Abstract
Background
Naltrexone is a nonaddictive medication that blocks the euphoric effects of opioids. However, naltrexone treatment is associated with high rates of noncompliance and opioid relapse, possibly because it does not reduce stress and protracted withdrawal symptoms during early recovery. Prior clinical and preclinical research has indicated that both stress and drug-cue-related arousal response is associated with craving and vulnerability to relapse in a range of drug-using populations.
Aims
To examine opioid craving and the subjective and cardiovascular response to stress and drug cues in naltrexone-treated opioid abusers.
Method
Eleven men and three women engaged in naltrexone treatment for opioid dependence. They were exposed to personalized stress, drug-cue, and neutral-relaxing imagery in a single laboratory session. Subjective (craving, emotion) and cardiovascular (heart rate, systolic blood pressure, and diastolic blood pressure) measures were assessed.
Results
Stress and drug-cue-related imagery significantly increased opioid craving, anxiety, and negative emotions and significantly decreased positive emotions compared to neutral imagery. Selective emotional responses were greater in the stress condition than in the drug-cue condition. Only stress-related imagery was associated with an increased cardiovascular response.
Conclusions
Naltrexone-treated opioid abusers demonstrate vulnerability to stress and drug-cue-induced craving and arousal responses that may contribute to the high rates of noncompliance and relapse among opioid-dependent individuals undergoing naltrexone treatment. Pharmacological and behavioral interventions that specifically target the negative affectivity that co-occurs with drug-cue and stress-induced craving could be of benefit in improving naltrexone treatment outcomes in opioid dependence.
Keywords: naltrexone, opioid dependence, stress, drug cue, opioid craving
The profile of opioid addiction has changed considerably in recent years. While heroin abuse remains a significant public health concern (Brown, 2004; Kirchmayer et al., 2002), nonmedical use and abuse of prescription opioids is increasing, and the prevalence of prescription opioid abuse has become comparable to that of heroin and cocaine abuse (Zacny et al., 2003). Children are especially at risk, given that the number of 12- to 17-year-olds abusing controlled prescription drugs increased by 212% between 1992 and 2003 (National Center on Addiction and Substance Abuse at Columbia University, 2005). These findings are alarming and indicate a need for the development of additional and more comprehensive treatments that may be better suited for this growing cohort of young opioid abusers.
Naltrexone, a medication that blocks the euphoric effects of opioids, is a nonaddictive alternative to agonist maintenance treatments such as methadone and buprenorphine. The potential usefulness of naltrexone follows from operant conditioning models of behavior, which postulate that drug-seeking will extinguish if drug use is not reinforced by euphoric drug effects (Tucker & Ritter, 2000). However, its treatment efficacy has been questioned due to poor compliance and high drop-out rates (Kirchmayer et al., 2002; Rounsaville, 1995; Tucker & Ritter, 2000). Reasons given for poor compliance include its absence of euphoria-producing properties, the requirement of an opioid-free period prior to initiation, the fear of using a new drug, and a lack of genuine motivation to achieve abstinence (Tucker & Ritter, 2000). Another possible explanation for the high drop-out rates is that opioid-dependent individuals experience stress and protracted withdrawal symptoms in early recovery, which may increase craving and relapse susceptibility.
Indeed, prior research has implicated stress in the perpetuation of drug use and relapse (Bradley, Phillips, Green, & Gossop, 1989; Goeders, 2003; Sinha, 2001; Wallace, 1989). Animal studies have shown that acute physical and social stress facilitates drug self-administration and reinstatement to drug-seeking behavior in drug-addicted animals that have been drug free for extended time periods (Do Couto et al., 2006; Shaham & Stewart, 1995). Although naltrexone attenuates opioid-induced drug seeking in heroin-dependent animals, it does not alter stress-induced drug seeking in the same animals (Shaham et al., 1997). This finding may be related to the possible existence of somewhat different neurochemical pathways underlying stress and drug-cue-related drug seeking and relapse (see Stewart, 2003, for review). It is important to note that this finding may also be related to the fact that both acute and chronic administration of opioid receptor antagonists, such as naltrexone and naloxone, increase stress markers and/or cardiovascular responses in healthy volunteers and ex-opioid addicts (Klein, Jamner, Alberts, Ornstein, & Leigh, 2000; Kosten et al., 1986; McCubbin et al., 1998; Uhart, Chong, Oswald, Lin, & Wand, 2006), raising the question of whether naltrexone addresses stress-induced opioid craving and associated arousal responses.
Previous work has shown that imagery exposure to stressful and drug-cue experiences increase drug craving, negative affect, and physiological reactivity in cocaine-and alcohol-dependent individuals (Breese et al., 2005; Sinha, Catapano, & O’Malley, 1999, Sinha, Fuse, Aubin, & O’Malley, 2000, Sinha et al., 2003). Moreover, recent findings have indicated that stress-induced craving and hypothalamic–pituitary–adrenal responses significantly predicted relapse outcomes in both alcohol- and cocaine-dependent individuals (Brady et al., 2006; Sinha, Garcia, Paliwal, Kreek, & Rounsaville, 2006). With regard to opioid addiction, opioid-dependent individuals undergoing inpatient detoxification have been found to experience an increase in craving and physiological reactivity while imagining and describing situations involving strong urges to use opioids (Weinstein, Wilson, Bailey, Myles, & Nutt, 1997). Detoxified opioid-dependent individuals exposed to drug-related stimuli have also been shown to experience an increase in craving, dysphoria, and withdrawal-like symptoms (Powell, Bradley, & Gray, 1992). Even after detoxification and 12 weeks of intensive inpatient treatment, opioid-dependent patients continued to demonstrate increased craving and negative affect when exposed to drug-related stimuli (Franken, De Hann, Van Der Meer, Haffmans, & Hendriks, 1999). Regarding stress-induced craving responses, imagery induction of negative mood states has been found to increase opioid craving and withdrawal symptoms in detoxified opioid abusers (Childress et al., 1994).
