Abstract
The past two decades have greatly improved our knowledge of vertebrate skeletal morphogenesis. It is now clear that bony morphology lacks individual descriptive specification and instead results from an interplay between positional information assigned during early limb bud deployment and its “execution” by highly conserved cellular response programs of derived connective tissue cells (e.g., chondroblasts and osteoblasts). Selection must therefore act on positional information and its apportionment, rather than on more individuated aspects of presumptive adult morphology. We suggest a trait classification system that can help integrate these findings in both functional and phylogenetic examinations of fossil mammals and provide examples from the human fossil record.
Although molecular data have at least partially revolutionized the systematics of living mammals (1–2), except in rare instances (3), species known only from fossils lie beyond the scope of this revolution. For them, a careful evaluation of morphology is the only means of elucidating phylogenetic and functional relationships. The power of such analyses is directly related to the number and independence of the characters they employ. However, we do not yet understand the genetic basis of most complex morphological characters, and most analysts still proceed under the sub rosa presumption that whatever a trait’s genetic basis, natural selection must have acted on its variation to guide its evolution. However, studies are often conducted in such detail that many of the morphological characters discussed are unlikely to have ever been separately heritable. Such interdependent characters are of dubious functional and phyletic significance. These observations are not new. Over the years there have been prominent and longstanding warnings that serious errors emanate from an uncontrolled disarticulation of continuous anatomical structure (4–7). However, these warnings continue to be systematically ignored.
Such trait “atomization” is due largely to the presumption that particulate inheritance ultimately underlies morphological characters. However, the view that a trait is independently heritable (or heritable at all) simply because it can be separately defined and analyzed has been rendered largely obsolete by modern developmental biology. As Thorogood put it, we must “move away from the rather dated analogy of a descriptive specification and think of the genome and its implementation as a generative program” (ref. 8, p. 4). Making an animal is not just “painting by genes” (9). Indeed, the traditional analogy of a genetic “blueprint” in which some form of “descriptive specification” underlies anatomical characters is no longer tenable and should not be an a priori basis for character definition (8). Put quite simply, “there are no genes for bones” (ref. 10, p. 1815) (see also refs. 11 and 12). But how should we proceed?
We believe that our improved understanding of developmental biology now offers an initial solution to some of these problems. Here we propose a classification system for mammalian postcranial traits derived from vertebrate limb development and show how its application can clarify functional and phylogenetic interpretations.
Recent Improvements in Our Understanding of Limb Development.
Recent dramatic improvements in our knowledge of the molecular basis of limb development (13–15) have the potential to greatly enrich our understanding of mammalian postcranial evolution. Although advances in pattern formation have outpaced those that illuminate growth control mechanisms, it is now clear that the former directly influence the latter.
During embryogenesis, paired forelimb and hindlimb buds emerge from the lateral plate mesoderm, each consisting of a homogeneous population of mesenchyme cells jacketed in ectoderm. Cellular interactions within the bud, orchestrated by specialized signaling regions, including the apical ectodermal ridge and zone of polarizing activity, specify limb pattern. These interactions are mediated by both short and long range signaling molecules, which assign positional information (PI) to cells within the bud. Establishment of this three-dimensional system of molecular coordinates polarizes the limb along the three primary anatomical axes (reviewed in ref. 16). The cellular interpretation of these signals is highly complex and involves activation of various combinations of transcription factors that will, in turn, influence the differentiation pathway of the cell (reviewed in ref. 14).
From a morphological perspective, it is important to be continuously vigilant of the fact that patterning in the limb is determined at a cellular, and not an anatomical, level. Morphology is the ultimate read-out of these complex molecular interactions. Although their precise roles in anatomical ontogeny are still poorly understood, earliest skeletogenesis can now be viewed as a distinct two-step process (17): (i) PI is first assigned to mesenchymal cells via a three-dimensional coordinate system, and (ii) that information is then interpreted by cells by using highly specific differentiation programs.
Such programs are themselves of two distinct types. Many are highly conserved “housekeeping” functions on which cells and their descendants (i.e., tissues and organs) rely to canalize and manifest their PI. These are redundant throughout the musculoskeletal system (though their effects will vary depending on the locality of the tissue in which they are expressed) and constitute a primary “raw material” by which changes in PI can be channeled. We will refer to them as systemic assembly mechanisms (SAMs).
A probable example of a typical SAM is the parathyroid hormone-related protein–Indian hedgehog positive feedback loop that regulates chondroblast differentiation in growth plates (18, 19). How the implementation of this loop is influenced by earlier pattern formation is as yet unclear, but, because modification of the parathyroid hormone-related protein–Indian hedgehog loop perturbs all endochondral bone, local changes to the skeleton must be achieved by altering either the molecular and/or mechanical context in which such systems operate or by altering regulatory control of the loop locally rather than globally.
