Abstract
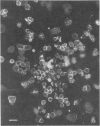
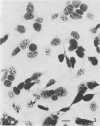
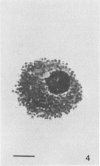
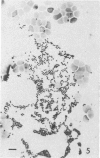
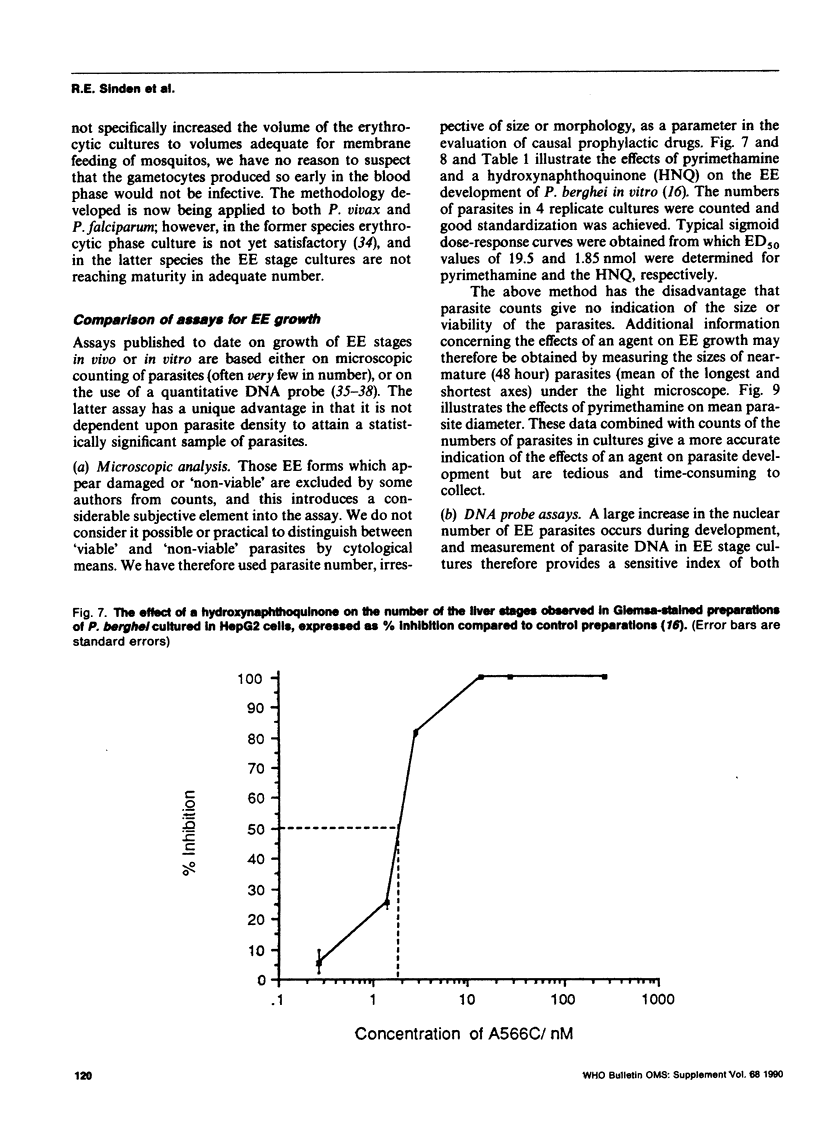
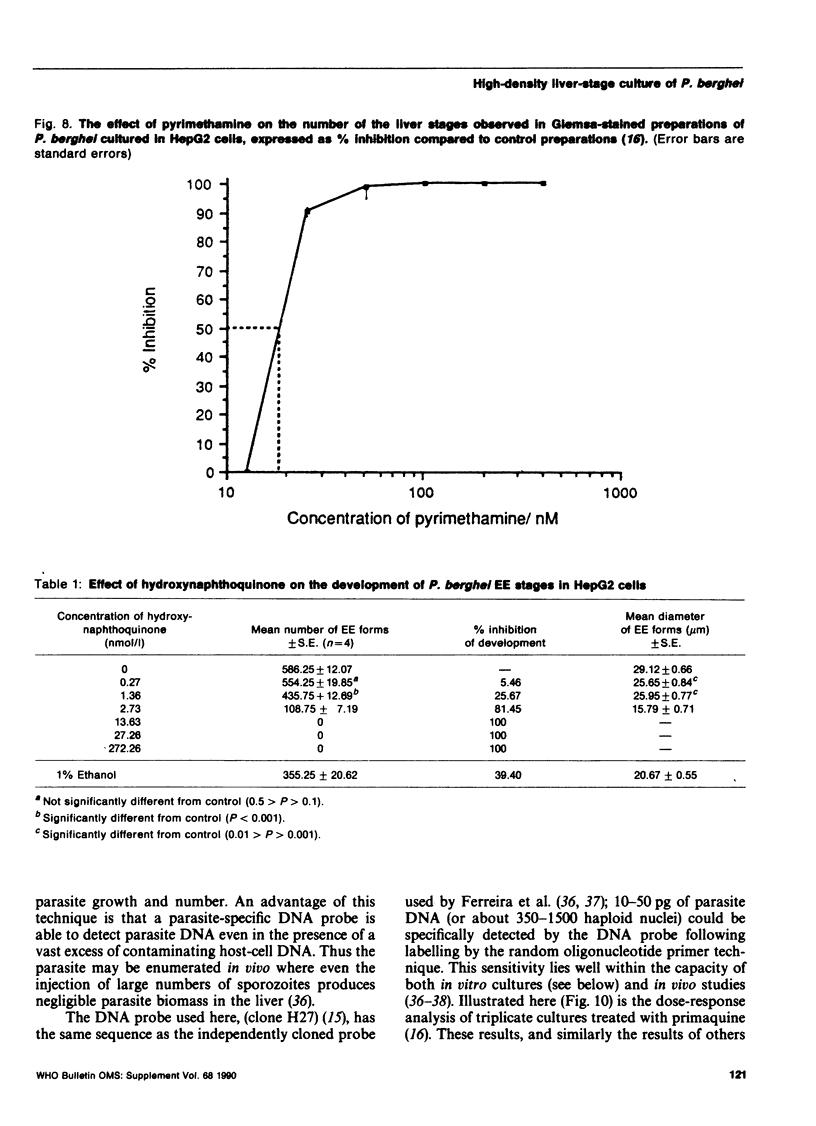
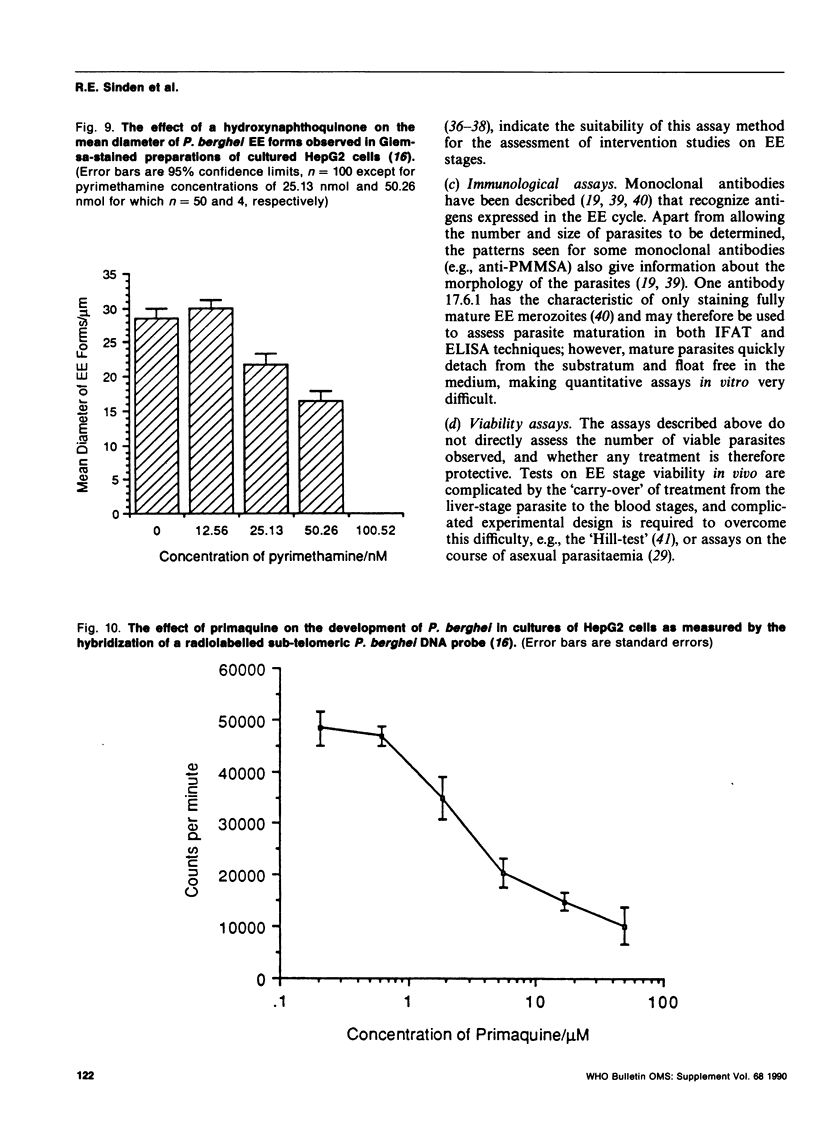
Methods are reviewed for the culture of the exoerythrocytic stages of Plasmodium berghei wherein development reproducibly reflects growth observed in vivo in laboratory rodents. The combination of these methods with the culture of both asexual and sexual blood stages has allowed the completion of the entire vertebrate phase of malaria development in vitro. The development of new methods for high-density exoerythrocytic-stage culture combined with robust statistical analysis of parasite growth by morphological (light microscopy), or DNA probe methods now allows the critical and precise evaluation of chemotherapeutic or