Abstract
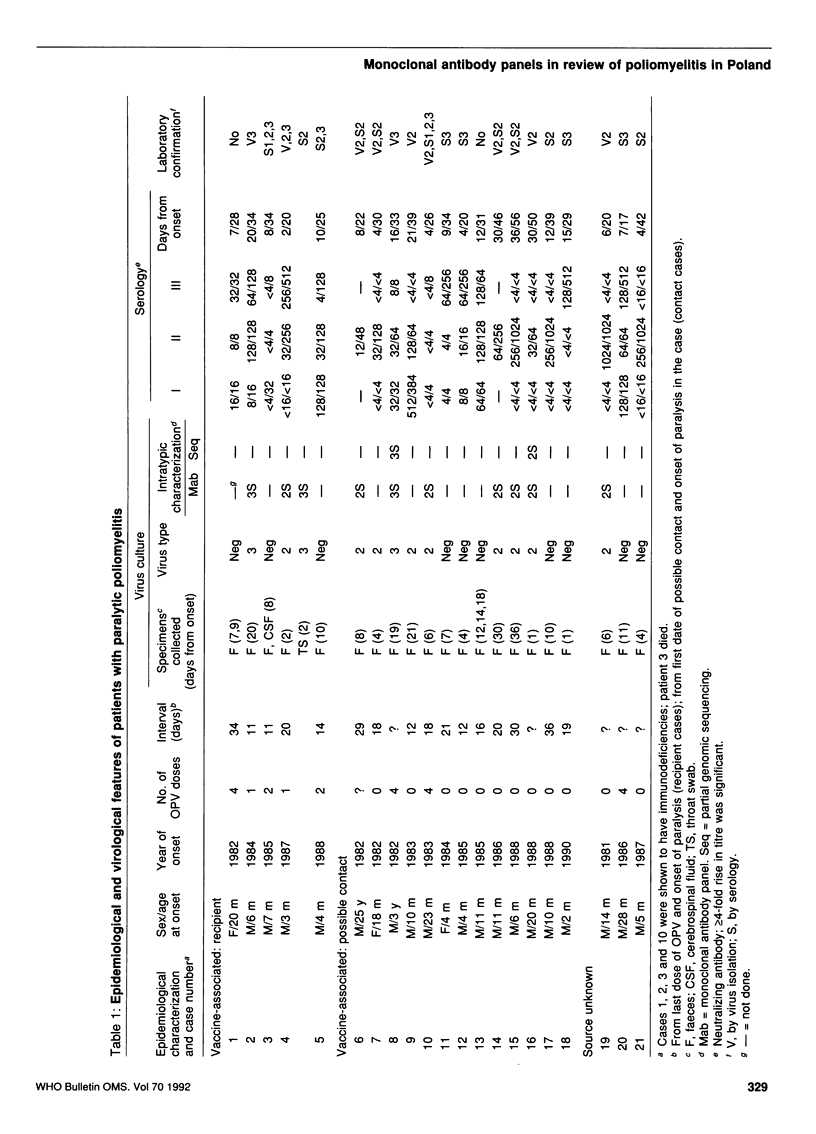
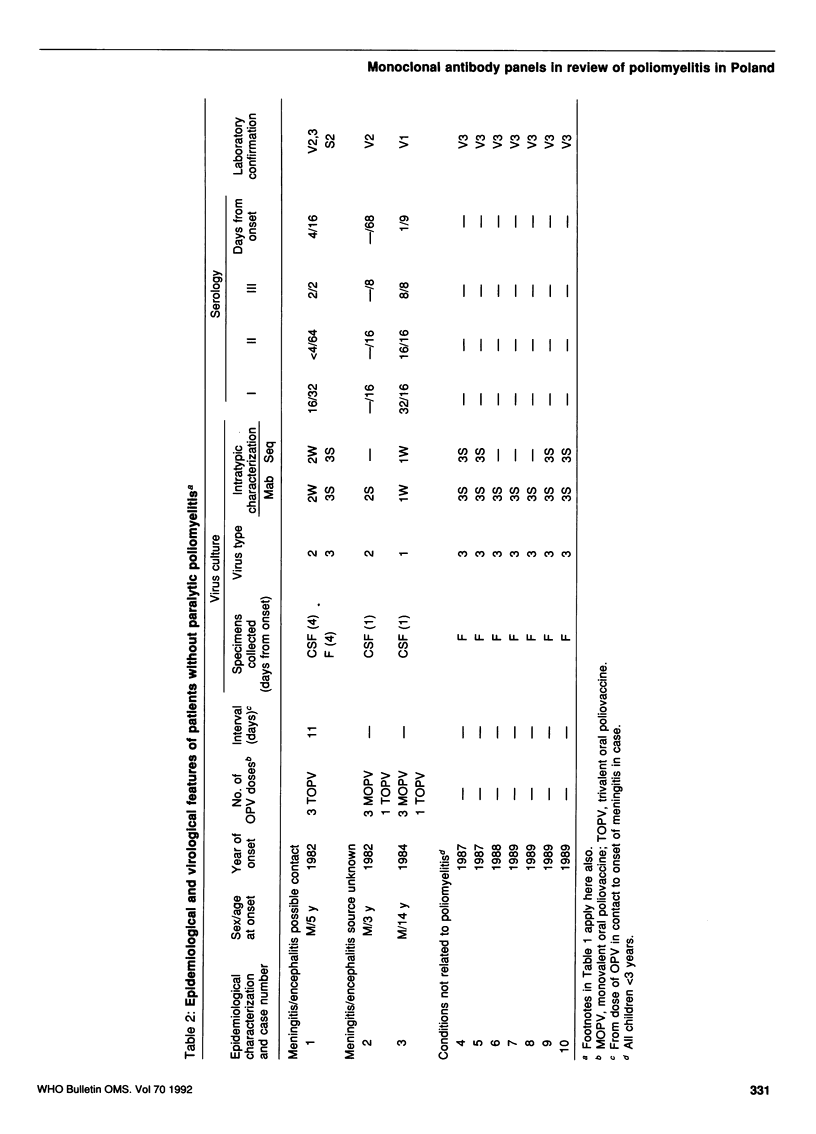
Monoclonal antibody panels developed to differentiate vaccine-derived and wild-type strains of polio-viruses were applied to isolates from cases of paralytic poliomyelitis, non-paralytic poliomyelitis, and healthy excreters of poliovirus from Poland. All isolates from poliomyelitis cases were shown to be vaccine-derived, as were most other strains. However, two strains associated with meningitis had wild-type antigenic phenotypes and, as shown by partial genomic sequencing, wild-type genotypes. Correlation of laboratory and epidemiological data suggested that residual cases of paralytic poliomyelitis in Poland between 1981 and 1990 were vaccine-related. Study of the non-paralytic cases, however, helped identify the circulation of endemic wild-type viruses in a well-vaccinated community.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dunn G., Begg N. T., Cammack N., Minor P. D. Virus excretion and mutation by infants following primary vaccination with live oral poliovaccine from two sources. J Med Virol. 1990 Oct;32(2):92–95. doi: 10.1002/jmv.1890320205. [DOI] [PubMed] [Google Scholar]
- Esteves K. Safety of oral poliomyelitis vaccine: results of a WHO enquiry. Bull World Health Organ. 1988;66(6):739–746. [PMC free article] [PubMed] [Google Scholar]
- Evans D. M., Dunn G., Minor P. D., Schild G. C., Cann A. J., Stanway G., Almond J. W., Currey K., Maizel J. V., Jr Increased neurovirulence associated with a single nucleotide change in a noncoding region of the Sabin type 3 poliovaccine genome. Nature. 1985 Apr 11;314(6011):548–550. doi: 10.1038/314548a0. [DOI] [PubMed] [Google Scholar]