Other types of SAMs are related to the specific capacity of mesenchymal cells and their descendants to transduce mechanical stimuli into the controlled production of extracellular matrix. It has recently been demonstrated, for example, that even the cells of undifferentiated mesenchyme respond differentially to imposed local compression by up-regulating and down-regulating (respectively) the Sox9 and IL-1β (interleukin 1β) genes. Changes in the form and dimensions of anlagen (as guided by PI; see below) would therefore differentially affect such cell behaviors, which result in both cellular positional changes and the production of Type II collagen (20). At the same time, adult bones of mammals ranging from mice to elephants appear to undergo microstrain within surprisingly narrow limits (reviewed in refs. 21 and 22). This indicates a high degree of conservation of the response sensitivities of their osteoblasts. Such conservatism implies that these kinds of response systems are again unlikely candidates for local skeletal evolution because alterations of such systems would tend to produce systemic, rather than local, skeletal effects.
The other basic “program” by which PI is manifested is local regulation of tissue growth and cellular differentiation. In the limbs, for example, PI appears to be integrally related to the manifestation and patterning of posterior Hox gene expression territories (23–27). These genes are now thought to help regulate growth in multiple ways and to have demonstrable effects during both initial cartilage condensation and the later specification of growth within different regions of the anlagen (28). Hox interactions and their effect on probable downstream genes (e.g., bone morphogenetic protein(s) and growth and development factor(s), fibroblast growth factor(s), Noggin, Chordin, etc.) can thus directly affect both size and shape of presumptive bones (29). Their expression can in turn be regulated in a number of ways. Hoxd-11 is an excellent example.
Targeted disruptions of Hoxd-11 in mice result in severe limb malformations, axial skeletal defects, and male infertility (27). However, deletion of one Hoxd-11 transcriptional enhancer leads simply to a caudal transposition of the sacrum; the limbs and genitals remain unaffected (30). It is worthy of note that, because enhancer activity is influenced by other nuclear factors, actual evolutionary changes in morphology may not require mutations in the enhancers themselves. Localized changes in gene expression can also be generated by modulating the distribution of factors such as retinoic acid in a developmental field (see below).
This example emphasizes again the singularly most important aspect that an appreciation of the role that PI can play in improving our understanding of morphological evolution: that, whatever pathways are eventually found to be essential to the emergence of novel morphology, all must be assigned and initiated before earliest cartilage condensation, when cells lie within communicable distances from one another. Developing a general view of the mechanisms involved in the heritable transmission of morphology therefore requires at least a hypothesis as to the primary means by which PI is assigned to cells of the presumptive musculoskeletal system.
Currently there is abundant evidence that such assignment is made via morphogenetic fields (9, 31, 32), and much of what has been uncovered about vertebrate morphogenesis points to the validity of extending the morphogenetic field hypothesis to more detailed aspects of morphology, to what might be called “tertiary” (and higher order) fields (ref. 9; see also refs. 31, 33, and 34). Cell populations that constitute the presumptive cellular condensations that ultimately become the bones of the vertebrate skeleton would seem particularly good candidates for such tertiary field status (35). Evidence justifying this extension is of two distinct types—that which has emerged from several decades of experimental embryology of chick limb buds, and, as noted earlier, recent discoveries about the molecular mechanisms by which genes influence structure of the developing limb. Both have been the subject of numerous and comprehensive reviews and need not be considered in further detail here (the reader is directed to refs. 14, 36, 37, and 38).
In summary, (i) limb structure is primarily determined by an interpretation of PI by mesenchyme cells and not by descriptive specification; and (ii) the manner in which PI is assigned to cells (i.e., pattern formation) is the key to the hereditary basis of vertebrate limb morphology (39). We suggest that it is reasonable to use current knowledge about the interplay of PI and SAMs within the sequentially nested fields of the developing limb to construct general but meaningful hypotheses about morphological evolution in mammalian limbs.
The Evolution of the Human Pelvis.
Based on the principles reviewed above, adult bone evolution is best analyzed from the perspective of changes in the assignment of PI. The hominid pelvis is an important case of rapid and dramatic morphological change (Fig. 1) and can therefore serve as a good example (40).
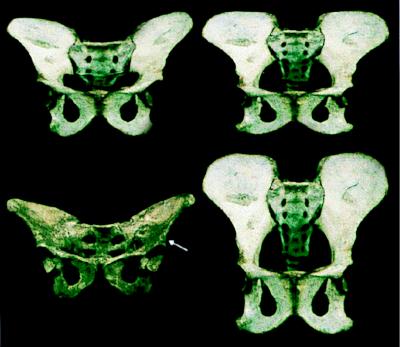
Figure 1.
