Abstract
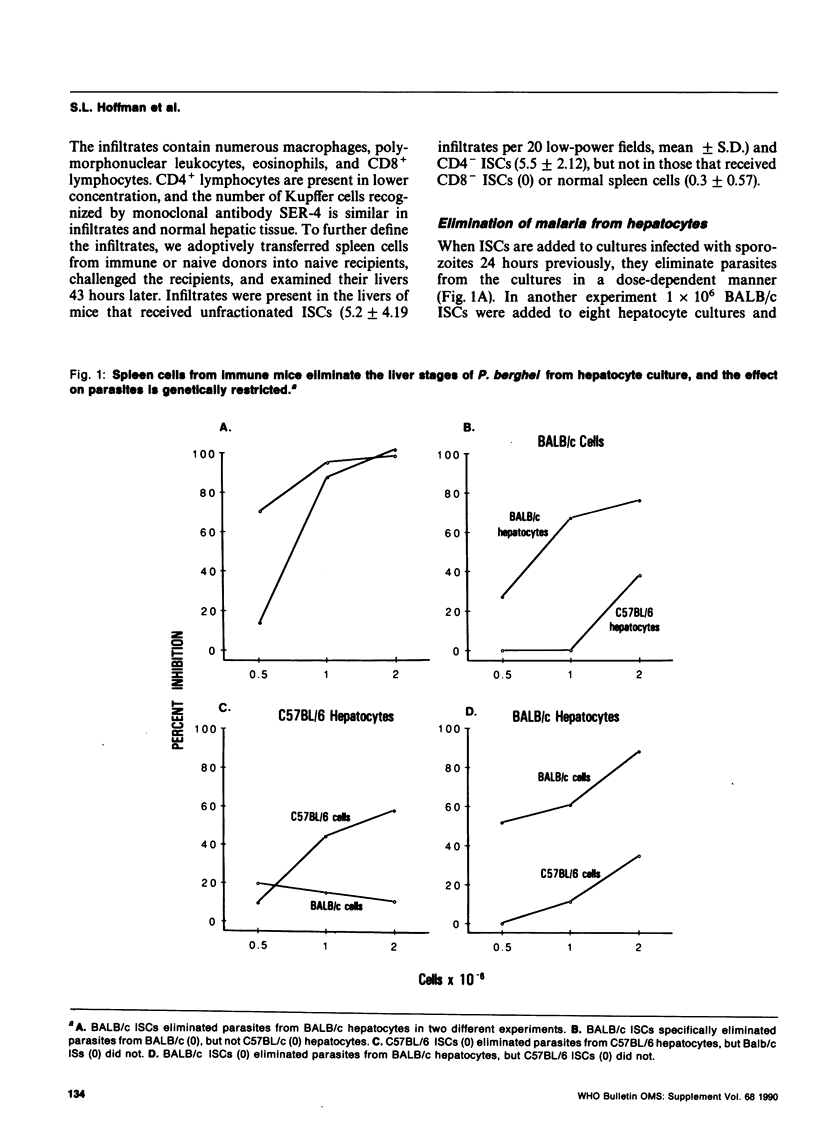
When mice are immunized with radiation-attenuated sporozoites they are solidly protected against sporozoite challenge by an immune response that has been shown to require CD8+ lymphocytes in several strains of mice. The target of this CD8+ T-cell-dependent immunity has not been established. Immune BALB/c mice were shown to develop malaria-specific, CD8+ T-cell-dependent inflammatory infiltrates in their livers after challenge with Plasmodium berghei sporozoites. Spleen cells from immune BALB/c and C57BL/6 mice eliminated hepatocytes infected with the liver stage of P. berghei in vitro. The activity against infected hepatocytes is not inhibited by antibodies to interferon-gamma and is not present in culture supernatants. It is genetically restricted, an indication that malaria antigens on the hepatocyte surface are recognized by immune T-effector cells. Further subunit pre-erythrocytic stage malaria vaccine development will require identification of the antigens recognized by these T cells and a method of immunization that induces such immunity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell J. R., Paleologo F. P., Franke E. D., Ratiwayanto S., Hadiputranto H., Kurniawan L., Wistar R., Jr, Hoffman S. L., Annis B. A., Wasserman G. Immune response of humans to the circumsporozoite protein of Plasmodium falciparum: limited T cell response to the immunodominant central repeat region. Am J Trop Med Hyg. 1988 Sep;39(3):232–235. doi: 10.4269/ajtmh.1988.39.232. [DOI] [PubMed] [Google Scholar]
- Chen D. H., Tigelaar R. E., Weinbaum F. I. Immunity to sporozoite-induced malaria infeciton in mice. I. The effect of immunization of T and B cell-deficient mice. J Immunol. 1977 Apr;118(4):1322–1327. [PubMed] [Google Scholar]
- Clyde D. F. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am J Trop Med Hyg. 1975 May;24(3):397–401. doi: 10.4269/ajtmh.1975.24.397. [DOI] [PubMed] [Google Scholar]
- Clyde D. F., McCarthy V. C., Miller R. M., Hornick R. B. Specificity of protection of man immunized against sporozoite-induced falciparum malaria. Am J Med Sci. 1973 Dec;266(6):398–403. doi: 10.1097/00000441-197312000-00001. [DOI] [PubMed] [Google Scholar]
- Del Giudice G., Cooper J. A., Merino J., Verdini A. S., Pessi A., Togna A. R., Engers H. D., Corradin G., Lambert P. H. The antibody response in mice to carrier-free synthetic polymers of Plasmodium falciparum circumsporozoite repetitive epitope is I-Ab-restricted: possible implications for malaria vaccines. J Immunol. 1986 Nov 1;137(9):2952–2955. [PubMed] [Google Scholar]
- Good M. F., Berzofsky J. A., Maloy W. L., Hayashi Y., Fujii N., Hockmeyer W. T., Miller L. H. Genetic control of the immune response in mice to a Plasmodium falciparum sporozoite vaccine. Widespread nonresponsiveness to single malaria T epitope in highly repetitive vaccine. J Exp Med. 1986 Aug 1;164(2):655–660. doi: 10.1084/jem.164.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M. F., Pombo D., Quakyi I. A., Riley E. M., Houghten R. A., Menon A., Alling D. W., Berzofsky J. A., Miller L. H. Human T-cell recognition of the circumsporozoite protein of Plasmodium falciparum: immunodominant T-cell domains map to the polymorphic regions of the molecule. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1199–1203. doi: 10.1073/pnas.85.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman S. L., Berzofsky J. A., Isenbarger D., Zeltser E., Majarian W. R., Gross M., Ballou W. R. Immune response gene regulation of immunity to Plasmodium berghei sporozoites and circumsporozoite protein vaccines. Overcoming genetic restriction with whole organism and subunit vaccines. J Immunol. 1989 May 15;142(10):3581–3584. [PubMed] [Google Scholar]
