Abstract
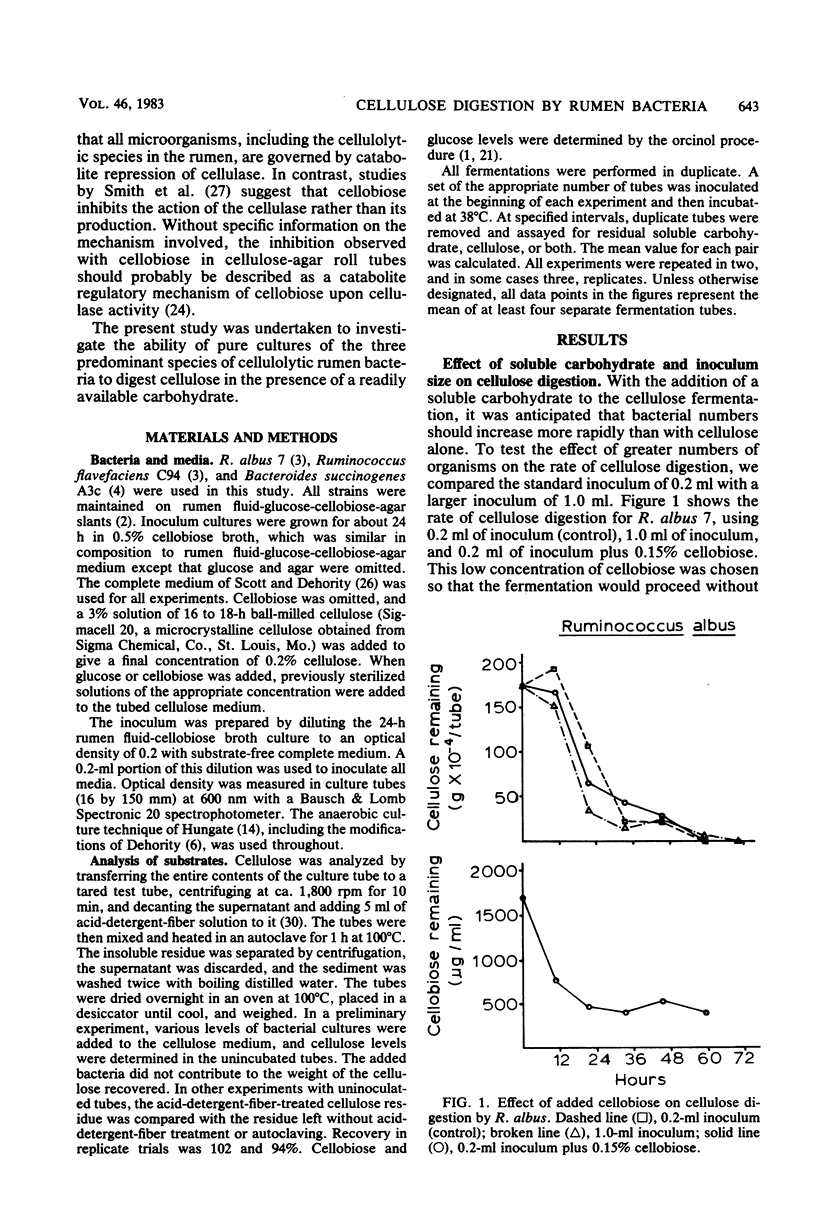
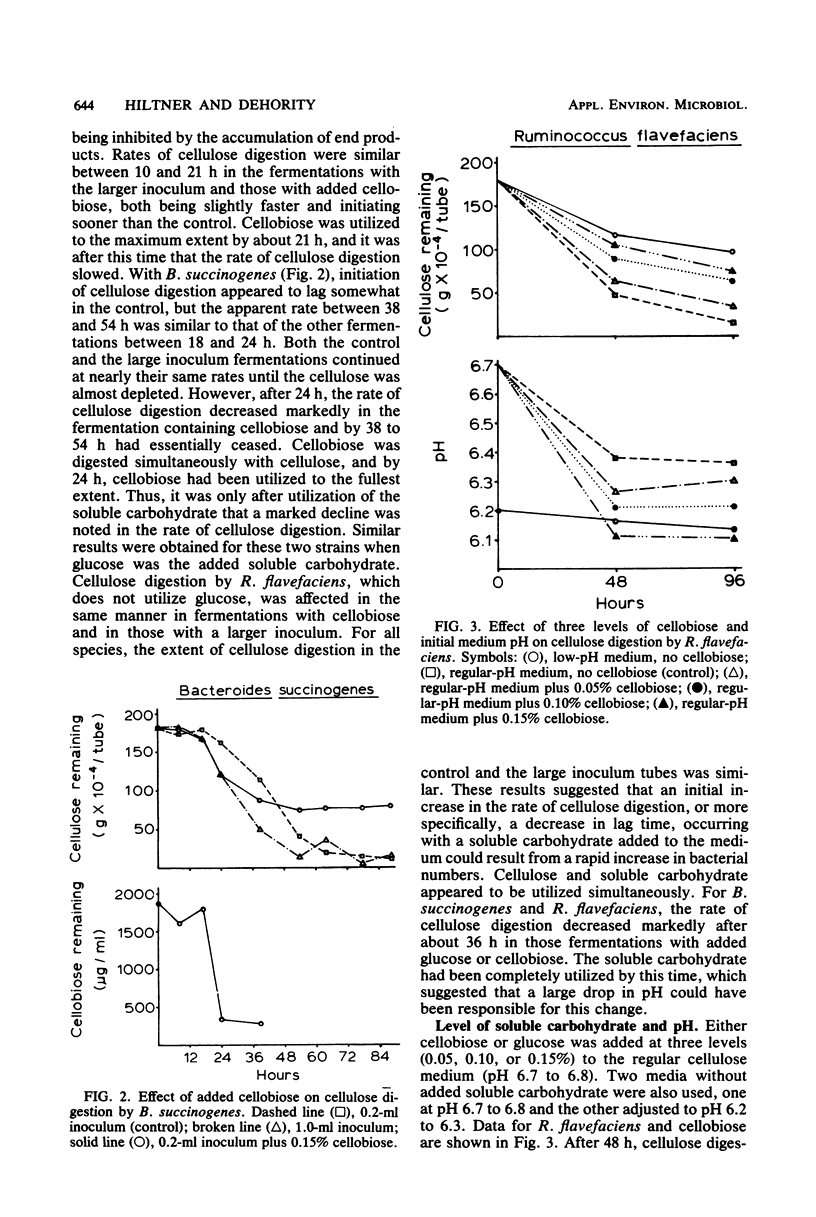
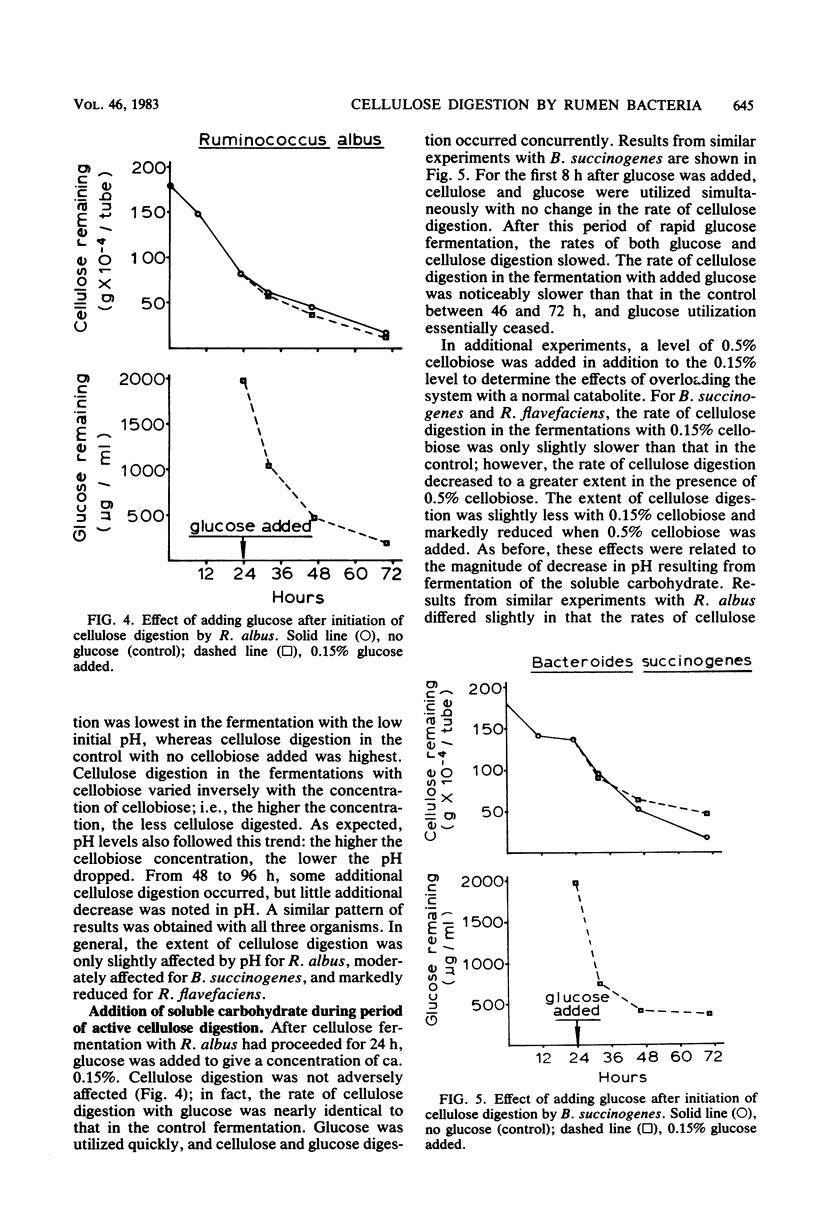
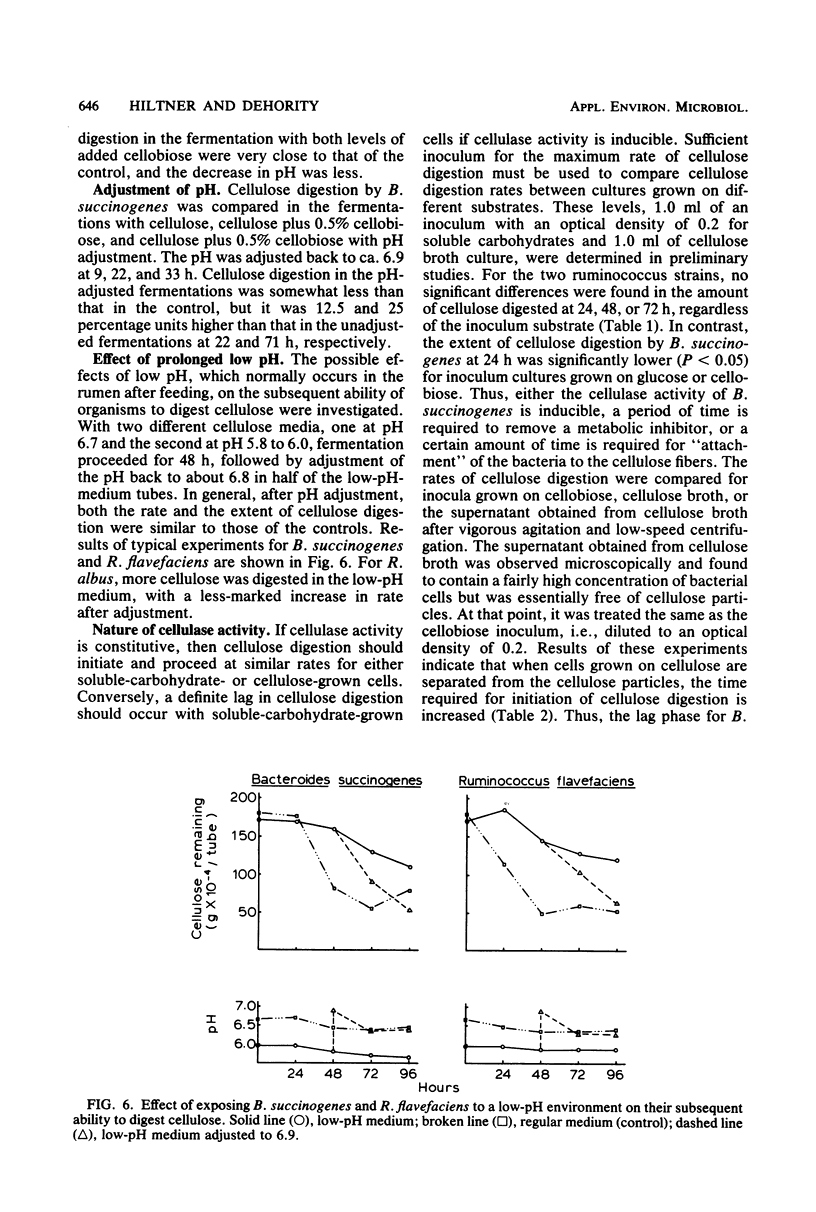
The rate of cellulose digestion in the presence of either glucose or cellobiose was studied for the three predominant species of cellulolytic rumen bacteria: Ruminococcus albus, Ruminococcus flavefaciens, and Bacteroides succinogenes. When a soluble carbohydrate was added to cellulose broth, the lag phase of cellulose digestion was shortened. Presumably, this was due to greater numbers of bacteria, because increasing the size of the inoculum had a similar effect. Cellulose digestion occurred simultaneously with utilization of the soluble carbohydrate. The rate of cellulose digestion slowed markedly for B. succinogenes and R. flavefaciens and slowed less for R. albus after the cellobiose or glucose had been utilized, and was accompanied by a decrease in pH. Both the rate and the extent of cellulose digestion were partially inhibited when the initial pH of the medium was 6.3 or below. R. albus appeared to be less affected by a low-pH medium than were B. succinogenes and R. flavefaciens. When a soluble carbohydrate was added to the fermentation during the maximum-rate phase of cellulose digestion, the rate of cellulose digestion was not affected until after the soluble carbohydrate had been depleted