Hypothetical transitional emergence of the hominid pelvis. Anterior photographs of a chimpanzee pelvis (lower right) and that of A.L. 288-1 (“Lucy”) (lower left) were scanned. Using a sliding scale, the upper left image was then obtained by digital morphing to a transitional stage 75% of the distance between the chimpanzee and A.L. 288-1. The upper right image is a simple superoinferior digital distortion of the chimpanzee without any reference to a known “end product” (Photoshop “scale function”). Image breadth was not altered; its superoinferior height was simply reduced by 2/3. (Note: the upper right image appears somewhat less “transitional” than the one at the upper left because the latter benefits from the three-dimensionality of the two images being morphed; i.e., our distortion was only two-dimensional.) We do not suggest that either image constitutes an actual “intermediate” pelvic form. We wish only to demonstrate that a simple dimensional change in one hypothetical adult form is very similar to that which has been morphed by using the known adult “final outcome” and that it might be achieved by a simple underlying mechanism such as a progressive increase or decrease in the slopes of cell response gradients (see text). We suggest that this is the most probable morphogenetic mode by which the many anatomical differences between A.L.-288-1 and the chimpanzee pelves evolved. Therefore, the isolated definition and separate analysis of each of the many traits that differ between these pelves is likely to greatly distort their functional and phyletic significance (see especially ref. 7, pp. 359–361 for discussion). Note, for example, that a number of the unusual distinguishing characters of the australopithecine pelvis, including its exceptionally broad sacrum, platypelloid birth canal (i.e., anteroposterior dimension/mediolateral dimension × 100 ≈ 50–60), short pubic symphysis, elongated superior and inferior pubic rami, ovoid obturator foramina, etc., have all been reproduced by this simple, relatively crude, linear distortion.
Two novel features of the hominid pelvis dramatically improved its function during upright running and walking. First, the entire pelvis (especially the ilium) is greatly reduced in superoinferior height. Second, virtually all of its individual “elements” are broader than in quadrupedal primates (compare, for example, the sacra of chimpanzees and hominids in Fig. 1). In addition, the iliac blade, which is virtually parallel with the coronal plane in quadrupeds, has been “twisted” into the sagittal plane. This transformed its attached muscles (gluteus minimus and medius) into the novel role of abductors that can prevent the pelvis from dropping to the unsupported side during the single leg phase of bipedal walking and running. How were all of these relatively dramatic changes most likely to have been achieved morphogenetically?
Given what we now know of the manner in which bone growth and morphology are specified, a likely scenario can be garnered from Fig. 1, which demonstrates that the differences between hominid and chimpanzee pelves are remarkably morphologically “linear.” This suggests that the morphogenetic basis of these anatomical changes may also be linear [in the sense of Zakany et al. (41)] and that they could have been achieved by changes in the geometry of pattern formation such as a progressive increase or decrease in the slopes of molecular gradients in the limb bud. Although evidence for active morphogen (sensu stricto) gradients in vertebrate limb development remains equivocal, numerous pattern forming genes have graded distribution patterns in limb buds, including homeobox genes (e.g., HoxC6, HoxD9, Dlx3) (42), cell adhesion molecules, fibroblast growth factor receptor 2 (43), wnt5a (44), and cellular retinoic acid binding protein (45). Therefore, the transformation of the common ancestral pelvis into that of early hominids may have been as “simple” as a slight modification of a gradient. For example, if a particular PI gradient were to span n cell diameters, and those cells defined the ultimate anteroposterior dimension of the presumptive ilium (superoinferior in the adult human), then a slight increase in the steepness of its slope would cause that signal to span fewer cells, “distorting” the presumptive anlagen and substantially altering downstream adult morphology. Fig. 1 is intended simply to illustrate this argument and is therefore necessarily restricted to two dimensions. In reality, condensations are three-dimensional, and iliac rotation would be equally subject to modification during field specification. In this manner, the breadth, height, and depth of the entire pelvic field could have been simultaneously altered. Our argument is not that a change in anlagen specification necessarily underlies hominid pelvic evolution. A variety of other kinds of PI “read-outs,” such as a substantial alteration of downstream growth rates in an otherwise unperturbed anlagen, could have had the same effects. Rather, we are suggesting that subtle shifts in the disposition of PI are the most probable morphogenetic mode of evolution of the hominid pelvis, and that such shifts are the primary source of most anatomical changes that have been achieved in mammalian bones.
Consider how profoundly such a hypothesis affects the manner in which the differences between the pelves of Australopithecus and a chimpanzee (as an example) are interpreted (Fig. 1). In both functional and phylogenetic analyses of this transition using conventional methods, each of many anatomical differences would typically be isolated and treated independently (see, e.g., refs. 46–48). However, the fundamental differences are that the hominid ilium and sacrum are dramatically shorter (superoinferiorly) and broader (mediolaterally). The neck of the hominid femur and the anterior parts of its pelvis (the pubic and ischial rami) have all participated in these same dimensional changes. Collectively, all are consistent either with a broadening and shortening of the morphogenetic field(s) responsible for the initial form of the entire pelvic region or by a systematic change in postanlagen growth with similar geometric effects. None of these individual differences is likely to have been specifically and separately fixed in the genome because virtually none is likely to have been a consequence of localized gene expression specific to each defined trait. Subtle changes in presumptive tissue fields such as those hypothesized here will typically yield many downstream effects, but only the most prominent are likely to have had a sufficiently significant effect on function to actually affect fitness. Except in rare instances (which can possibly serve as examples of punctuated “breakthrough” adaptations), most others will merely be retained byproducts of the primary changes. The transverse distance between the hip joints of the early hominid pelvis can serve to illustrate this important point.