Although these studies indicated that exposure to drug cues and stress can increase opioid craving, the pattern of stress and drug-cue-induced craving in opioid-dependent individuals undergoing naltrexone treatment has not been previously investigated. The potential inability of naltrexone to attenuate stress-induced responses may be a primary reason for the high relapse rates found with naltrexone treatment. Thus, we tested the hypothesis that opioid-dependent participants would demonstrate increased opioid craving, negative emotion, and cardiovascular arousal following exposure to both stress and drug-cue-related imagery compared to neutral imagery.
METHOD
Participants
Opioid-dependent individuals (11 men and 3 women) engaged in a standard outpatient naltrexone treatment program were invited to participate in the study. The standard naltrexone treatment regimen enrolled patients who had recently completed opioid detoxification and were opioid free (as assessed by an opioid-free urine toxicology screen) upon presentation to the outpatient facility. Initially, participants self-administered 25 mg of naltrexone under observation at the clinic on their first day of induction. Their dose was increased on subsequent days until they reached the optimum dosing schedule of three times per week. Under this dosing schedule, patients visited the clinic three times per week (Monday, Wednesday, and Friday) to self-administer their naltrexone; 100 mg was self-administered on Mondays and Wednesdays, and 150 mg was self-administered on Fridays, on the basis of previous data supporting the safety and effectiveness of this dosing and self-administration schedule (Carroll, Sinha, Nich, Babuscio, & Rounsaville, 2002; Landsberg, Taintor, Plumb, Amico, & Wicks, 1976; Rounsaville, 1995). Naltrexone self-administration was conducted at the clinic under observation of clinic staff in order to ensure medication compliance. All participants had been successfully treated with naltrexone for at least 4 weeks and no more than 6 weeks for participation in the laboratory session (tested between Days 28 and 40 of naltrexone treatment). In addition, all participants provided a negative urine screen (for all substances) on the day of the laboratory session.
A psychiatrist evaluated all potential participants, and those not requiring medication for comorbid psychiatric problems were invited to participate in the study. All participants completed the opioid-dependence section of the Structured Clinical Interview for DSM–IV Axis I Disorders (First, Spitzer, Gibbon, & Williams, 1997) to confirm dependence criteria. Those failing to meet health requirements were also deemed ineligible. All participants provided written informed consents and were paid for their participation. The study protocol was approved by the Human Investigation Committee of the Yale University School of Medicine.
Procedure
Imagery Script Development Session
In a meeting prior to the laboratory session, we developed imagery scripts for the guided imagery induction as described in previous studies (Sinha et al., 2000, 2003). The stress imagery scripts were based on participants’ detailed descriptions of a recent personal stressful event. Participants rated their perceived stress on a Likert point scale in which 0 = not at all stressful and 10 = the most stress I have felt recently in my life. Only situations rated as 8 or above were accepted as appropriate for script development. Any scenario that comprised a stressful situation related to drug use (e.g., being arrested for possession of drugs) was disallowed. Examples of acceptable stressful situations included a breakup with a significant other, a verbal argument with a significant other or family member, or an employment-related stress, such as being fired or laid off from work. The drug-cues script was developed by having participants identify a recent situation that included drug-related stimuli and resulted in subsequent opioid use (e.g., watching others shoot heroin or meeting a friend to buy OxyContin). Drug-related situations that were associated with negative affect or psychological distress were not allowed (i.e., calling a drug buddy after having a fight with a parent). A neutral-relaxing script was also developed from participants’ descriptions of a personal non-drug-related neutral-relaxing situation (e.g., sitting at the beach, taking a hot bath or shower). The scripts were developed by obtaining specific stimulus and response details, including physical and interpersonal context details, verbal–cognitive attributions regarding the people involved, and physiological and bodily sensations that were experienced while the participant was in the situation.
Laboratory Sessions
Upon arrival to the laboratory, participants took urine toxicology and breathalyzer tests to ensure that they were in a drug-free state. After participants were brought into the testing room and seated in a comfortable chair, a blood pressure cuff was placed around their preferred arm, and a pulse rate sensor was placed on the forefinger of their nonpreferred arm. The experimental procedure was based on the following format: a 5-min baseline period, a 5-min imagery period, and a 5-min recovery period. During the baseline period, participants were given instructions to relax for a few minutes, to clear their mind of any worrying thoughts, and to focus on deep breathing. After the baseline period, participants were given the following instructions prior to imagery presentation: “You will soon hear a situation being described to you. Your task is to close your eyes and imagine yourself in the situation being described as if it were happening right now. Allow yourself to become completely involved in the situation, by involving your mind and body in actually doing what is being described.” The experimenter then read the imagery script, which lasted approximately 5 min, at the end of which participants were asked to stop imagining, and a 5-min recovery period followed. The scripts were read by the same person for all participants. Following each baseline, imagery, and recovery period, opioid craving, anxiety, and specific emotion ratings were obtained. Heart rate and blood pressure were monitored throughout with three readings taken each for baseline, imagery, and recovery periods.
Following each imagery session, participants were given a 10-min break and again instructed to relax and focus on deep breathing. Cardiovascular measures were monitored during this time, and the second imagery session was not conducted until heart rate, blood pressure, subjective anxiety, and craving had returned to baseline levels. The second and third imagery conditions followed the same procedures described above. The presentation of all imagery scripts was randomized and counterbalanced across participants (the schedule of the laboratory session is shown in Table 1).
Table 1.
