Abstract
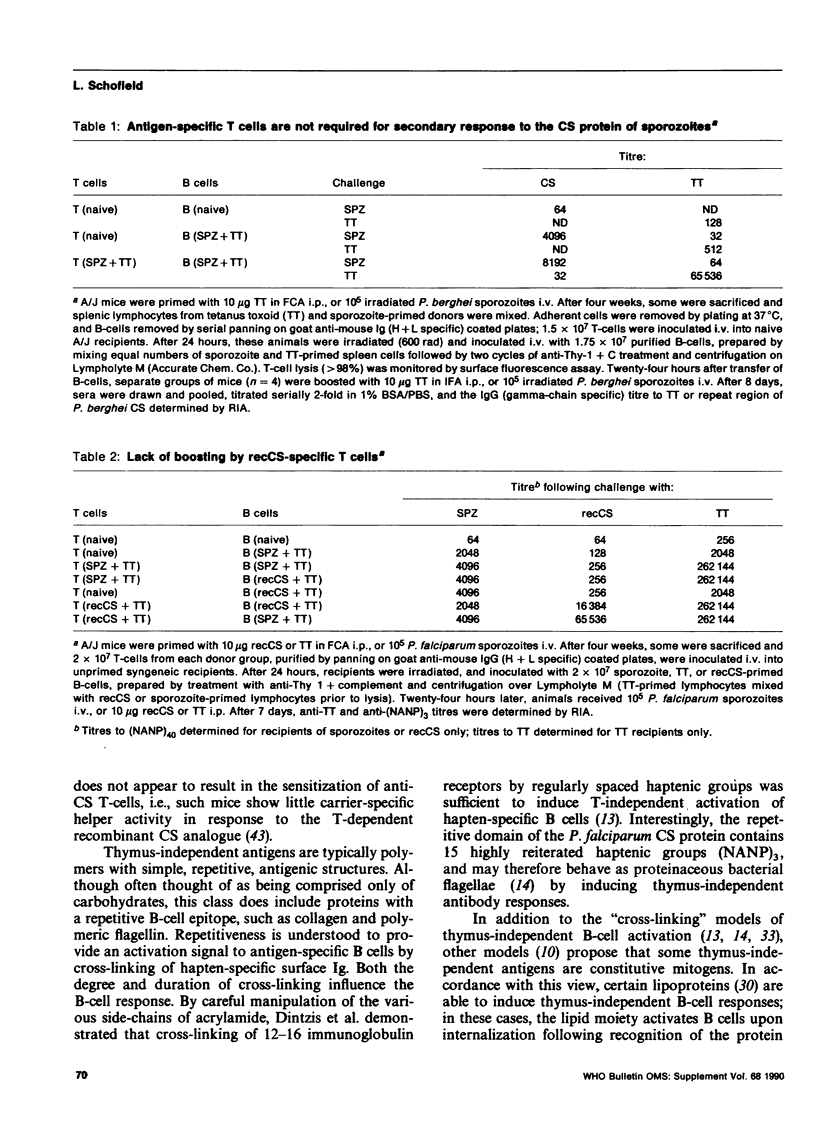
Sporozoites of malaria are covered with a repetitive surface antigen, the circumsporozoite (CS) protein. This antigen also appears to be a major target of the host immune response. The natural immunogenicity of the CS protein has led to attempts to develop the molecule as a vaccine candidate. It seems paradoxical, however, that a successful parasite should present to the host an immunogenic surface molecule which would induce protective immunity. In this paper we suggest that the CS protein is not the target of protective immunity under natural conditions, and that naturally immunogenic repetitive antigens in malaria and other parasites have evolved as a mechanism of immune evasion, via the induction of thymus-independent B-cell responses.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbas A. K. A reassessment of the mechanisms of antigen-specific T-cell-dependent B-cell activation. Immunol Today. 1988 Mar;9(3):89–94. doi: 10.1016/0167-5699(88)91271-6. [DOI] [PubMed] [Google Scholar]
- Arnot D. Malaria and the major histocompatibility complex. Parasitol Today. 1989 May;5(5):138–142. doi: 10.1016/0169-4758(89)90077-x. [DOI] [PubMed] [Google Scholar]
- Ballou W. R., Hoffman S. L., Sherwood J. A., Hollingdale M. R., Neva F. A., Hockmeyer W. T., Gordon D. M., Schneider I., Wirtz R. A., Young J. F. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet. 1987 Jun 6;1(8545):1277–1281. doi: 10.1016/s0140-6736(87)90540-x. [DOI] [PubMed] [Google Scholar]
- Chesnut R. W., Grey H. M. Studies on the capacity of B cells to serve as antigen-presenting cells. J Immunol. 1981 Mar;126(3):1075–1079. [PubMed] [Google Scholar]
- Clyde D. F., Most H., McCarthy V. C., Vanderberg J. P. Immunization of man against sporozite-induced falciparum malaria. Am J Med Sci. 1973 Sep;266(3):169–177. doi: 10.1097/00000441-197309000-00002. [DOI] [PubMed] [Google Scholar]
- Collins W. E., Anders R. F., Pappaioanou M., Campbell G. H., Brown G. V., Kemp D. J., Coppel R. L., Skinner J. C., Andrysiak P. M., Favaloro J. M. Immunization of Aotus monkeys with recombinant proteins of an erythrocyte surface antigen of Plasmodium falciparum. Nature. 1986 Sep 18;323(6085):259–262. doi: 10.1038/323259a0. [DOI] [PubMed] [Google Scholar]
- Collins W. E., Contacos P. G. Immunization of monkeys against Plasmodium cynomolgi by X-irradiated sporozoites. Nat New Biol. 1972 Apr 12;236(67):176–177. doi: 10.1038/newbio236176a0. [DOI] [PubMed] [Google Scholar]
- Del Giudice G., Cooper J. A., Merino J., Verdini A. S., Pessi A., Togna A. R., Engers H. D., Corradin G., Lambert P. H. The antibody response in mice to carrier-free synthetic polymers of Plasmodium falciparum circumsporozoite repetitive epitope is I-Ab-restricted: possible implications for malaria vaccines. J Immunol. 1986 Nov 1;137(9):2952–2955. [PubMed] [Google Scholar]