and the pH had decreased markedly. Prolonged exposure of the bacteria to a low pH had little if any effect on their subsequent ability to digest cellulose. Cellulase activity of intact bacterial cells appeared to be constitutive in nature for these three species of rumen bacteria.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRYANT M. P., SMALL N., BOUMA C., ROBINSON I. M. Characteristics of ruminal anaerobic celluloytic cocci and Cillobacterium cellulosolvens n. sp. J Bacteriol. 1958 Nov;76(5):529–537. doi: 10.1128/jb.76.5.529-537.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEHORITY B. A. DEGRADATION AND UTILIZATION OF ISOLATED HEMICELLULOSE BY PURE CULTURES OF CELLULOLYTIC RUMEN BACTERIA. J Bacteriol. 1965 Jun;89:1515–1520. doi: 10.1128/jb.89.6.1515-1520.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehority B. A. Pectin-fermenting bacteria isolated from the bovine rumen. J Bacteriol. 1969 Jul;99(1):189–196. doi: 10.1128/jb.99.1.189-196.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusee M. C., Leatherwood J. M. Regulation of cellulase from Ruminococcus. Can J Microbiol. 1972 Mar;18(3):347–353. doi: 10.1139/m72-053. [DOI] [PubMed] [Google Scholar]
- Groleau D., Forsberg C. W. Cellulolytic activity of the rumen bacterium Bacteroides succinogenes. Can J Microbiol. 1981 May;27(5):517–530. doi: 10.1139/m81-077. [DOI] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchner E. V., Leatherwood J. M. Use of a cellulase-derepressed mutant of cellulomonas in the production of a single-cell protein product from cellulose. Appl Environ Microbiol. 1980 Feb;39(2):382–386. doi: 10.1128/aem.39.2.382-386.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungate R. E. Studies on Cellulose Fermentation: III. The Culture and Isolation for Cellulose-decomposing Bacteria from the Rumen of Cattle. J Bacteriol. 1947 May;53(5):631–645. doi: 10.1128/jb.53.5.631-645.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham M. J., Brooker B. E., Pettipher G. L., Harris P. J. Adhesion of Bacteroides succinogenes in pure culture and in the presence of Ruminococcus flavefaciens to cell walls in leaves of perennial ryegrass (Lolium perenne). Appl Environ Microbiol. 1978 Jun;35(6):1166–1173. doi: 10.1128/aem.35.6.1166-1173.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham M. J., Brooker B. E., Pettipher G. L., Harris P. J. Ruminococcus flavefaciens Cell Coat and Adhesion to Cotton Cellulose and to Cell Walls in Leaves of Perennial Ryegrass (Lolium perenne). Appl Environ Microbiol. 1978 Jan;35(1):156–165. doi: 10.1128/aem.35.1.156-165.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson H., Irvin R., Costerton J. W., Cheng K. J. Ultrastructure and adhesion properties of Ruminococcus albus. J Bacteriol. 