Laboratory Schedule for Guided-Imagery Exposure
| Time | Activity |
|---|---|
| 2:45 p.m. | Participant arrived; urine and BAC check; laboratory setup |
|
| |
| Condition 1: stress/drug cue/neutral imagery | |
| 3:00 p.m. | Baseline period; heart rate/BP every minute |
| 3:05 p.m. | Craving, anxiety, and DES ratings; |
| 3:10 p.m. | Image period; heart rate/BP every minute |
| 3:15 p.m. | Craving, anxiety, and DES ratings; |
| 3:20 p.m. | Recovery period; heart rate/BP every minute |
| 3:25 p.m. | Craving, anxiety, and DES ratings; |
| 3:30 p.m. | 10-min relaxation period |
|
| |
| Condition 2: stress/drug cue/neutral imagery | |
| 3:40 p.m. | Schedule as in Condition 1 |
|
| |
| Condition 3: stress/drug cue/neutral imagery | |
| 4:20 p.m. | Schedule as in Condition 1 |
| 5:00 p.m. | Participant unhooked from lab apparatus—end of laboratory session |
Note. BAC = blood alcohol content; BP = blood pressure; DES = Differential Emotion Scale.
Laboratory Assessments (Subjective Ratings)
Vividness Rating
Following the imagery condition, participants rated how “clearly and vividly” they were able to imagine the scenario on a visual analogue scale of 0 (not at all clear) to 10 (extremely clear).
Drug Craving and Anxiety Ratings
The extent of opioid craving was measured with a visual analogue scale. Participants rated the current intensity of their desire to use opioids from 0 (none at all) to 10 (more than ever). Subjective anxiety was also measured with an identical rating scale. Participants rated how “tense, anxious, and/or jittery” they were at that time, from 0 (none at all) to 10 (more than ever). Ratings were taken at baseline and immediately following imagery and recovery for each imagery condition.
Emotion State Ratings
An abbreviated version of the Differential Emotion Scale (Izard, 1972) was administered in order to assess specific positive and negative emotional states at baseline and immediately following imagery and recovery. The scale requires participants to rate the extent to which various emotional words were able to describe their feelings at the present time on a 5-point Likert scale ranging from 1 = very slightly or not at all to 5 = very strongly. Five items were attributed to five subscales comprising anger, fear, sadness, joy, and neutral/relaxed state. Scores ranged from 5 to 25 on each subscale. Ratings were taken at baseline and immediately following imagery and recovery for each imagery condition.
Laboratory Assessments (Cardiovascular Measures)
Blood Pressure
An SD-700 Monitor (IBS Corporation, MA) with arm cuff was used to measure systolic and diastolic blood pressure at baseline, during imagery, and following recovery. Blood pressure measures were taken each minute for 5 min at baseline, and an average measure was used. This was also done at the imagery and recovery time points (see Table 1).
Heart Rate
A pulse sensor was attached to the forefinger of the participant’s nonpreferred hand and connected to the SD-700 Monitor. Heart rate measures were taken each minute for 5 min at baseline, and an average measure was used. This was also done at the imagery and recovery time points (see Table 1).
Design and Statistical Analyses
A within-group design was used with time point (baseline, imagery, and recovery) and condition (stress, drug, and neutral) representing within-group factors. We performed all statistical analyses using linear mixed effects models (SAS, Version 8.0). The within-group factors of time point (baseline, imagery, and recovery) and condition (stress, drug, and neutral) represented the fixed effects, and subjects represented the random effect. Linear mixed effects models are particularly well-suited when the design calls for repeated measurements within the same individual that can lead to positive correlations between measurements (Littell, Milliken, Strout, & Wolfinger, 1996).
We also performed a series of Pearson product–moment correlations in order to assess the associations between stress and drug-cue-related craving and all emotional and cardiovascular measures. We correlated stress and drug-cue-induced craving responses with years of regular (three times per week) opioid use. All measures were collapsed across time points for each condition.
RESULTS
Participant Characteristics
The participant sample comprised 11 men and 3 women, with a mean (± SD) age of 29.6 ± 8.1 years and a mean of 12.4 ± 1.6 years of education. Eighty-six percent were Caucasian, and 14% were African American. The average age of first opioid use was 19.1 ± 4.5 years. The average age of onset of regular opioid use (defined as using at least three times per week) was 20.1 ± 4.4 years. On average, participants had used opioids for 10.4 ± 9.3 years and had used them regularly for 9.4 ± 8.8 years. All participants were seeking treatment for primary opioid dependence and opioids were the drug of choice for all participants, although some had reported a history of other drug use. Nine participants (69%) had reported a history of regular (three times per week) cocaine use at some point in their past, 8 participants (57%) had reported a history of regular (three times per week) alcohol use, and 12 participants (86%) had reported a history of regular (three times per week) cannabis use. All participants were participating in an abstinence-based substance abuse treatment program, and they were able to provide a negative urine toxicology screen on the day of the laboratory session.