- Feldmann M. Induction of immunity and tolerance in vitro by hapten protein conjugates. I. The relationship between the degree of hapten conjugation and the immunogenicity of dinitrophenylated polymerized flagellin. J Exp Med. 1972 Apr 1;135(4):735–753. doi: 10.1084/jem.135.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M. F., Berzofsky J. A., Maloy W. L., Hayashi Y., Fujii N., Hockmeyer W. T., Miller L. H. Genetic control of the immune response in mice to a Plasmodium falciparum sporozoite vaccine. Widespread nonresponsiveness to single malaria T epitope in highly repetitive vaccine. J Exp Med. 1986 Aug 1;164(2):655–660. doi: 10.1084/jem.164.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M. F., Kumar S., Miller L. H. The real difficulties for malaria sporozoite vaccine development: nonresponsiveness and antigenic variation. Immunol Today. 1988 Nov;9(11):351–355. doi: 10.1016/0167-5699(88)91336-9. [DOI] [PubMed] [Google Scholar]
- Good M. F., Maloy W. L., Lunde M. N., Margalit H., Cornette J. L., Smith G. L., Moss B., Miller L. H., Berzofsky J. A. Construction of synthetic immunogen: use of new T-helper epitope on malaria circumsporozoite protein. Science. 1987 Feb 27;235(4792):1059–1062. doi: 10.1126/science.2434994. [DOI] [PubMed] [Google Scholar]
- Good M. F., Pombo D., Maloy W. L., de la Cruz V. F., Miller L. H., Berzofsky J. A. Parasite polymorphism present within minimal T cell epitopes of Plasmodium falciparum circumsporozoite protein. J Immunol. 1988 Mar 1;140(5):1645–1650. [PubMed] [Google Scholar]
- Good M. F., Pombo D., Quakyi I. A., Riley E. M., Houghten R. A., Menon A., Alling D. W., Berzofsky J. A., Miller L. H. Human T-cell recognition of the circumsporozoite protein of Plasmodium falciparum: immunodominant T-cell domains map to the polymorphic regions of the molecule. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1199–1203. doi: 10.1073/pnas.85.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttinger M., Caspers P., Takacs B., Trzeciak A., Gillessen D., Pink J. R., Sinigaglia F. Human T cells recognize polymorphic and non-polymorphic regions of the Plasmodium falciparum circumsporozoite protein. EMBO J. 1988 Aug;7(8):2555–2558. doi: 10.1002/j.1460-2075.1988.tb03104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar K., Ferguson M. A., Cross G. A. Acylation of a Plasmodium falciparum merozoite surface antigen via sn-1,2-diacyl glycerol. J Biol Chem. 1985 Apr 25;260(8):4969–4974. [PubMed] [Google Scholar]
- Hoffman S. L., Berzofsky J. A., Isenbarger D., Zeltser E., Majarian W. R., Gross M., Ballou W. R. Immune response gene regulation of immunity to Plasmodium berghei sporozoites and circumsporozoite protein vaccines. Overcoming genetic restriction with whole organism and subunit vaccines. J Immunol. 1989 May 15;142(10):3581–3584. [PubMed] [Google Scholar]