A notable consequence of the overall broadening of the early hominid pelvis is that the relative distance between the two hip joints was also increased. This is an apparent disadvantage during bipedal locomotion because it requires greater abductor contraction during the single leg phase (i.e., it reduces the lever arm length of the pelvic stabilizers). Does this increased distance therefore “demand some special functional explanation” as several authors have insisted (ref. 49, p. 285; see also ref. 50)? The answer is very probably no, so long as consideration is given to the manner in which the ancestral–descendant transition is likely to have been achieved morphogenetically, and the entire pelvis is not atomized into component parts that, in fact, probably have no individual, separable, heritability (51). By what other genetic means than that outlined here was the hominid pelvis so systematically and rapidly altered? By separate, independent fixation of all of its novel anatomical features [broader ilium, broader sacrum, longer (i.e., broader) femoral neck, longer (i.e., broader) pubic rami, shorter ilium, shorter pubic joint, etc.]? Are we to presume that each such isolated change in pelvic structure had a sufficiently strong effect on reproductive success to have been independently altered and fixed in the genome, even if realistic genetic models for the individual specification of each such feature were available? Many such complicated (even labyrinthine) biomechanical explanations of these pelvic traits have been posited (49, 50), but, in light of what we have discovered about morphogenesis in limbs, such analyses have been rendered unreasonable.
Cartilage Modeling and the Evolution of the Human Knee.
As noted earlier, the tissues of the musculoskeletal system are exquisitely sensitive to mechanical loading. This sensitivity can be “exploited” by selection to produce relatively profound anatomical changes with only minimal changes in PI. The evolution of the human knee (Fig. 2) can serve as an excellent example of the potential role of such SAMs in the evolution of the mammalian postcranium.
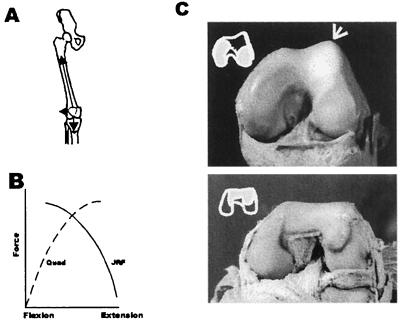
Figure 2.
The human knee exhibits specialized features that can be directly attributed to its role in upright walking. These include a bicondylar angle [the knee angle that places the foot beneath the trunk’s center of mass (A)], an elevated lateral condylar lip [which counteracts the tendency for patellar dislocation by the quadriceps (C Upper)], and elliptically shaped femoral condyles [which increase cartilage contact in full extension during the primary periods of ground contact]. In addition, human knees are tibial dominant (C Upper) whereas those of quadrupedal primates are patellar dominant (C Lower). The latter features require more explanation. The patella is lodged within the quadriceps, which is the principal extensor of the knee (A). When the knee is in flexion, a large component of extensor force compresses the patella against the femur. The resultant stress is determined by the congruity of the two mated surfaces. However, in extension, knee extensor force (plus body mass) generates compression between the femur and tibia; in this position, their area of contact determines joint stress (These relationships are graphed in B). Primates have a great range of motion in the knee. Therefore, unlike many other mammals, there is a significant part of their distal femoral surface that must contact the patella during flexion and the tibia in extension. The shape of this “shared” region (C) differs radically in chimpanzee and human distal femora. In chimpanzees (C Lower and Inset), it is simple and mirror images the discoid surface of the patella (not shown). In the human (C Upper and Inset), it instead conforms to the shapes of the medial and lateral tibial condyles (as deepened by their respective menisci). There is also a dramatic anteroposterior elongation of the human lateral condyle (not shown). This increases the area of cartilage contact in the last 20 degrees or less of knee extension [the chimpanzee’s is circular and does not reflect any single joint position of increased cartilage contact]. The chimpanzee knee is clearly patellar dominant whereas the human knee is tibial dominant. Given the plasticity of developing joint cartilage, all of these individual morphological differences could have been elicited by elongating the presumptive prechondrogenic condylar mesenchyme (especially that of the lateral condyle) by a few cell diameters. This, in conjunction with a habitual bipedal gait (which generates continuously high levels of tibiofemoral force in full extension), can account for virtually all of these unique human characters. What at first appears to be a profusion of separate traits more probably reflects a profoundly simpler change in the pattern formation field of the human femur.
When two or more bones that comprise a synovial joint move relative to one another, they must do so in a manner that generates velocity vectors that are continuously tangential to their contacting surfaces. If this is not the case, the two rigid bodies will deform and degrade their contacting surfaces. This results in their eventual destruction and further kinematic derangement (52, 53). In addition, the joint’s inherent tensile restraint system of ligaments and tendons must also be in exacting compliance with the joint’s pathway of motion, so that it can maintain that pathway in the face of any external forces that tend to dislodge it.