Laboratory Assessments
Subjective Measures
Opioid Craving
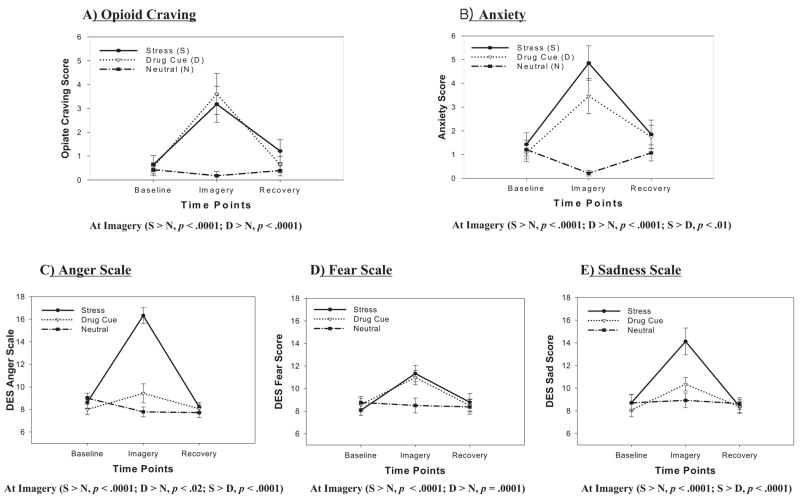
A main effect of condition, F(2, 26) = 11.3, p = .0003, indicated that opioid craving was significantly higher in the stress (p = .0002) and drug-cue (p = .0005) conditions compared with the neutral condition. A main effect of time point, F(2, 26) = 18.9, p < .0001, also indicated that craving scores were significantly higher at the imagery time point compared with both baseline (p < .0001) and recovery (p < .0001). A significant Condition × Time-Point interaction, F(4, 52) = 6.6, p = .0002, indicated that significantly increased stress-induced (p < .0001) and drug-cue-induced (p < .0001) opioid craving was reported at the imagery time point, and there were no differences between imagery conditions at baseline or at recovery time points (see Figure 1A).
Figure 1.
Subjective rating scores for craving and negative affect across baseline, imagery, and recovery time points for all three conditions. DES = Differential Emotion Scale.
Anxiety
A main effect of condition, F(2, 26) = 18.7, p < .0001, indicated significantly higher anxiety in the stress (p < .0001) and drug-cue (p = .0004) conditions compared with the neutral condition. A main effect of time point, F(2, 26) = 14.7, p < .0001, indicated that anxiety scores were significantly higher at the imagery time point compared with baseline (p < .0001) and recovery (p = .0004). A significant Condition × Time-Point interaction, F(4, 52) = 10.7, p < .0001, indicated significantly increased anxiety at the imagery time point for the stress (p < .0001) and drug-cue (p < .0001) conditions compared to the neutral condition and increased anxiety levels in the stress condition compared to the drug-cue condition (p < .01), but there was no difference between conditions at the baseline and recovery time points (see Figure 1B).
Differential Emotion Scale—Negative Emotion Scales
Anger
A main effect of condition, F(2, 26) = 34.8, p < .0001, indicated significantly greater anger in the stress condition compared with both the drug-cue (p < .0001) and neutral (p < .0001) conditions. A main effect of time point, F(2, 26) = 40.1, p < .0001, indicated significantly higher anger at the imagery time point compared with both the baseline (p < .0001) and recovery (p < .0001) time points. A significant Condition × Time-Point interaction, F(4, 52) = 30.7, p < .0001, indicated that higher anger was reported in the stress condition compared with both the neutral (p < .0001) and the drug-cue (p < .0001) conditions at the imagery time point only. Significantly higher anger was also reported in the drug-cue condition compared with the neutral condition (p < .02) at the imagery time point (see Figure 1C).
Fear
A main effect of condition, F(2, 26) = 3.8, p < .04, indicated significantly greater fear in the stress (p = .02) and drug-cue (p < .03) conditions compared with the neutral condition. A main effect of time point, F(2, 26) = 17.1, p < .0001, also showed that significantly higher fear was reported at the imagery time point compared with both the baseline (p < .0001) and recovery (p < .0001) time points. A significant Condition × Time-Point interaction, F(4, 52) = 5.2, p = .001, indicated that the increases in fear ratings after stress and drug-cue exposure compared to the neutral imagery exposure were only evident at the imagery time point, with no significant differences in fear ratings across conditions at the baseline and recovery time points (see Figure 1D).
Sadness
A main effect of condition, F(2, 26) = 8.2, p < .002, indicated significantly greater sadness in the stress condition compared with both the drug-cue (p < .003) and neutral (p < .001) imagery conditions. A main effect of time point, F(2, 26) = 23.6, p < .0001, also indicated significantly higher sadness at the imagery time point compared with both the baseline (p < .0001) and recovery (p < .0001) time points. A significant Condition × Time-Point interaction, F(4, 52) = 8.2, p < .0001, was also observed. This interaction was indicative of significantly greater stress-induced sadness at the imagery time point compared with sadness ratings in the drug-cue and neutral conditions. No differences in sadness ratings were observed across conditions at the baseline and recovery time points (see Figure 1E).
Differential Emotion Scale—Positive Emotion Scales
Joy
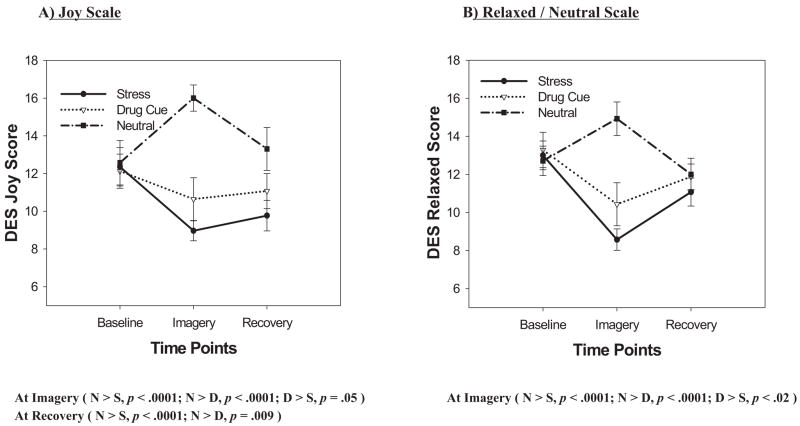
A main effect of condition, F(2, 26) = 30.6, p < .0001, indicated significantly decreased joy in the stress (p < .0001) and drug-cue (p < .0001) imagery conditions compared with the neutral condition. A significant Condition × Time-Point interaction, F(4, 52) = 9.2, p < .0001, was observed indicating that at both the imagery and the recovery time points, joy ratings significantly increased in the neutral condition compared to the stress and drug-cue conditions, but there were no differences across conditions at the baseline time point (see Figure 2A).