- Hoffman S. L., Oster C. N., Plowe C. V., Woollett G. R., Beier J. C., Chulay J. D., Wirtz R. A., Hollingdale M. R., Mugambi M. Naturally acquired antibodies to sporozoites do not prevent malaria: vaccine development implications. Science. 1987 Aug 7;237(4815):639–642. doi: 10.1126/science.3299709. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Hamaoka T., Benacerraf B. Cell interactions between histoincompatible T and B lymphocytes. II. Failure of physiologic cooperative interactions between T and B lymphocytes from allogeneic donor strains in humoral response to hapten-protein conjugates. J Exp Med. 1973 Jun 1;137(6):1405–1418. doi: 10.1084/jem.137.6.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Liano D., HayGlass K. A., Benacerraf B., Sy M. S., Abbas A. K. The role of antigen-presenting B cells in T cell priming in vivo. Studies of B cell-deficient mice. J Immunol. 1988 Jun 1;140(11):3773–3778. [PubMed] [Google Scholar]
- Lockyer M. J., Marsh K., Newbold C. I. Wild isolates of Plasmodium falciparum show extensive polymorphism in T cell epitopes of the circumsporozoite protein. Mol Biochem Parasitol. 1989 Dec;37(2):275–280. doi: 10.1016/0166-6851(89)90159-x. [DOI] [PubMed] [Google Scholar]
- Mellouk S., Mazier D., Druilhe P., Berbiguier N., Danis M. In vitro and in vivo results suggesting that anti-sporozoite antibodies do not totally block Plasmodium falciparum sporozoite infectivity. N Engl J Med. 1986 Sep 4;315(10):648–648. doi: 10.1056/NEJM198609043151016. [DOI] [PubMed] [Google Scholar]
- Nussenzweig R. S., Vanderberg J. P., Sanabria Y., Most H. Plasmodium berghei: accelerated clearance of sporozoites from blood as part of immune-mechanism in mice. Exp Parasitol. 1972 Feb;31(1):88–97. doi: 10.1016/0014-4894(72)90051-3. [DOI] [PubMed] [Google Scholar]
- Nussenzweig R. S., Vanderberg J., Most H., Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967 Oct 14;216(5111):160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- Pike B. L., Nossal G. J. A reappraisal of "T-independent" antigens. I. Effect of lymphokines on the response of single adult hapten-specific B lymphocytes. J Immunol. 1984 Apr;132(4):1687–1695. [PubMed] [Google Scholar]
- Potocnjak P., Yoshida N., Nussenzweig R. S., Nussenzweig V. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J Exp Med. 1980 Jun 1;151(6):1504–1513. doi: 10.1084/jem.151.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero P., Maryanski J. L., Corradin G., Nussenzweig R. S., Nussenzweig V., Zavala F. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature. 1989 Sep 28;341(6240):323–326. doi: 10.1038/341323a0. [DOI] [PubMed] [Google Scholar]
- Schofield L. T cell immunity to malaria sporozoites. Exp Parasitol. 1989 Apr;68(3):357–364. doi: 10.1016/0014-4894(89)90118-5. [DOI] [PubMed] [Google Scholar]