Mammalian joints, therefore, must develop inviolate coordination between their surface geometries and soft tissue restraint systems (54) (Fig. 2). It is virtually inconceivable that such exact conformity between the mating surfaces of a synovial joint could be dictated in some directly heritable fashion (i.e., by descriptive specification). This would require not only an exact ordination of the three-dimensional form of the mated surfaces but equally exacting construction of the network of individual fiber bundles that compose each of the joint’s ligaments, all of which would be in some way subject to the process of genetic recombination. Clearly, such exacting geometries are not directly heritable.
We have long known, since the classic tissue culture experiments of Murray (55) and Fell (56), that cartilage exhibits considerable mechanical reactivity, and there is now substantial direct experimental evidence of its robust modeling capacity, including recent demonstrations that chondrocytes are “very sensitive to loading pattern” (ref. 57, p. 906) and that imposed loads act to calibrate their metabolic activity, including the up- and down-regulation of matrix synthesis (reviewed in ref. 57). It is therefore increasingly likely that joint geometry is quite plastic even though such plasticity ceases at adulthood (there are no repair mechanisms). This SAM has great import with respect to the evolution of joint structure.
The human knee differs dramatically from that of other primates (Fig. 2), reflecting a highly specialized adaptation to bipedality. How were all of these dramatic changes acquired by humans and their ancestors? The bicondylar angle may simply be a modeling effect of differential induction of regional mitosis in the distal femoral growth plate, the chondral epiphysis, or both in response to a medial joint position required during habitual upright gait (58). Other unique characters, such as tibial dominance, however, would at first seem to require much more complicated origins.
At the same time, given the plasticity of developing joint cartilage, relatively simple changes in pattern formation can underlie profound downstream changes. This type of pathway is the most probable means by which the generalized morphology of the hominoid distal femur was transformed into its highly specialized human form. Specifically, elongation of the lateral femoral condyle could have been introduced by a slight increase in the anteroposterior length of its prechondrogenic mesenchymal field by only a few cell diameters or by a slight respecification of postanlagen growth. Such a change, in conjunction with cartilage plasticity and a habitual bipedal gait (which generates continuously high levels of tibiofemoral force in and about full extension) (Fig. 2), can account for virtually the entirety of the unique morphology of the human knee. Therefore, what appears to be a profusion of separate traits may very well reflect a profoundly simpler, albeit highly influential, change in the pattern formation field of the human distal femur.
Although the above examples have been restricted to issues of human evolution, expositions directly linking equally dramatic anatomical changes to pattern formation shifts are readily available for other mammals and even classes [the origin of the fibular crest of birds and of the elongated tarsus of frogs are particularly striking examples (59–62)].
A Systematic Classification of Traits Based on Their Developmental Etiology.
Let us summarize the discussion to this point. A mammalian bone is ultimately the product of coding specified in its positional information (PI). That PI is manifested downstream by specific local growth and development regimens specific to each presumptive bone. Those regimens are in turn expressed by employment of a variety of highly conserved systemic assembly mechanisms (SAMs). Although subtle differences in PI can be unique to a species, the SAMs are not. They are shared among large numbers of taxa, and probably do not differ significantly among most mammals. This is highly probable simply because any alteration of such tissue and cellular response protocols would systemically alter the entire skeleton whereas subtle, local changes in the growth of a primordium have only limited effects. SAMs are therefore almost certainly highly conserved as they constitute a fundamental raw material of musculoskeletal evolution and mammalian “evolvability” (63). On the other hand, changes in such “rules” may have served to generate some major adaptive radiations.
In many cases of anatomical trait analysis, it will be difficult to distinguish among possible etiological pathways for the features being studied. However, the difficulty of this task does not make it insoluble, nor does it permit us to merely ignore its potentially profound implications, as viable hypotheses can now be constructed from our expanding knowledge of vertebrate development. For example, studies of the acquisition of the bicondylar angle in normal and myelodysplastic children suggest that this character is progressively acquired by responsive modeling in the joint cartilage, growth plate, or both whereas the effects of a variety of HOX knockouts clearly imply that bony superstructures such as the deltoid crest are at least partially read-outs of PI (23). We have known for years that the sagittal crest is merely a direct consequence of tension developed by fusion of the contralateral temporalis fascias (64). These represent the kinds of information that can be gleaned from investigations of development and applied toward a more informed interpretation of the fossil record.