Figure 2.
Subjective rating scores for positive affect across baseline, imagery, and recovery time points for all three conditions. DES = Differential Emotion Scale.
Neutral/relaxed state
A main effect of condition, F(2, 26) = 14.5, p < .0001, indicated that significantly decreased relaxed-state ratings were reported in both the stress (p < .0001) and drug-cue (p < .005) imagery conditions compared with the neutral imagery condition. Decreased relaxed state was also reported in the stress condition compared with the drug-cue condition (p = .03). A main effect of time point, F(2, 26) = 8.4, p < .002, also indicated that significantly greater relaxed state was reported at the baseline time point compared with both the imagery (p = .0006) and recovery (p < .005) time points. A significant Condition × Time-Point interaction, F(4, 52) = 12.2, p < .0001, was indicative of significantly decreased relaxed ratings reported at the imagery time point in the stress and drug-cue conditions compared with the neutral condition, but there were no differences between conditions at the baseline and recovery time points (see Figure 2B).
Cardiovascular Measures
Heart Rate
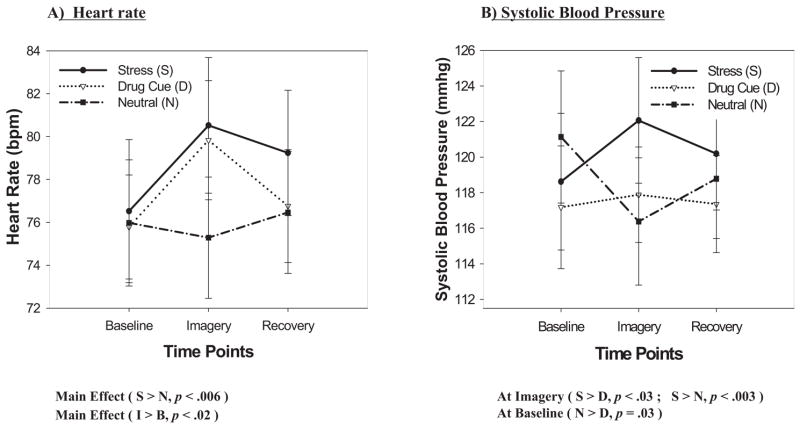
A significant main effect of condition, F(2, 26) = 4.5, p = .02, indicated that participants demonstrated significantly increased heart rates in the stress imagery condition compared with the neutral imagery condition (p < .006). A main effect of time point, F(2, 26) = 3.3, p = .05, also showed increased heart rate at the imagery time point compared with the baseline time point (p < .02). No Condition × Time-Point interaction was observed (see Figure 3A).
Figure 3.
Cardiovascular measures across baseline, imagery, and recovery time points for all three conditions.
Systolic Blood Pressure (SBP)
A significant main effect of condition, F(2, 26) = 3.6, p = .04, indicated that participants demonstrated significantly increased SBP in the stress imagery condition compared with the drug-cue imagery condition (p = .01; see Figure 3B). There was no main effect of time point. A significant Condition × Time-Point interaction, F(4, 52) = 2.7, p = .04, indicated that in the neutral imagery condition, significantly decreased SBP was observed at the imagery time point compared with the baseline time point (p = .01). At the imagery time point, there was a significantly greater SBP in the stress condition compared with the drug-cue (p < .03) and neutral (p < .003) conditions. Greater SBP was also observed in the neutral imagery condition compared with the drug-cue condition at the baseline time point (p = .03). These effects of condition were not observed at the recovery time point (see Figure 3B).1
Diastolic Blood Pressure
A significant main effect of time point, F(2, 26) = 4.7, p < .02, indicated that participants demonstrated significantly increased diastolic blood pressure at the imagery time point compared with the baseline time point (p < .005). There was no main effect of condition or significant interactions.
Correlational Analyses
Moderate but significant correlations were obtained between stress-induced craving and self-reported anxiety (r = .54, p < .05), fear (r = .61, p = .02), and sadness (r = .56, p < .04) when averaged across each time point. No significant correlations were observed between stress-induced craving and cardiovascular measures or between drug-cue-induced craving and any emotional or cardiovascular measures. No significant correlations were observed between years of regular opioid use and stress-induced craving or years of regular opioid use and drug-cue-induced craving.
DISCUSSION
We examined subjective and cardiovascular responses to stress and drug-cue imagery in recently abstinent opioid-dependent individuals being treated with naltrexone. Findings indicated significant increases in stress-induced and drug-cue-induced craving alongside increases in negative emotions and decreases in positive emotions. A more intense negative emotional response was reported in the stress condition compared with the drug-cue condition, as participants reported significantly increased anger and sadness as well as significantly decreased joy and relaxation following exposure to stressful imagery compared with drug-cue-related imagery. It is important that extended correlational analyses also indicated that stress-induced opioid craving was significantly associated with increased anxiety, fear, and sadness, whereas these increases in negative emotion were not associated with drug-cue-induced craving. Moreover, significant increases in heart rate and SBP were observed only in the stress condition.