immunological treatments. These methods are illustrated by data obtained on pyrimethamine, primaquine and a hydroxynaphthoquinone. Some of the new avenues of research made feasible by the high-density cultures, e.g., direct immunization to produce monoclonal antibodies and biochemical studies are discussed.
Full text
PDF

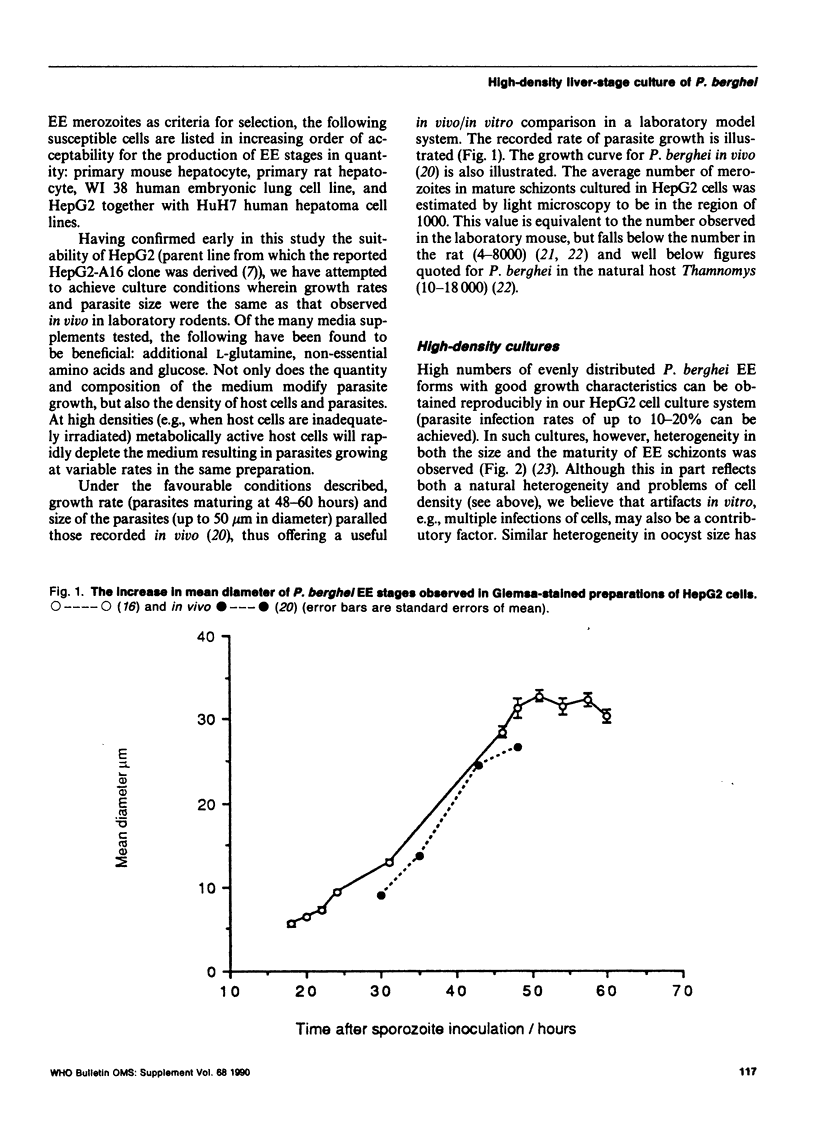
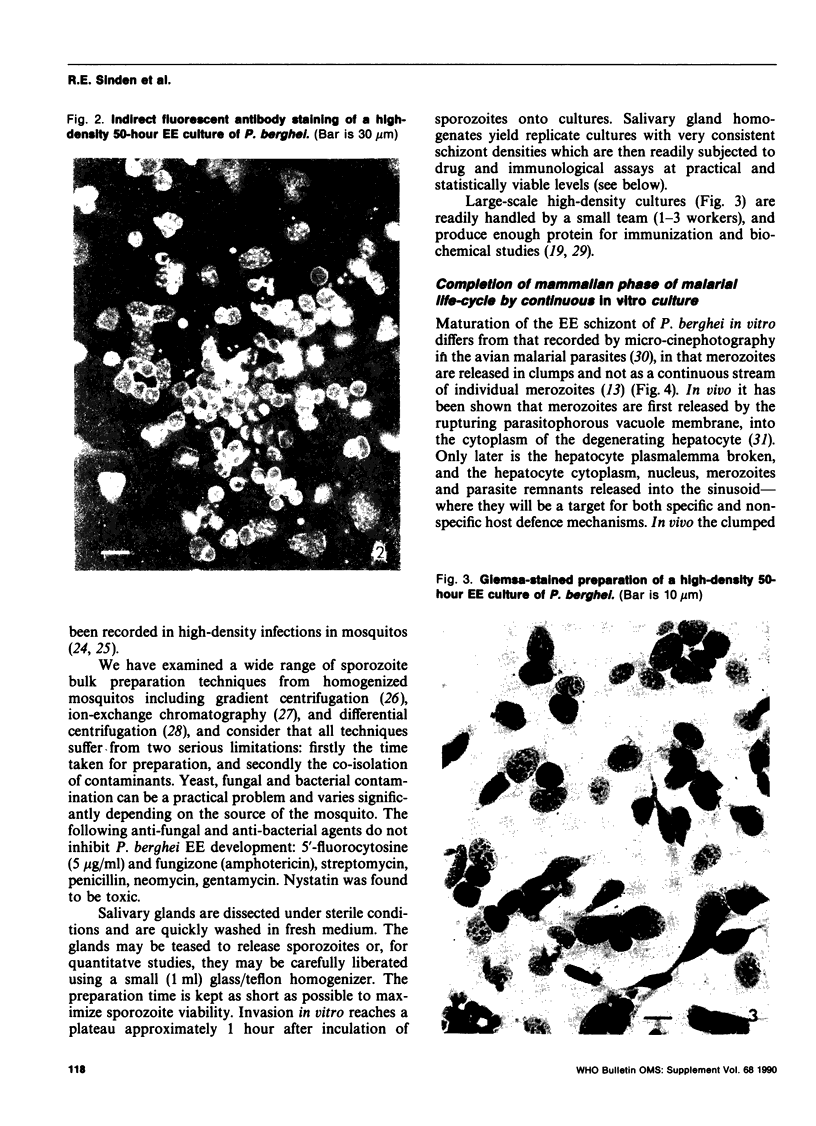
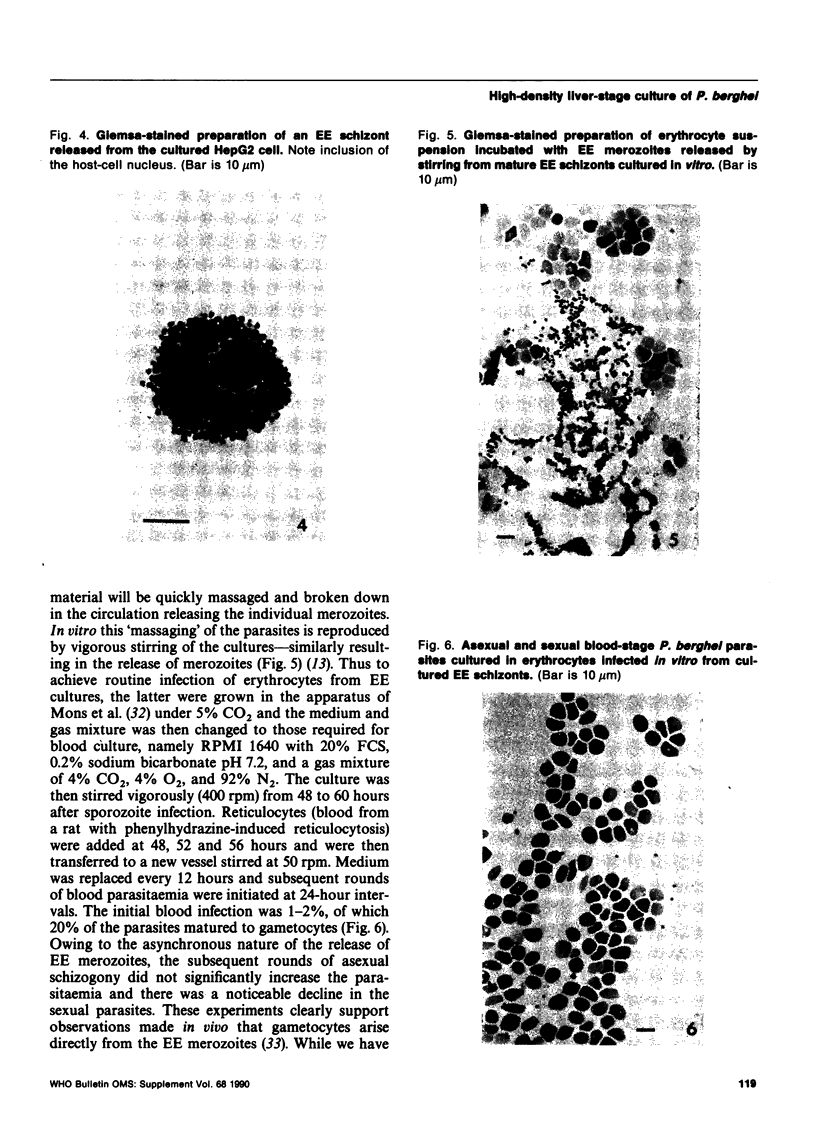



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DUBIN I. N. The cultivation of the exoerythrocytic forms of Plasmodium gallinaceum in tissue culture. J Infect Dis. 1952 Jul-Aug;91(1):33–49. doi: 10.1093/infdis/91.1.33. [DOI] [PubMed] [Google Scholar]
- Dawson J. R., Adams D. J., Wolf C. R. Induction of drug metabolizing enzymes in human liver cell line Hep G2. FEBS Lett. 1985 Apr 22;183(2):219–222. doi: 10.1016/0014-5793(85)80780-8. [DOI] [PubMed] [Google Scholar]