We believe that the foregoing discussions provide the basis for a classification system that can greatly facilitate the decision-making process and thereby improve the accuracy of phylogenetic and functional analyses. We propose that differences in adult traits between taxa be formally classified, whenever possible, into the five categories defined in Table 1. Types 1 and 2 are traits that can, in theory, be traced to changes in PI. Type 3 traits are those that have systemic effects because of the alteration of a SAM or other systemic, genetically determined, effect. Alternatively, Type 3 traits may be the product of unmodified but currently poorly understood SAMs, such as allometric adjustments to simple changes in body size. Types 1 and 2 are true individually heritable genomic differences. Type 3 traits may be specific heritable changes or conversely, as just noted, the product of the interplay of SAMs with Types 1 and/or 2. Types 4 and 5 reflect the nonheritable effects of the interplay between unmodified SAMs, local mechanical environments, and the epigenetic effects of changes at other sites.
Table 1.
Proposed analytical trait types
| Type 1 |
| A trait that differs in two taxa because its presence and/or expression are downstream consequences of differences in the PI of its cells and its resultant effects on local pattern formation. Type 1 traits are fixed by directional and/or stabilizing selection because their primary functional features have a real effect on fitness (65), and result largely from a direct interaction between genes expressed during field deployment and the functional biology of their adult product. Example: the superoinferior shortening of the ilium in hominids. |
| Type 2 |
| A trait that is a collateral byproduct of field changes whose principal morphological consequences are selected. Type 2 traits differ in two taxa because of differences in pattern formation (as in Type 1 traits), but their functional effect is so minimal as to have had no probable real interaction with natural selection. Unlike Types 4 and 5, they represent true field derived pleiotropy. Example: the superoinferiorly shortened pubic symphyseal joint of hominids (for discussion see text). |
| Type 3 |
| A trait that differs in two taxa because of modification of a systemic factor that affects multiple elements, such as an anabolic steroid. Example: Body size and its allometric effects. Growth hormone indirectly regulates growth by controlling levels of secondary signals such as insulin-like growth factor 1. Zaire pygmies exhibit reduced levels of growth hormone binding protein and, therefore, lower levels of insulin-like growth factor 1 (reviewed in refs. 66 and 67). In giant transgenic mice, altered growth hormone levels increase the growth rate without changing the period over which growth occurs (68). This generates shape changes by allometric scaling effects: i.e., differential growth rates of elements (66). Such growth response mechanisms are also likely to be controlled by highly conserved SAMs (69). Allometric shifts, therefore, usually reflect slight changes of systemic control factors during development: e.g., small modulations of growth hormone and/or its related factors can generate fully coordinated morphological change. |
| Type 4 |
| A trait that differs between taxa because its presence/absence and/or “grade” are attributable exclusively to phenotypic effects of the interaction of SAMs and environmental stimuli. Such traits have no antecedent differences in pattern formation and therefore have no value in phyletic analysis. They are epigenetic and not pleiotropic. However, they provide significant behavioral information and are therefore of expository or evidentiary value in interpreting fossils. They result from habitual behaviors during development. Example: the bicondylar angle of the femur. |
| Type 5 |
| Traits arising by the same process as Type 4 traits but that have no reliable diagnostic value with respect to significant behavior. Such traits are not consistently expressed within species and often show marked variation of expression within individuals and local populations. Example: femoral anteversion. |
How are traits to be allocated to one of these five categories, given that virtually nothing is known about their actual genetic basis and that such knowledge is virtually unobtainable for extinct organisms? Our proposed classification is not intended to require such knowledge, but only to encourage observers to formally state the presumed morphogenetic basis of each of the traits they choose to include in a functional or phyletic analysis. Such basis should be consistent with our current understanding of postcranial morphogenesis; i.e., a hypothesis should be congruent with possible morphogenetic pathways, such as changes in the distribution pattern of PI, alterations of anlagen shape, or the composition of compartments. Resulting classifications may then be treated as hypotheses for further study and analysis.
Conclusion.
The recognition of biologically independent characters is fundamental to the analysis of fossils, and character atomization can lead to serious errors of over (or under) weighting in both phylogenetics and the interpretation of functional behavior and adaptation. We believe that careful categorization of characters using a system like that provided here may lead to more reliable phyletic (especially cladistic) analyses by allowing both neontologists and paleontologists to more accurately assess the characters they use. We suggest, for example, that multiple cladograms be constructed using different combinations of assigned trait types (using the system defined here or in some other broadly similar way) and then compared as a means of determining the possible effects of differing interpretations of character morphogenesis (e.g., cladograms based only on traits assigned to Types 1 and 3 versus others that include Types 1, 2, and 3, Types 1–4, etc). A comparison of their results can provide substantial information about the effects of morphogenetic explication on phyletic interpretation. The differences among such cladograms will be enlightening. In addition, the process may be reversed and phylogeny presumed to test functional hypotheses (see, for example, ref. 41). The methods proposed here thus increase the prospect of using phyletic information to improve our understanding of morphology and development.
Finally, many current hypotheses about musculoskeletal function require a particulate genomic basis for individual anatomical characters in order for them to be regarded as adaptations. Such interpretations conflict markedly with our current knowledge of morphogenesis, and explications of mammalian musculoskeletal morphology must now be made more congruent with what we know about pattern formation. The system presented here is designed to facilitate this process. We find it likely that morphogenetic statements of hypothesis, when constructed by using some form of system similar to that proposed here (and when subjected to systematic testing), will lead to substantial improvements in our understanding of mammalian postcranial function, phylogeny, and development and their respective interactions and coevolution.
