Abstract
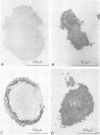
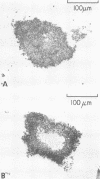
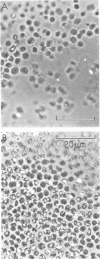
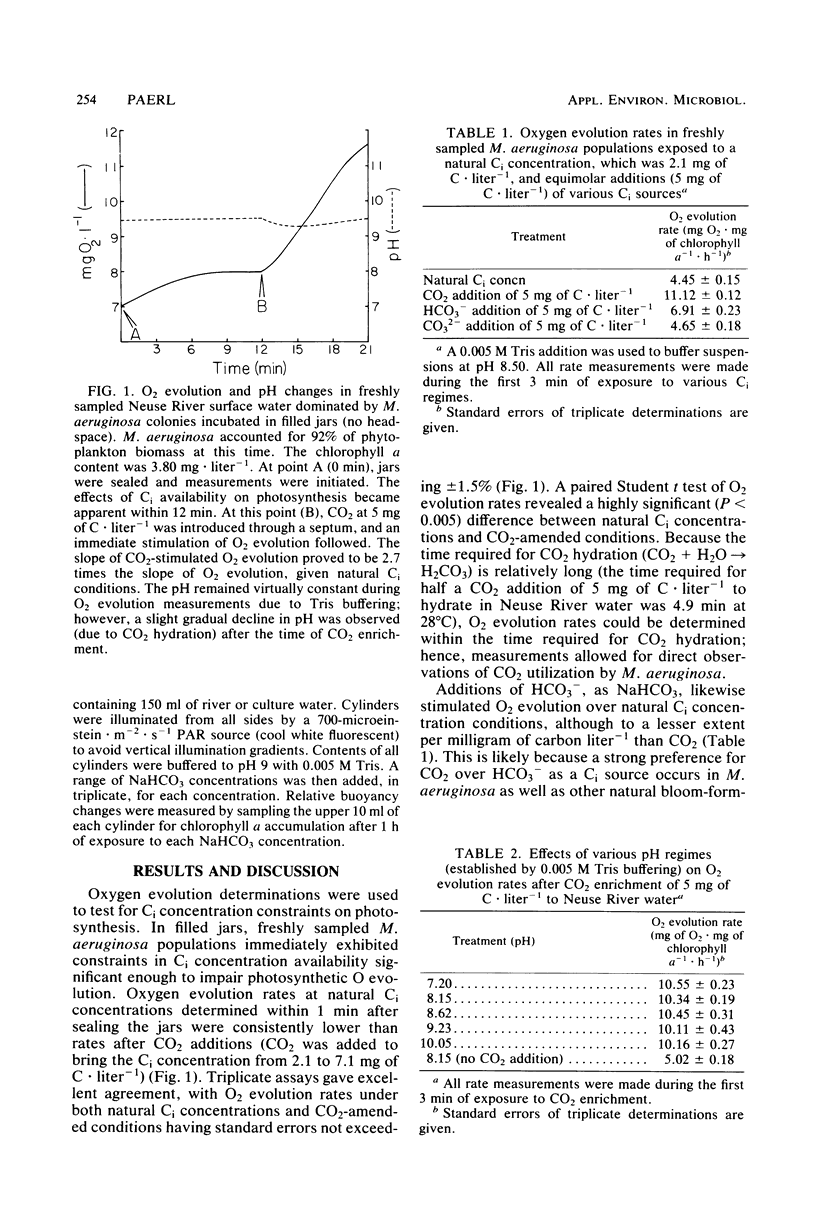
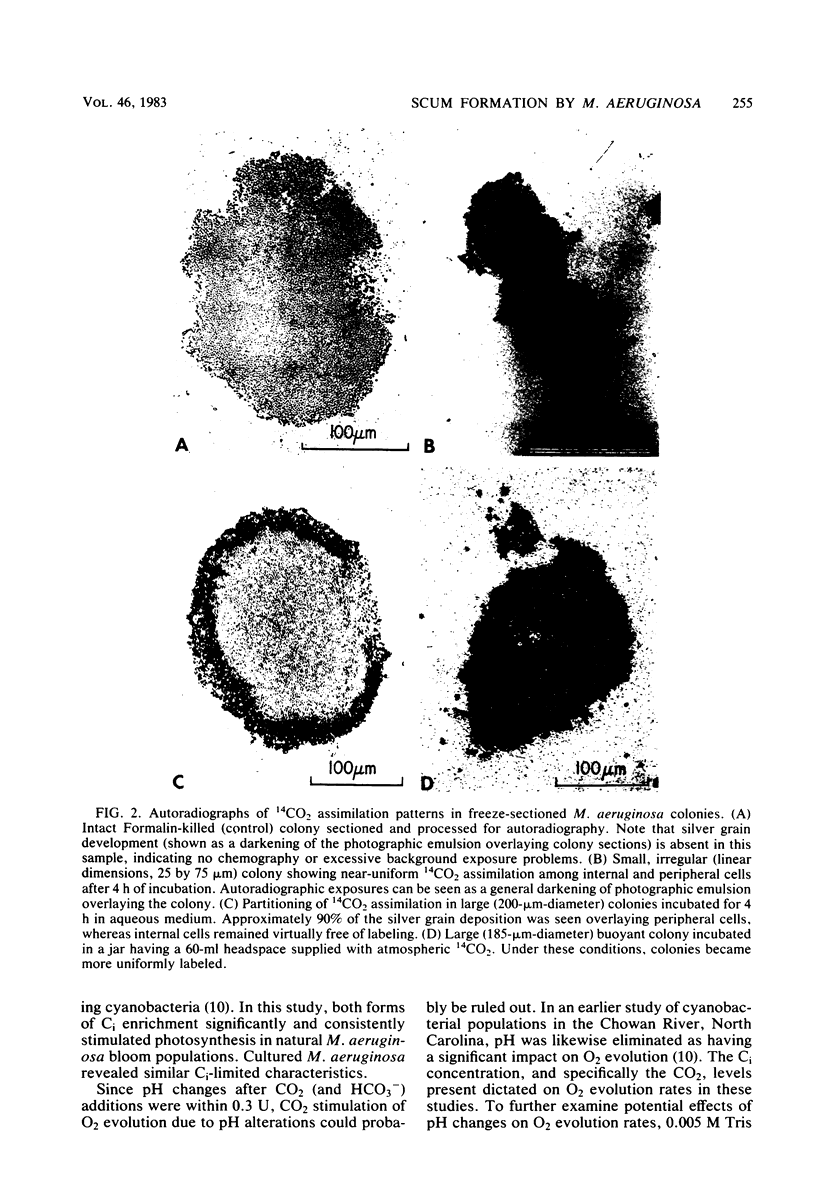
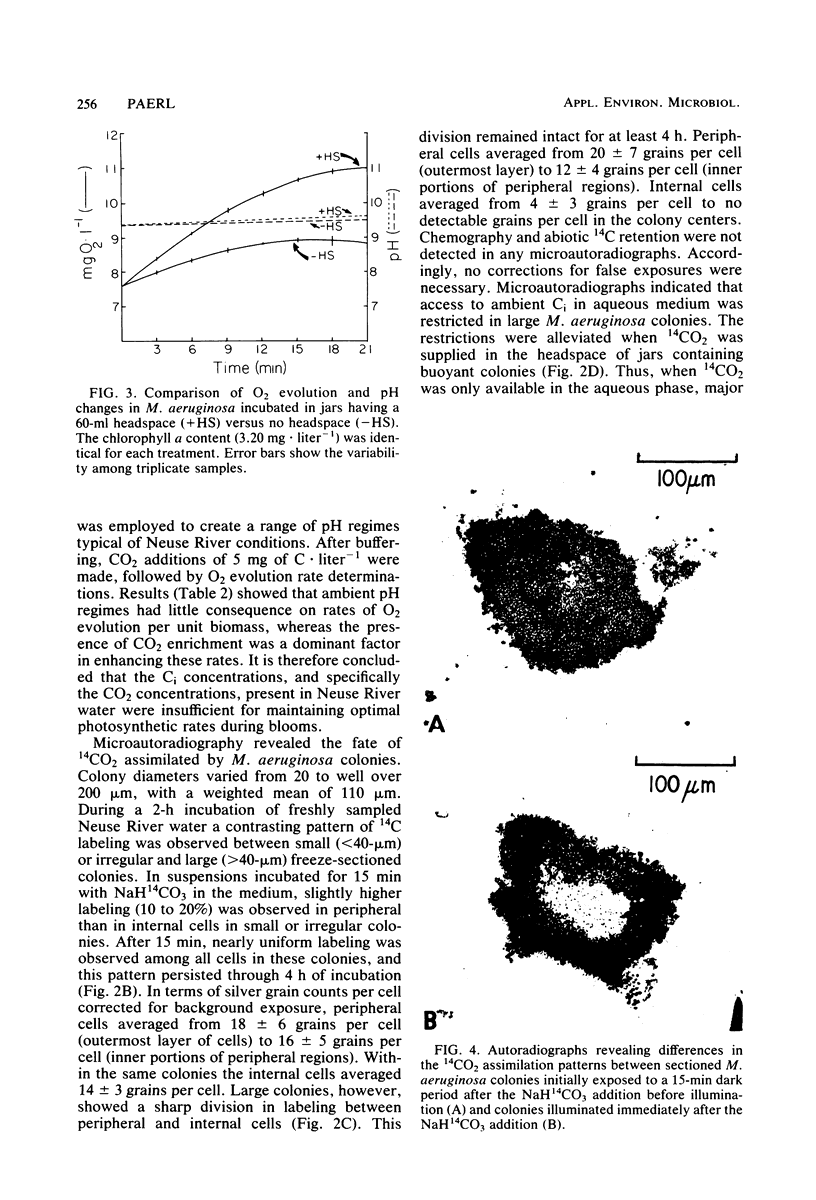
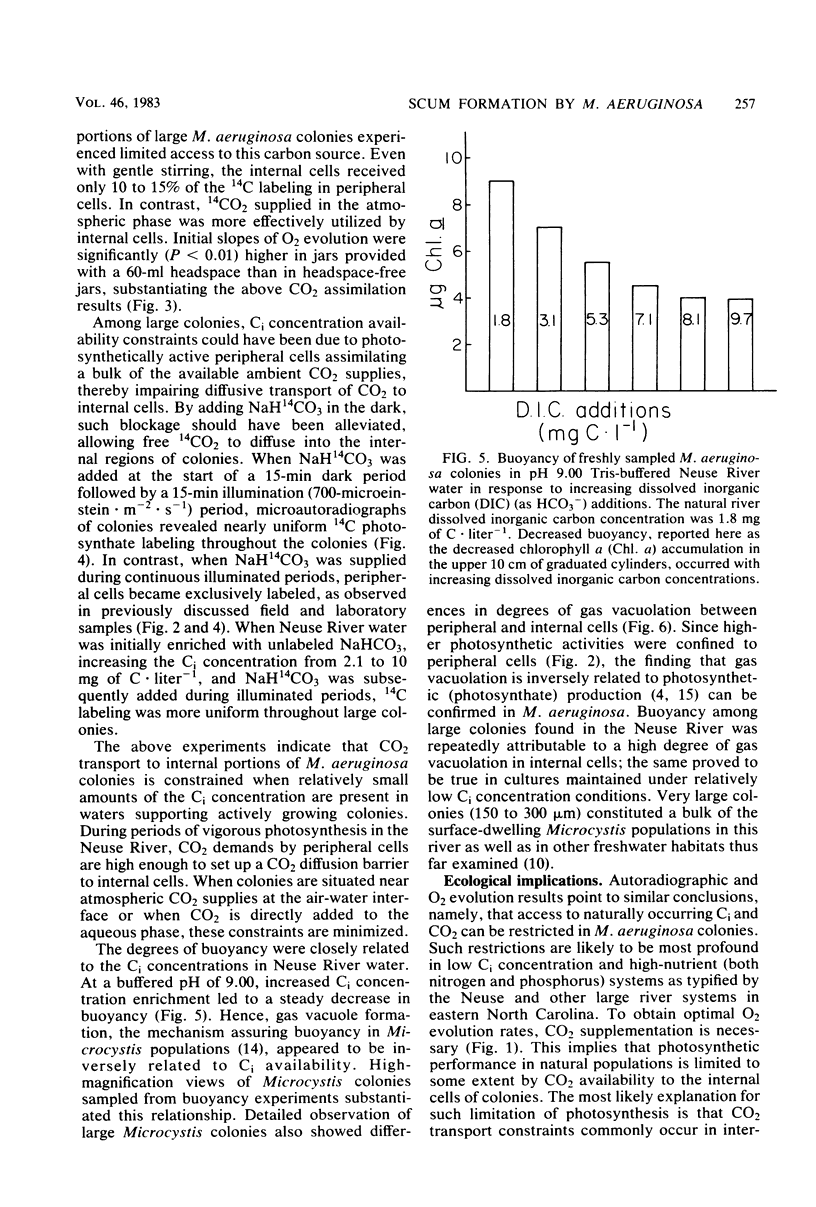
Constraints on inorganic carbon (Ci) availability stimulated buoyancy in natural, photosynthetically active populations of the colonial blue-green alga (cyanobacterium) Microcystis aeruginosa. In nonmixed eutrophic river water and cultures, O2 evolution determinations indicated Ci limitation of photosynthesis, which was overcome either by CO2 additions to the aqueous phase or by exposure of buoyant colonies to atmospheric CO2. Microautoradiographs of M. aeruginosa colonies revealed partitioning of 14CO2 fixation and photosynthate