- HUFF C. G., PIPKIN A. C., WEATHERSBY A. B., JENSEN D. V. The morphology and behavior of living exoerythrocytic stages of Plasmodium gallinaceum and P. fallax and their host cells. J Biophys Biochem Cytol. 1960 Feb;7:93–102. doi: 10.1083/jcb.7.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killick-Kendrick R., Warren M. Primary exoerythrocytic schizonts of a mammalian Plasmodium as a source of gametocytes. Nature. 1968 Oct 12;220(5163):191–192. doi: 10.1038/220191a0. [DOI] [PubMed] [Google Scholar]
- Landau I., Killick-Kendrick R. Rodent plasmodia of the République Centrafricaine: the sporogony and tissue stages of Plasmodium chabaudi and P. berghei yoelii. Trans R Soc Trop Med Hyg. 1966;60(5):633–649. doi: 10.1016/0035-9203(66)90010-1. [DOI] [PubMed] [Google Scholar]
- Millet P., Landau I., Baccam D., Miltgen F., Peters W. La culture des schizontes exo-érythocytaires des Plasmodium de rongeurs dans des hépatocytes: un nouveau modèle expérimental pour la chimiothérapie du paludisme. C R Acad Sci III. 1985;301(8):403–406. [PubMed] [Google Scholar]
- Mons B., Croon J. J., van der Star W., van der Kaay H. J. Erythrocytic schizogony and invasion of Plasmodium vivax in vitro. Int J Parasitol. 1988 Apr;18(3):307–311. doi: 10.1016/0020-7519(88)90138-5. [DOI] [PubMed] [Google Scholar]
- Mons B., Janse C. J., Croon J. J., van der Kaay H. J. In vitro culture of Plasmodium berghei using a new suspension system. Int J Parasitol. 1983 Apr;13(2):213–217. doi: 10.1016/0020-7519(83)90015-2. [DOI] [PubMed] [Google Scholar]
- Moser G., Brohn F. H., Danforth H. D., Nussenzweig R. S. Sporozoites of rodent and simian malaria, purified by anion exchangers, retain their immunogenicity and infectivity. J Protozool. 1978 Feb;25(1):119–124. doi: 10.1111/j.1550-7408.1978.tb03881.x. [DOI] [PubMed] [Google Scholar]