Acknowledgments
We thank Melanie McCollum, Neil Shubin, Richard Meindl, Phil Reno, Ketan Patel, David DeGusta, Henry Gilbert, and Burt Rosenman for critical readings and suggestions. This work was supported by National Science Foundation Grant SBR 9729060 and a Biotechnology and Biological Sciences Research Council (BBSRC) David Phillips Fellowship to M.J.C.
Abbreviations
- PI
positional information
- SAM
systemic assembly mechanism
References
- 1.Hillis D M, Huselsenbeck J P, Cunningham C W. Science. 1994;264:671–677. doi: 10.1126/science.8171318. [DOI] [PubMed] [Google Scholar]
- 2.Douzery E, Catzeflis F M. J Mol Evol. 1995;41:622–636. doi: 10.1007/BF00175821. [DOI] [PubMed] [Google Scholar]
- 3.Krings M, Stake A, Schmitz R W, Krainitzki H, Stoneking M, Paabo S. Cell. 1997;90:19–30. doi: 10.1016/s0092-8674(00)80310-4. [DOI] [PubMed] [Google Scholar]
- 4.Dobzhansky T. Am Nat. 1956;40:337–347. [Google Scholar]
- 5.Gould S J, Lewontin R C. Proc R Soc London Ser B. 1979;205:147–164. doi: 10.1098/rspb.1979.0086. [DOI] [PubMed] [Google Scholar]
- 6.Mayr E. Am Nat. 1983;121:324–334. [Google Scholar]
- 7.Raff R A. The Shape of Life. Chicago: Univ. of Chicago Press; 1996. [Google Scholar]
- 8.Thorogood P. In: Embryos, Genes and Birth Defects. Thorogood P, editor. Vol. 1. Chichester, U.K.: Wiley; 1997. , 1–16. [Google Scholar]
- 9.Akam M. Cell. 1998;92:153–155. doi: 10.1016/s0092-8674(00)80909-5. [DOI] [PubMed] [Google Scholar]
- 10.Moss-Salentijn L. J Dent Res. 1997;12:1814–1817. doi: 10.1177/00220345970760120201. [DOI] [PubMed] [Google Scholar]
- 11.Moss M L. Am J Orthod. 1981;80:366–375. doi: 10.1016/0002-9416(81)90172-x. [DOI] [PubMed] [Google Scholar]
- 12.Frost H M. Henry Ford Hosp Med J. 1983;31:3–9. [PubMed] [Google Scholar]
- 13.Cohn M J, Tickle C. Trends Genet. 1996;12:253–257. doi: 10.1016/0168-9525(96)10030-5. [DOI] [PubMed] [Google Scholar]
- 14.Johnson R L, Tabin C J. Cell. 1997;90:979–990. doi: 10.1016/s0092-8674(00)80364-5. [DOI] [PubMed] [Google Scholar]
- 15.Nelson C E, Morgan B A, Burke A C, Laufer E, DiMambro E, Murtaugh L C, Gonzales E, Tessarollo L, Parada L F, Tabin C. Development (Cambridge, UK) 1996;122:1449–1466. doi: 10.1242/dev.122.5.1449. [DOI] [PubMed] [Google Scholar]
- 16.Cohn M J, Bright P E. Cell Tissue Res. 1999;296:3–17. doi: 10.1007/s004410051261. [DOI] [PubMed] [Google Scholar]
- 17.Eriebacher A, Filvaroff E H, Gitelman S E, Derynck R. Cell. 1995;80:371–378. doi: 10.1016/0092-8674(95)90487-5. [DOI] [PubMed] [Google Scholar]
- 18.Vortkamp A, Lee K, Lanske B, Segre G V, Kronenberg H M, Tabin C J. Science. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- 19.Lanske B, Karaplis A, Lee K, Luz A, Vortkamp A, Pirro A, Karperien M, Defize L, Ho C, Mulligan R, et al. Science. 1996;273:663–666. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi I, Nuckolls G H, Takahashi K, Tanaka O, Semba I, Dashner R, Shum L, Stavkin C. J Cell Sci. 1998;111:2067–2076. doi: 10.1242/jcs.111.14.2067. [DOI] [PubMed] [Google Scholar]
- 21.Biewener A A, Fazzalari N, Konieczynski D, Baudinette R. Bone. 1996;19:1–8. doi: 10.1016/8756-3282(96)00116-0. [DOI] [PubMed] [Google Scholar]
- 22.Biewener A A. J Exp Biol. 1983;105:147–171. doi: 10.1242/jeb.105.1.147. [DOI] [PubMed] [Google Scholar]
- 23.Fromental-Ramain C, Warot X, Lakkaraju S, Favier B, Haack H, Birling C, Dierich A, Dolle P, Chambon P. Development (Cambridge, UK) 1996;122:461–472. doi: 10.1242/dev.122.2.461. [DOI] [PubMed] [Google Scholar]