1975 Apr;122(1):278–287. doi: 10.1128/jb.122.1.278-287.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Baldwin R. L. Substrate preferences in rumen bacteria: evidence of catabolite regulatory mechanisms. Appl Environ Microbiol. 1978 Aug;36(2):319–329. doi: 10.1128/aem.36.2.319-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Dombrowski D. B. Effect of pH on the efficiency of growth by pure cultures of rumen bacteria in continuous culture. Appl Environ Microbiol. 1980 Mar;39(3):604–610. doi: 10.1128/aem.39.3.604-610.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT H. W., DEHORITY B. A. VITAMIN REQUIREMENTS OF SEVERAL CELLULOLYTIC RUMEN BACTERIA. J Bacteriol. 1965 May;89:1169–1175. doi: 10.1128/jb.89.5.1169-1175.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. R., Yu I., Hungate R. E. Factors affecting cellulolysis by Ruminococcus albus. J Bacteriol. 1973 May;114(2):729–737. doi: 10.1128/jb.114.2.729-737.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. S. Factors affecting the cellulolytic activity of rumen contents. Appl Environ Microbiol. 1977 Mar;33(3):497–502. doi: 10.1128/aem.33.3.497-502.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry R. A., Tilley J. M., Outen G. E. Effect of pH on cellulose distion under in vitro conditions. J Sci Food Agric. 1969 May;20(5):317–320. doi: 10.1002/jsfa.2740200514. [DOI] [PubMed] [Google Scholar]