Findings indicate that both imagery conditions elicited an increase in negative emotion. Moreover, craving was elevated to similar levels in both conditions. This finding is important, as craving has been associated with relapse factors in both recently abstinent cocaine abusers (Sinha et al., 2006) and alcoholics (Brady et al., 2006; Breese et al., 2005; Cooney, Litt, Morse, Bauer, & Gaupp, 1997). However, although comparable increases in stress- and drug-cue-induced craving were observed, some of the negative emotional components accompanying drug-cue-induced craving were less elevated. This finding is consistent with our correlational findings indicating that stress- and drug-cue-related opioid craving comprises different emotional distress components, which could suggest different neural correlates for stress- and drug-cue-related craving states.
Overall, the study findings indicate that naltrexone-treated opioid users in early recovery continue to be vulnerable to stress- and drug-cue-induced craving and stress-related hyperarousal responses despite opioid effects being blocked by chronic naltrexone treatment. These findings may help clarify why patients continue to report opioid craving despite the rewarding effects of opioids being blocked by naltrexone (Alderson, Robbins, & Everitt, 2000; Mello & Negus, 1996). They also suggest that the negative affectivity that co-occurs with the opioid-craving state may be an important target in the treatment of opioid dependence.
It is possible that the lower emotional and cardiovascular response to drug cues versus stress was an effect of naltrexone treatment attenuating drug-cue-related hyperarousal but not stress-related hyperarousal in opioid-dependent individuals. Previous preclinical research indicates the existence of somewhat different neurochemical pathways underlying stress- and drug-cue-related drug seeking and relapse (see Stewart, 2003, for review). For example, studies have shown that animals pretreated with naloxone showed decreased responding for drugs in the context of drug-related stimuli such as light (Alderson et al., 2000; Killian, Bonese, & Schuster, 1978). Yet other animal studies have also shown that naltrexone treatment has no effect on foot-shock stress-induced reinstatement of heroin seeking (Shaham & Stewart, 1996). Alternatively, the greater negative emotion and physiological arousal response in the stress condition could have also been a result of participants’ experiencing the stress condition as more distressing than the drug-cue condition.
Our data suggest that naltrexone alone may not be an adequate treatment for opioid addiction because it does not in itself protect against subjective and cardiovascular stress and drug-cue-related opioid craving and arousal responses. On this basis, pharmacological treatments that specifically target stress-induced opioid craving and related arousal may be combined with naltrexone to improve treatment outcomes in opioid dependence. This approach may prove more effective than simple administration of opioid antagonists and opioid agonists and partial agonists such as methadone and buprenorphine, all of which have been shown to successfully reduce heroin-seeking in rats following heroin priming but which have not been shown to be effective in relation to stress-induced reinstatement (Leri, Tremblay, Sorge, & Stewart, 2004; Shaham & Stewart, 1996; Sorge, Rajabi, & Stewart, 2005).
Recent preclinical research suggests the involvement of brain stress pathways in stress-induced opioid, cocaine, and alcohol reinstatement as well as in protracted withdrawal symptoms (Aston-Jones & Harris, 2004; Le et al., 2000; Liu & Weiss, 2002; Shaham, Erb, Leung, Buczek, & Stewart, 1998; Shaham et al., 1997). For example, central norepinephrine activation of corticotropin-releasing factor receptors in the bed nucleus of the stria terminalis has been shown to mediate stress-induced reinstatement (Leri, Flores, Rodaros, & Stewart, 2002; Stewart, 2003, for review). In view of this, the administration of naltrexone with alpha 2-adrenoceptor agonists such as lofexidine and clonidine have been shown to effectively block stress-induced reinstatement of heroin in laboratory rats (Highfield, Yap, Grimm, Shalev, & Shaham, 2001; Shaham, Highfield, Delfs, Leung, & Stewart, 2000), as well as to facilitate the acceptance of naltrexone treatment in heroin-dependent humans (Gerra et al., 2001; Lin, Strang, Su, Tsai, & Hu, 1997). Administration of these agents may enhance naltrexone treatment outcomes.
Cognitive–behavioral and mindfulness-based interventions combined with stress-reducing pharmacological agents may improve outcomes as well. For instance, mindfulness-based stress reduction has demonstrated success in reducing stress and psychiatric symptoms in a number of studies (e.g., Carlson, Ursuliak, Goodey, Angen, & Speca, 2001; Kabat-Zinn et al., 1992; Rosenzweig, Reibel, Greeson, Brainard, & Hojat, 2003) and has been advocated for use in the treatment of addictive disorders (Marlatt, 2002). Other stress-reducing interventions, such as stress inoculation training (Meichenbaum, 1985), relaxation training (see Lehrer, 1996), and exercise (see Hays, 1999), may also help reduce stress-related craving and hyperarousal responses and improve naltrexone treatment outcomes.
The main study limitation is the lack of a placebo-treated control group to fully compare the naltrexone versus placebo effects on stress- and drug-cue-induced craving and arousal. However, it is important to note that due to the extremely high rates of opioid relapse in unmedicated opioid addicts (over 90%), maintaining placebo-treated opioid-dependent individuals in outpatient treatment is largely unfeasible. Our findings should also be considered preliminary and limited due to the small sample size. Despite these limitations, the current findings are the first to indicate that chronic naltrexone treatment is associated with increases in stress- and drug-cue-induced craving and associated arousal, suggesting the need for specific attention to stress and drug-cue reactivity in opioid relapse prevention. It is possible that naltrexone treatment may be improved by addressing the stress-related drug craving and arousal responses commonly observed in opioid-dependent patients early in recovery.
Acknowledgments
This research was supported by National Institutes of Health Grants R01-DA18219, P50DA16556, and K02-DA17232. We thank the staff at the Substance Abuse Treatment Unit of the Connecticut Mental Health Center for their support of this work.
Footnotes
Because baseline differences were observed for SBP, we conducted a second analysis using change from baseline data. The findings still indicated a significant effect of condition, F(2, 26) = 6.0, p = .01, where higher SBP was demonstrated in the stress condition compared with the neutral condition ( p = .03).
