Abstract
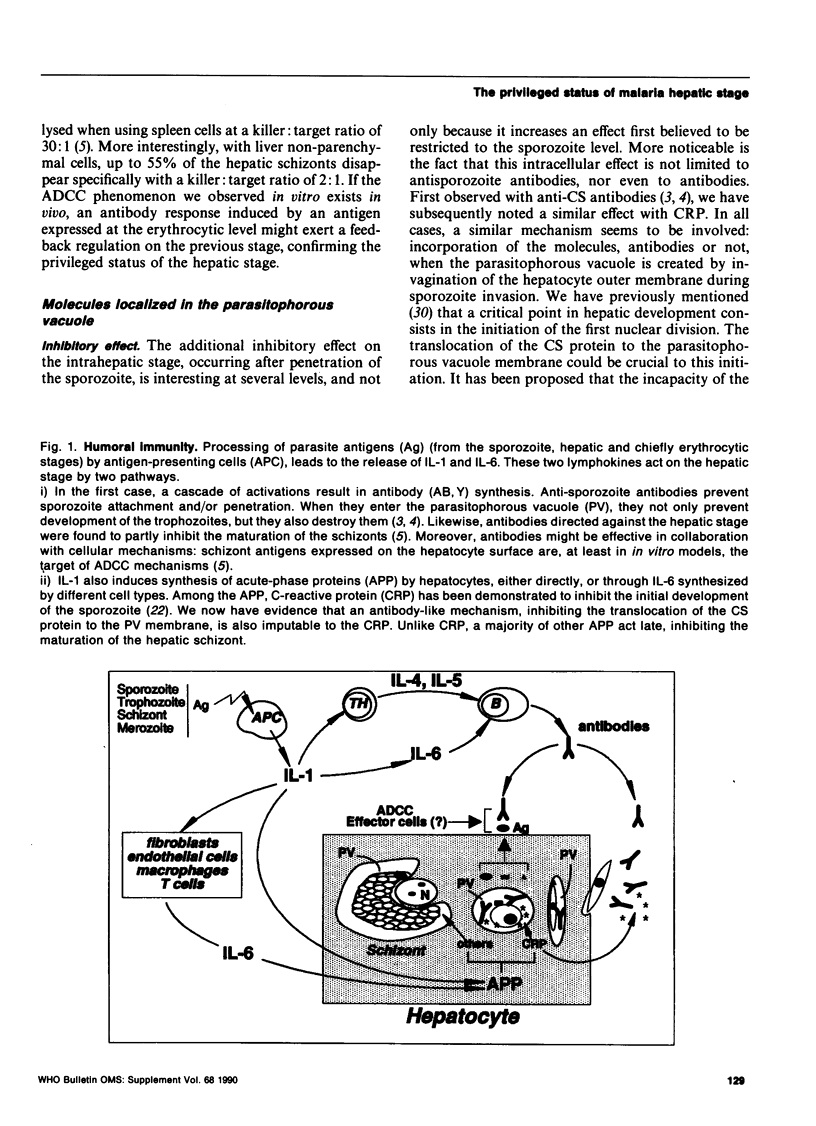
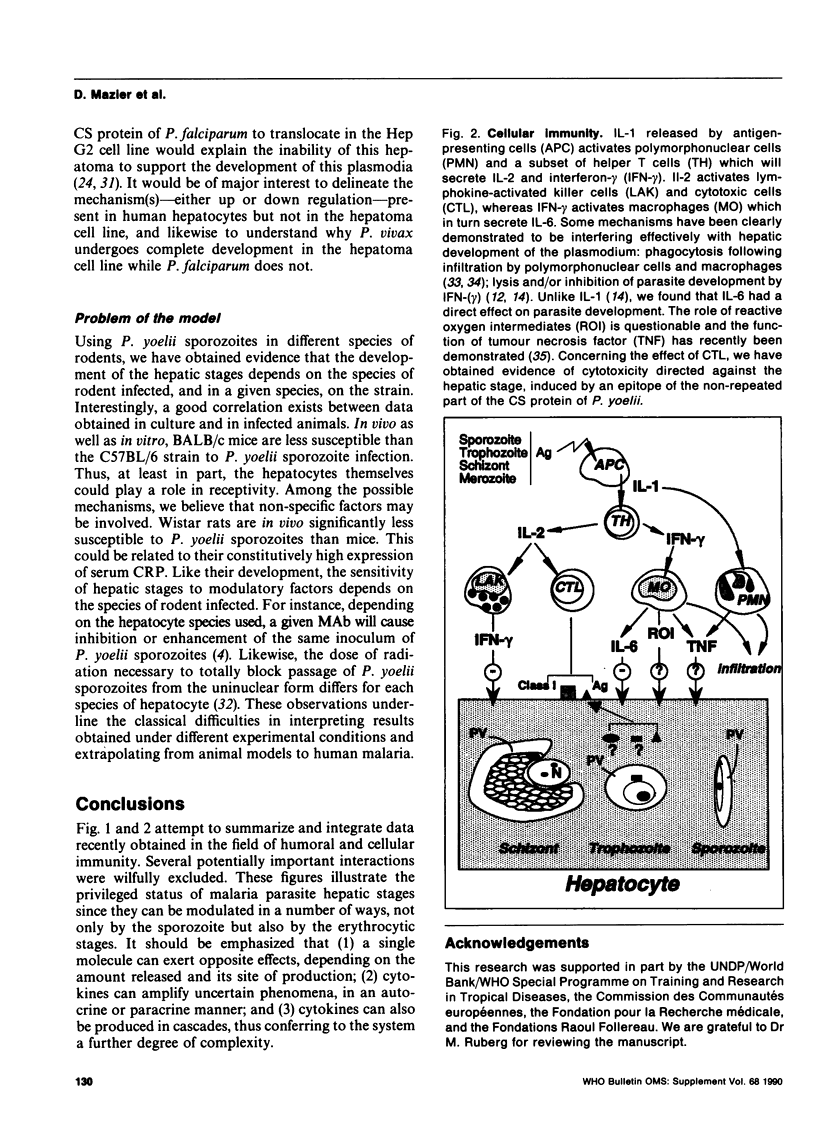
Besides potential interest in itself, the hepatic stage of malaria might play a crucial role as "go-between" with other stages. When present in the parasitophorous vacuole, antibodies induced by both sporozoite and erythrocytic stages efficiently disturb hepatic development of the parasite. Likewise previous and ensuing erythrocytic stages can modulate the "shielded" phase by cytokines, directly or as a result of a cascade of events, and by MHC-restricted or antibody-dependent cytotoxic mechanisms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aley S. B., Atkinson C. T., Aikawa M., Maloy W. L., Hollingdale M. R. Ultrastructural localization of Plasmodium falciparum circumsporozoite protein in newly invaded hepatoma cells. J Parasitol. 1987 Dec;73(6):1241–1245. [PubMed] [Google Scholar]
- Ardeshir F., Flint J. E., Richman S. J., Reese R. T. A 75 kd merozoite surface protein of Plasmodium falciparum which is related to the 70 kd heat-shock proteins. EMBO J. 1987 Feb;6(2):493–499. doi: 10.1002/j.1460-2075.1987.tb04780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardosa M. J., Porterfield J. S., Gordon S. Complement receptor mediates enhanced flavivirus replication in macrophages. J Exp Med. 1983 Jul 1;158(1):258–263. doi: 10.1084/jem.158.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Virelizier J. L., Carswell E. A., Wood P. R. Possible importance of macrophage-derived mediators in acute malaria. Infect Immun. 1981 Jun;32(3):1058–1066. doi: 10.1128/iai.32.3.1058-1066.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain R. N. Immunology. The ins and outs of antigen processing and presentation. Nature. 1986 Aug 21;322(6081):687–689. doi: 10.1038/322687a0. [DOI] [PubMed] [Google Scholar]
- Guerin-Marchand C., Druilhe P., Galey B., Londono A., Patarapotikul J., Beaudoin R. L., Dubeaux C., Tartar A., Mercereau-Puijalon O., Langsley G. A liver-stage-specific antigen of Plasmodium falciparum characterized by gene cloning. Nature. 1987 Sep 10;329(6135):164–167. doi: 10.1038/329164a0. [DOI] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977 Jul 1;146(1):201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jap P. H., Meis J. F., Verhave J. P., Meuwissen J. H. Degenerating exo-erythrocytic forms of Plasmodium berghei in rat liver: an ultrastructural and cytochemical study. Parasitology. 1982 Oct;85(Pt 2):263–269. doi: 10.1017/s0031182000055244. [DOI] [PubMed] [Google Scholar]
