]
- Dimmick R. L., Wolochow H., Chatigny M. A. Evidence for more than one division of bacteria within airborne particles. Appl Environ Microbiol. 1979 Oct;38(4):642–643. doi: 10.1128/aem.38.4.642-643.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich R., Miller S. Effect of NO 2 on airborne Venezuelan equine encephalomyelitis virus. Appl Microbiol. 1972 Mar;23(3):481–484. doi: 10.1128/am.23.3.481-484.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY E. L. Oxides of nitrogen: their occurrence, toxicity, hazard; a brief review. AMA Arch Ind Health. 1959 May;19(5):479–486. [PubMed] [Google Scholar]
- Lee R. E., Jr, Harris K., Akland G. Relationship between viable bacteria and air pollutants in an urban atmosphere. Am Ind Hyg Assoc J. 1973 Apr;34(4):164–170. doi: 10.1080/0002889738506826. [DOI] [PubMed] [Google Scholar]
- Lewis W. M., Jr, Grant M. C. Acid precipitation in the Western United States. Science. 1980 Jan 11;207(4427):176–177. doi: 10.1126/science.207.4427.176. [DOI] [PubMed] [Google Scholar]
- Mancinelli R. L., Shulls W. A. Airborne bacteria in an urban environment. Appl Environ Microbiol. 1978 Jun;35(6):1095–1101. doi: 10.1128/aem.35.6.1095-1101.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J. Reduction of nitrogenous oxides by microorganisms. Bacteriol Rev. 1973 Dec;37(4):409–452. doi: 10.1128/br.37.4.409-452.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor W. A., Lightsey J. W. Mechanisms of nitrogen dioxide reactions: initiation of lipid peroxidation and the production of nitrous Acid. Science. 1981 Oct 23;214(4519):435–437. doi: 10.1126/science.214.4519.435. [DOI] [PubMed] [Google Scholar]
- SHANK J. L., SILLIKER J. H., HARPER R. H. The effect of nitric oxide on bacteria. Appl Microbiol. 1962 May;10:185–189. doi: 10.1128/am.10.3.185-189.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodzinski R. S., Labeda D. P., Alexander M. Effects of low concentrations of bisulfite-sulfite and nitrite on microorganisms. Appl Environ Microbiol. 1978 Apr;35(4):718–723. doi: 10.1128/aem.35.4.718-723.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won W. D., Ross H. Reaction of airborne Rhizobium meliloti to some environmental factors. Appl Microbiol. 1969 Oct;18(4):555–557. doi: 10.1128/am.18.4.555-557.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