accumulation between peripheral and internal cells, particularly in large colonies. When illuminated colonies were suspended in the aqueous phase, peripheral cells accounted for at least 90% of the 14CO2 assimilation, whereas internal cells remained unlabeled. However, when 14CO2 was allowed to diffuse into colonies 15 min before illumination, a more uniform distribution of labeling was observed. Resultant differences in labeling patterns were most likely due to peripheral cells more exclusively utilizing CO2 when ambient Ci concentrations were low. Among colonies located at the air-water interface, internal cells showed an increased share of photosynthate production when atmospheric 14CO2 was supplied. This indicated that Ci transport was restricted in large colonies below the water surface, forcing internal cells to maintain a high degree of buoyancy, thus promoting the formation of surface scums. At the surface, Ci restrictions were alleviated. Accordingly, scum formation appears to have an ecological function, allowing cyanobacteria access to atmospheric CO2 when the Ci concentration is growth limiting in the water column.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpenter E. J., Price C. C. Marine oscillatoria (Trichodesmium): explanation for aerobic nitrogen fixation without heterocysts. Science. 1976 Mar 26;191(4233):1278–1280. doi: 10.1126/science.1257749. [DOI] [PubMed] [Google Scholar]
- Goldman J. C. Letter: Carbon dioxide and pH: effect on species succession of algae. Science. 1973 Oct 19;182(4109):306–307. doi: 10.1126/science.182.4109.306. [DOI] [PubMed] [Google Scholar]
- Kellar P. E., Paerl H. W. Physiological adaptations in response to environmental stress during an n(2)-fixing anabaena bloom. Appl Environ Microbiol. 1980 Sep;40(3):587–595. doi: 10.1128/aem.40.3.587-595.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemer A. R., Feuillade J., Feuillade M. Cyanobacterial blooms: carbon and nitrogen limitation have opposite effects on the buoyancy of oscillatoria. Science. 1982 Mar 26;215(4540):1629–1631. doi: 10.1126/science.215.4540.1629. [DOI] [PubMed] [Google Scholar]
- Shapiro J. Blue-green algae: why they become dominant. Science. 1973 Jan 26;179(4071):382–384. doi: 10.1126/science.179.4071.382. [DOI] [PubMed] [Google Scholar]
- Walsby A. E. Structure and function of gas vacuoles. Bacteriol Rev. 1972 Mar;36(1):1–32. doi: 10.1128/br.36.1.1-32.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]