References
- Alderson HL, Robbins TW, Everitt BJ. Heroin self-administration under a second-order schedule of reinforcement: Acquisition and maintenance of heroin-seeking behaviour in rats. Psychopharmacology (Berl) 2000;153:120–133. doi: 10.1007/s002130000429. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47(Suppl 1):167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Phillips G, Green L, Gossop M. Circumstances surrounding the initial relapse to opiate use following detoxification. British Journal of Psychiatry. 1989;154:354–359. doi: 10.1192/bjp.154.3.354. [DOI] [PubMed] [Google Scholar]
- Brady KT, Back SE, Waldrop AE, McRae AL, Anton RF, Upadhyaya HP, et al. Cold pressor task reactivity: Predictors of alcohol use among alcohol-dependent individuals with and without comorbid posttraumatic stress disorder. Alcoholism: Clinical and Experimental Research. 2006;30:938–946. doi: 10.1111/j.1530-0277.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, et al. Stress enhancement of craving during sobriety: A risk for relapse. Alcoholism: Clinical and Experimental Research. 2005;29:185–195. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. Heroin dependence. Wisconsin Medical Journal. 2004;103(4):20–26. [PubMed] [Google Scholar]
- Carlson LE, Ursuliak Z, Goodey E, Angen M, Speca M. The effects of a mindfulness meditation-based stress reduction program on mood and symptoms of stress in cancer outpatients: 6-month follow-up. Supportive Care in Cancer. 2001;9:112–123. doi: 10.1007/s005200000206. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Sinha R, Nich C, Babuscio T, Rounsaville BJ. Contingency management to enhance naltrexone treatment of opioid dependence: A randomized clinical trial of reinforcement magnitude. Experimental and Clinical Psychopharmacology. 2002;10:54–63. doi: 10.1037//1064-1297.10.1.54. [DOI] [PubMed] [Google Scholar]
- Childress AR, Ehrman R, McLellan T, MacRae J, Natale M, O’Brien CP. Can induced moods trigger drug-related responses in opiate abuse patients? Journal of Substance Abuse Treatment. 1994;11:17–23. doi: 10.1016/0740-5472(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. Journal of Abnormal Psychology. 1997;106:243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Do Couto BR, Aguilar MA, Manzanedo C, Rodriguez-Arias M, Armario A, Minarro J. Social stress is as effective as physical stress in reinstating morphine-induced place preference in mice. Psychopharmacology (Berl) 2006;185:459–470. doi: 10.1007/s00213-006-0345-z. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV Axis I disorders–Patient edition (SCID–I/P, Version 2.0) New York: New York State Psychiatric Institute; 1997. [Google Scholar]
- Franken IHA, De Hann HA, Van Der Meer CW, Haffmans PMJ, Hendriks VM. Cue reactivity and effects of cue exposure in abstinent posttreatment drug users. Journal of Substance Abuse Treatment. 1999;16:81–85. doi: 10.1016/s0740-5472(98)00004-x. [DOI] [PubMed] [Google Scholar]
- Gerra G, Zaimovic A, Giusti F, Di Gennaro C, Zambelli U, Gardini S, Delsignore R. Lofexidine versus clonidine in rapid opiate detoxification. Journal of Substance Abuse Treatment. 2001;21:11–17. doi: 10.1016/s0740-5472(01)00178-7. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The impact of stress on addiction. European Neuropsychopharmacology. 2003;13:435–441. doi: 10.1016/j.euroneuro.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Hays KF. Working it out: Using exercise in psychotherapy. Washington, DC: American Psychological Association; 1999. [Google Scholar]
- Highfield D, Yap J, Grimm JW, Shalev U, Shaham Y. Repeated lofexidine treatment attenuates stress-induced, but not drug cues-induced reinstatement of a heroin-cocaine mixture (speedball) seeking in rats. Neuropsychopharmacology. 2001;25:320–331. doi: 10.1016/S0893-133X(01)00227-5. [DOI] [PubMed] [Google Scholar]
- Izard C. Patterns of emotions: A new analysis of anxiety and depression. New York: Academic Press; 1972. [Google Scholar]
- Kabat-Zinn J, Massion AO, Kristeller J, Peterson LG, Fletcher KE, Pbert L, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. American Journal of Psychiatry. 1992;149:936–943. doi: 10.1176/ajp.149.7.936. [DOI] [PubMed] [Google Scholar]
- Killian AK, Bonese K, Schuster CR. The effects of naloxone on behavior maintained by cocaine and heroin injections in the rhesus monkey. Drug and Alcohol Dependence. 1978;3:243–251. doi: 10.1016/0376-8716(78)90078-9. [DOI] [PubMed] [Google Scholar]
- Kirchmayer U, Davoli M, Verster AD, Amato L, Ferri M, Perucci CA. A systematic review on the efficacy of naltrexone maintenance treatment in opioid dependence. Addiction. 2002;97:1241–1249. doi: 10.1046/j.1360-0443.2002.00217.x. [DOI] [PubMed] [Google Scholar]
- Klein LC, Jamner LD, Alberts J, Ornstein MD, Leigh H. Sex differences in salivary cortisol levels following naltrexone administration. Journal of Applied Biobehavioral Research. 2000;5:144–153. [Google Scholar]
- Kosten TR, Kreek MJ, Ragunath J, Kleber HD. Cortisol levels during chronic naltrexone maintenance treatment in ex-opiate addicts. Biological Psychiatry. 1986;21:217–220. doi: 10.1016/0006-3223(86)90150-2. [DOI] [PubMed] [Google Scholar]