- Hoffman S. L., Oster C. N., Mason C., Beier J. C., Sherwood J. A., Ballou W. R., Mugambi M., Chulay J. D. Human lymphocyte proliferative response to a sporozoite T cell epitope correlates with resistance to falciparum malaria. J Immunol. 1989 Feb 15;142(4):1299–1303. [PubMed] [Google Scholar]
- Kumar S., Miller L. H., Quakyi I. A., Keister D. B., Houghten R. A., Maloy W. L., Moss B., Berzofsky J. A., Good M. F. Cytotoxic T cells specific for the circumsporozoite protein of Plasmodium falciparum. Nature. 1988 Jul 21;334(6179):258–260. doi: 10.1038/334258a0. [DOI] [PubMed] [Google Scholar]
- Long G. W., Leath S., Schuman R., Hollingdale M. R., Ballou W. R., Sim B. K., Hoffman S. L. Cultivation of the exoerythrocytic stage of Plasmodium berghei in primary cultures of mouse hepatocytes and continuous mouse cell lines. In Vitro Cell Dev Biol. 1989 Sep;25(9):857–862. doi: 10.1007/BF02623670. [DOI] [PubMed] [Google Scholar]
- Mellouk S., Maheshwari R. K., Rhodes-Feuillette A., Beaudoin R. L., Berbiguier N., Matile H., Miltgen F., Landau I., Pied S., Chigot J. P. Inhibitory activity of interferons and interleukin 1 on the development of Plasmodium falciparum in human hepatocyte cultures. J Immunol. 1987 Dec 15;139(12):4192–4195. [PubMed] [Google Scholar]
- Schofield L., Ferreira A., Altszuler R., Nussenzweig V., Nussenzweig R. S. Interferon-gamma inhibits the intrahepatocytic development of malaria parasites in vitro. J Immunol. 1987 Sep 15;139(6):2020–2025. [PubMed] [Google Scholar]
- Schofield L., Villaquiran J., Ferreira A., Schellekens H., Nussenzweig R., Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987 Dec 17;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- Sinigaglia F., Guttinger M., Gillessen D., Doran D. M., Takacs B., Matile H., Trzeciak A., Pink J. R. Epitopes recognized by human T lymphocytes on malaria circumsporozoite protein. Eur J Immunol. 1988 Apr;18(4):633–636. doi: 10.1002/eji.1830180422. [DOI] [PubMed] [Google Scholar]
- Szarfman A., Lyon J. A., Walliker D., Quakyi I., Howard R. J., Sun S., Ballou W. R., Esser K., London W. T., Wirtz R. A. Mature liver stages of cloned Plasmodium falciparum share epitopes with proteins from sporozoites and asexual blood stages. Parasite Immunol. 1988 May;10(3):339–351. doi: 10.1111/j.1365-3024.1988.tb00225.x. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Bastin J., Gould K., Brownlee G. G. Cytotoxic T lymphocytes recognize influenza haemagglutinin that lacks a signal sequence. Nature. 1986 Dec 11;324(6097):575–577. doi: 10.1038/324575a0. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., McMichael A. J., Carter N. P., Huddleston J. A., Brownlee G. G. Cytotoxic T cell recognition of the influenza nucleoprotein and hemagglutinin expressed in transfected mouse L cells. Cell. 1984 Nov;39(1):13–25. doi: 10.1016/0092-8674(84)90187-9. [DOI] [PubMed] [Google Scholar]
- Weiss W. R., Sedegah M., Beaudoin R. L., Miller L. H., Good M. F. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci U S A. 1988 Jan;85(2):573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meide P. H., Dubbeld M., Vijverberg K., Kos T., Schellekens H. The purification and characterization of rat gamma interferon by use of two monoclonal antibodies. J Gen Virol. 1986 Jun;67(Pt 6):1059–1071. doi: 10.1099/0022-1317-67-6-1059. [DOI] [PubMed] [Google Scholar]