- Kumar N., Syin C. A., Carter R., Quakyi I., Miller L. H. Plasmodium falciparum gene encoding a protein similar to the 78-kDa rat glucose-regulated stress protein. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6277–6281. doi: 10.1073/pnas.85.17.6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellouk S., Maheshwari R. K., Rhodes-Feuillette A., Beaudoin R. L., Berbiguier N., Matile H., Miltgen F., Landau I., Pied S., Chigot J. P. Inhibitory activity of interferons and interleukin 1 on the development of Plasmodium falciparum in human hepatocyte cultures. J Immunol. 1987 Dec 15;139(12):4192–4195. [PubMed] [Google Scholar]
- Naik P., Voller A. Serum C-reactive protein levels and falciparum malaria. Trans R Soc Trop Med Hyg. 1984;78(6):812–813. doi: 10.1016/0035-9203(84)90027-0. [DOI] [PubMed] [Google Scholar]
- Nüssler A., Follezou J. Y., Miltgen F., Mazier D. Effect of irradiation on Plasmodium sporozoites depends on the species of hepatocyte infected. Trop Med Parasitol. 1989 Dec;40(4):468–469. [PubMed] [Google Scholar]
- Ojo-Amaize E. A., Salimonu L. S., Williams A. I., Akinwolere O. A., Shabo R., Alm G. V., Wigzell H. Positive correlation between degree of parasitemia, interferon titers, and natural killer cell activity in Plasmodium falciparum-infected children. J Immunol. 1981 Dec;127(6):2296–2300. [PubMed] [Google Scholar]
- Rhodes-Feuillette A., Bellosguardo M., Druilhe P., Ballet J. J., Chousterman S., Canivet M., Périès J. The interferon compartment of the immune response in human malaria: II. Presence of serum-interferon gamma following the acute attack. J Interferon Res. 1985 Winter;5(1):169–178. doi: 10.1089/jir.1985.5.169. [DOI] [PubMed] [Google Scholar]
- Schofield L., Villaquiran J., Ferreira A., Schellekens H., Nussenzweig R., Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987 Dec 17;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- Vergara U., Ferreira A., Schellekens H., Nussenzweig V. Mechanism of escape of exoerythrocytic forms (EEF) of malaria parasites from the inhibitory effects of interferon-gamma. J Immunol. 1987 Jun 15;138(12):4447–4449. [PubMed] [Google Scholar]
- Weiss W. R., Sedegah M., Beaudoin R. L., Miller L. H., Good M. F. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci U S A. 1988 Jan;85(2):573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]