- Ferguson M., Magrath D. I., Minor P. D., Schild G. C. WHO collaborative study on the use of monoclonal antibodies for the intratypic differentiation of poliovirus strains. Bull World Health Organ. 1986;64(2):239–246. [PMC free article] [PubMed] [Google Scholar]
- McBRIDE W. D. Antigenic analysis of polioviruses by kinetic studies of serum neutralization. Virology. 1959 Jan;7(1):45–58. doi: 10.1016/0042-6822(59)90176-x. [DOI] [PubMed] [Google Scholar]
- McBean A. M., Modlin J. F. Rationale for the sequential use of inactivated poliovirus vaccine and live attenuated poliovirus vaccine for routine poliomyelitis immunization in the United States. Pediatr Infect Dis J. 1987 Oct;6(10):881–887. doi: 10.1097/00006454-198710000-00001. [DOI] [PubMed] [Google Scholar]
- Minor P. D., John A., Ferguson M., Icenogle J. P. Antigenic and molecular evolution of the vaccine strain of type 3 poliovirus during the period of excretion by a primary vaccinee. J Gen Virol. 1986 Apr;67(Pt 4):693–706. doi: 10.1099/0022-1317-67-4-693. [DOI] [PubMed] [Google Scholar]
- Rantala H., Uhari M., Tuokko H., Stenvik M., Kinnunen L. Poliovaccine virus in the cerebrospinal fluid after oral polio vaccination. J Infect. 1989 Sep;19(2):173–176. doi: 10.1016/s0163-4453(89)92014-8. [DOI] [PubMed] [Google Scholar]
- Rico-Hesse R., Pallansch M. A., Nottay B. K., Kew O. M. Geographic distribution of wild poliovirus type 1 genotypes. Virology. 1987 Oct;160(2):311–322. doi: 10.1016/0042-6822(87)90001-8. [DOI] [PubMed] [Google Scholar]
- Sutter R. W., Brink E. W., Cochi S. L., Kew O. M., Orenstein W. A., Biellik R. J., Hinman A. R. A new epidemiologic and laboratory classification system for paralytic poliomyelitis cases. Am J Public Health. 1989 Apr;79(4):495–498. doi: 10.2105/ajph.79.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]