- Landsberg R, Taintor Z, Plumb M, Amico L, Wicks N. An analysis of naltrexone use: Its efficacy, safety and potential. National Institute on Drug Abuse Research Monograph. 1976;9:106–113. doi: 10.1037/e497452006-020. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Lehrer PM. Varieties of relaxation methods and their unique effects. International Journal of Stress Management. 1996;3:1–15. [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. Journal of Neuroscience. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Tremblay A, Sorge RE, Stewart J. Methadone maintenance reduces heroin- and cocaine-induced relapse without affecting stress-induced relapse in a rodent model of poly-drug use. Neuropsychopharmacology. 2004;29:1312–1320. doi: 10.1038/sj.npp.1300435. [DOI] [PubMed] [Google Scholar]
- Lin SK, Strang J, Su LW, Tsai CJ, Hu WH. Double-blind randomised controlled trial of lofexidine versus clonidine in the treatment of heroin withdrawal. Drug and Alcohol Dependence. 1997;48:127–133. doi: 10.1016/s0376-8716(97)00116-6. [DOI] [PubMed] [Google Scholar]
- Littell R, Milliken G, Strout W, Wolfinger R. SAS systems for mixed models. Cary, NC: SAS Institute; 1996. [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: Exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. Journal of Neuroscience. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt AG. Buddhist philosophy and the treatment of addictive behavior. Cognitive and Behavioral Practice. 2002;9:44–50. [Google Scholar]
- McCubbin JA, Bruehl S, Wilson JF, Sherman JJ, Norton JA, Colclough G. Endogenous opioids inhibit ambulatory blood pressure during naturally occurring stress. Psychosomatic Medicine. 1998;60:227–231. doi: 10.1097/00006842-199803000-00020. [DOI] [PubMed] [Google Scholar]
- Meichenbaum D. Stress inoculation training. New York: Pergamon Press; 1985. [Google Scholar]
- Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- National Center on Addiction and Substance Abuse at Columbia University. Under the counter: The diversion and abuse of controlled prescription drugs in the US. New York: Author; 2005. Jul, [Google Scholar]
- Powell J, Bradley B, Gray J. Classical conditioning and cognitive determinants of subjective craving for opiates: An investigation of their relative contributions. British Journal of Addiction. 1992;87:1133–1144. doi: 10.1111/j.1360-0443.1992.tb02000.x. [DOI] [PubMed] [Google Scholar]
- Rosenzweig S, Reibel DK, Greeson JM, Brainard GC, Hojat M. Mindfulness-based stress reduction lowers psychological distress in medical students. Teaching and Learning in Medicine. 2003;15:88–92. doi: 10.1207/S15328015TLM1502_03. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ. Can psychotherapy rescue naltrexone treatment of opioid addiction? In: Onken LS, Blaine JD, editors. Potentiating the efficacy of medications: Integrating psychosocial therapies with pharmacotherapies in the treatment of drug dependence (NIDA Research Monograph Series No. 105, NIH Publication No. 95–3899. Rockville, MD: National Institute on Drug Abuse; 1995. pp. 37–52. [PubMed] [Google Scholar]
- Shaham Y, Erb S, Leung S, Buczek Y, Stewart J. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor type 1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berl) 1998;137:184–190. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticosterone does not contribute to relapse to heroin seeking in rats induced by footshock stress or priming injections of heroin. Journal of Neuroscience. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Highfield D, Delfs J, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: An effect independent of locus coeruleus nor-adrenergic neurons. European Journal of Neuroscience. 2000;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: An effect mimicking heroin, not withdrawal. Psychopharmacologia. 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Effects of opioid and dopamine receptor antagonists on relapse induced by stress and re-exposure to heroin in rats. Psychopharmacology (Berl) 1996;125:385–391. doi: 10.1007/BF02246022. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin L, O’Malley S. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and HPA responses predict cocaine relapse outcomes. Archives of General Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug-cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Rajabi H, Stewart J. Rats maintained chronically on buprenorphine show reduced heroin and cocaine seeking in tests of extinction and drug-induced reinstatement. Neuropsychopharmacology. 2005;30:1681–1692. doi: 10.1038/sj.npp.1300712. [DOI] [PubMed] [Google Scholar]
- Stewart J. Stress and relapse to drug seeking: Studies in laboratory animals shed light on mechanisms and sources of long-term vulnerability. American Journal on Addictions. 2003;12:1–17. [PubMed] [Google Scholar]
- Tucker TK, Ritter AJ. Naltrexone in the treatment of heroin dependence: A literature review. Drug and Alcohol Review. 2000;19:73–82. [Google Scholar]
- Uhart M, Chong RY, Oswald L, Lin PI, Wand GS. Gender differences in hypothalamic-pituitary-adrenal (HPA) axis reactivity. Psychoneuroendocrinology. 2006;31:642–652. doi: 10.1016/j.psyneuen.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Wallace BC. Psychological and environmental determinants of relapse in crack cocaine smokers. Journal of Substance Abuse Treatment. 1989;6:95–106. doi: 10.1016/0740-5472(89)90036-6. [DOI] [PubMed] [Google Scholar]
- Weinstein A, Wilson S, Bailey J, Myles J, Nutt D. Imagery of craving in opiate addicts undergoing detoxification. Drug and Alcohol Dependence. 1997;48:25–31. doi: 10.1016/s0376-8716(97)00098-7. [DOI] [PubMed] [Google Scholar]
- Zacny J, Bigelow G, Compton P, Foley K, Iguchi M, Sannerud C. College on Problems of Drug Dependence taskforce on prescription opioid non-medical use and abuse: Position statement. Drug and Alcohol Dependence. 2003;69:215–232. doi: 10.1016/s0376-8716(03)00003-6. [DOI] [PubMed] [Google Scholar]