- Pace T., Ponzi M., Dore E., Frontali C. Telomeric motifs are present in a highly repetitive element in the Plasmodium berghei genome. Mol Biochem Parasitol. 1987 Jun;24(2):193–202. doi: 10.1016/0166-6851(87)90106-x. [DOI] [PubMed] [Google Scholar]
- Peters W., Robinson B. L. The chemotherapy of rodent malaria.XLIII. Indolo (3,2-c) quinoline-N-oxides. Ann Trop Med Parasitol. 1988 Oct;82(5):423–427. doi: 10.1080/00034983.1988.11812271. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Sinden R. E., Smith J. Culture of the liver stages (exoerythrocytic schizonts) of rodent malaria parasites from sporozoites in vitro. Trans R Soc Trop Med Hyg. 1980;74(1):134–136. doi: 10.1016/0035-9203(80)90033-4. [DOI] [PubMed] [Google Scholar]
- Strome C. P., De Santis P. L., Beaudoin R. L. The cultivation of the exoerythrocytic stages of Plasmodium berghei from sporozoites. In Vitro. 1979 Jul;15(7):531–536. doi: 10.1007/BF02618155. [DOI] [PubMed] [Google Scholar]
- Surerus Campos D. G., Queiroz da Cruz M., Rodrigues R. M., Tendler M., Katz N., Oliveira Lima A. Pesquisa simultãnea de antígeno, anticorpo e imunocomplexos específicos no soro de pacientes com esquistossomose crõnica. Mem Inst Oswaldo Cruz. 1986 Apr-Jun;81(2):135–144. [PubMed] [Google Scholar]
- Szarfman A., Lyon J. A., Walliker D., Quakyi I., Howard R. J., Sun S., Ballou W. R., Esser K., London W. T., Wirtz R. A. Mature liver stages of cloned Plasmodium falciparum share epitopes with proteins from sporozoites and asexual blood stages. Parasite Immunol. 1988 May;10(3):339–351. doi: 10.1111/j.1365-3024.1988.tb00225.x. [DOI] [PubMed] [Google Scholar]
- Turner D. P., Gregson N. A. The cell surface of Plasmodium gallinaceum sporozoites: microelectrophoretic and lectin-binding characteristics. Parasitology. 1982 Apr;84(Pt 2):227–238. doi: 10.1017/s0031182000044796. [DOI] [PubMed] [Google Scholar]