- Schofield L., Uadia P. Lack of Ir gene control in the immune response to malaria. I. A thymus-independent antibody response to the repetitive surface protein of sporozoites. J Immunol. 1990 Apr 1;144(7):2781–2788. [PubMed] [Google Scholar]
- Schofield L., Villaquiran J., Ferreira A., Schellekens H., Nussenzweig R., Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987 Dec 17;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- Sinigaglia F., Guttinger M., Gillessen D., Doran D. M., Takacs B., Matile H., Trzeciak A., Pink J. R. Epitopes recognized by human T lymphocytes on malaria circumsporozoite protein. Eur J Immunol. 1988 Apr;18(4):633–636. doi: 10.1002/eji.1830180422. [DOI] [PubMed] [Google Scholar]
- Sinigaglia F., Guttinger M., Kilgus J., Doran D. M., Matile H., Etlinger H., Trzeciak A., Gillessen D., Pink J. R. A malaria T-cell epitope recognized in association with most mouse and human MHC class II molecules. Nature. 1988 Dec 22;336(6201):778–780. doi: 10.1038/336778a0. [DOI] [PubMed] [Google Scholar]
- Smythe J. A., Coppel R. L., Brown G. V., Ramasamy R., Kemp D. J., Anders R. F. Identification of two integral membrane proteins of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5195–5199. doi: 10.1073/pnas.85.14.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. L. Molecular biology of malaria parasites. Exp Parasitol. 1988 Aug;66(2):143–170. doi: 10.1016/0014-4894(88)90087-2. [DOI] [PubMed] [Google Scholar]
- Weiss W. R., Good M. F., Hollingdale M. R., Miller L. H., Berzofsky J. A. Genetic control of immunity to Plasmodium yoelii sporozoites. J Immunol. 1989 Dec 15;143(12):4263–4266. [PubMed] [Google Scholar]
- Weiss W. R., Sedegah M., Beaudoin R. L., Miller L. H., Good M. F. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci U S A. 1988 Jan;85(2):573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida N., Nussenzweig R. S., Potocnjak P., Nussenzweig V., Aikawa M. Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science. 1980 Jan 4;207(4426):71–73. doi: 10.1126/science.6985745. [DOI] [PubMed] [Google Scholar]
- Zavala F., Tam J. P., Barr P. J., Romero P. J., Ley V., Nussenzweig R. S., Nussenzweig V. Synthetic peptide vaccine confers protection against murine malaria. J Exp Med. 1987 Nov 1;166(5):1591–1596. doi: 10.1084/jem.166.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala F., Tam J. P., Hollingdale M. R., Cochrane A. H., Quakyi I., Nussenzweig R. S., Nussenzweig V. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science. 1985 Jun 21;228(4706):1436–1440. doi: 10.1126/science.2409595. [DOI] [PubMed] [Google Scholar]
- de la Cruz V. F., Lal A. A., McCutchan T. F. Sequence variation in putative functional domains of the circumsporozoite protein of Plasmodium falciparum. Implications for vaccine development. J Biol Chem. 1987 Sep 5;262(25):11935–11939. [PubMed] [Google Scholar]