- 24.Rijli F, Chambon P. Curr Opin Genet Dev. 1997;7:481–487. doi: 10.1016/s0959-437x(97)80074-3. [DOI] [PubMed] [Google Scholar]
- 25.Small K, Potter S. Genes Dev. 1993;7:2318–2328. doi: 10.1101/gad.7.12a.2318. [DOI] [PubMed] [Google Scholar]
- 26.Fromental-Ramain C, Warot X, Messadecq N, LeMeur M, Dolle P, Chambon P. Development (Cambridge, UK) 1996;122:2997–3011. doi: 10.1242/dev.122.10.2997. [DOI] [PubMed] [Google Scholar]
- 27.Davis A, Capecchi M. Development (Cambridge, UK) 1994;120:2187–2198. doi: 10.1242/dev.120.8.2187. [DOI] [PubMed] [Google Scholar]
- 28.Goff D, Tabin J. Development (Cambridge, UK) 1997;124:627–636. doi: 10.1242/dev.124.3.627. [DOI] [PubMed] [Google Scholar]
- 29.Merino R, Ganan Y, Macias D, Economides A, Sampath K, Hurle J. Dev Biol. 1998;200:35–45. doi: 10.1006/dbio.1998.8946. [DOI] [PubMed] [Google Scholar]
- 30.Zakany J, Gerard M, Favier B, Duboule D. EMBO J. 1997;16:4393–4402. doi: 10.1093/emboj/16.14.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawrence P A, Struhl G. Cell. 1996;85:951–961. doi: 10.1016/s0092-8674(00)81297-0. [DOI] [PubMed] [Google Scholar]
- 32.Gilbert S F, Opitz J M, Raff R A. Dev Biol. 1996;173:357–372. doi: 10.1006/dbio.1996.0032. [DOI] [PubMed] [Google Scholar]
- 33.Goodwin B. BioEssays. 1985;3:32–36. doi: 10.1002/bies.950030109. [DOI] [PubMed] [Google Scholar]
- 34.Goodwin B. J Theor Biol. 1982;97:443–455. doi: 10.1016/0022-5193(82)90275-2. [DOI] [PubMed] [Google Scholar]
- 35.Hall B K, Miyake T. Acta Embryol. 1992;186:107–124. doi: 10.1007/BF00174948. [DOI] [PubMed] [Google Scholar]
- 36.Tickle C. Curr Opin Genet Dev. 1995;5:478–484. doi: 10.1016/0959-437x(95)90052-i. [DOI] [PubMed] [Google Scholar]
- 37.Hinchliffe J R, Hurle J M, Summerbell D. Developmental Patterning of the Vertebrate Limb. New York: Plenum; 1991. [Google Scholar]
- 38.Shubin N, Tabin C, Carroll S. Nature (London) 1997;388:639–648. doi: 10.1038/41710. [DOI] [PubMed] [Google Scholar]
- 39.Wolpert L, Beddington R, Brockes J, Jessell T, Lawrence P, Meyerowitz E. Principles of Development. Oxford: Oxford Univ. Press; 1998. pp. 1–484. [Google Scholar]
- 40.Lovejoy C O, Heiple K G, Burstein A H. Am J Phys Anthropol. 1973;38:757–780. doi: 10.1002/ajpa.1330380315. [DOI] [PubMed] [Google Scholar]
- 41.Zakany J, Fromental-Ramain C, Warot X, Duboule D. Proc Natl Acad. Sci. USA. 1997;94:13695–13700. doi: 10.1073/pnas.94.25.13695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oliver G, Sidell N, Fiske W, Heinzmann C, Mohandas T, Sparkes R S, De Robertis E M. Genes Dev. 1989;3:641–650. doi: 10.1101/gad.3.5.641. [DOI] [PubMed] [Google Scholar]
- 43.Orr-Urtreger A, Givol D, Yayon A, Yarden Y, Lonai P. Development (Cambridge, UK) 1991;113:1419–1434. doi: 10.1242/dev.113.4.1419. [DOI] [PubMed] [Google Scholar]
- 44.Parr B A, Shea M J, Vassileva G, McMahon A P. Development (Cambridge, UK) 1993;119:247–261. doi: 10.1242/dev.119.1.247. [DOI] [PubMed] [Google Scholar]
- 45.Dolle P, Rubert E, Kastner P, Petkovich M, Stoner C, Gudas L, Chambon P. Nature. 1989;342:702–705. doi: 10.1038/342702a0. [DOI] [PubMed] [Google Scholar]
- 46.McHenry H. In: Origines de la Bipedie chez les Hominides. Coppens Y, Senut B, editors. Paris: Éditioas du Centre National de la Recherche Scientifique; 1991. pp. 133–142. [Google Scholar]
- 47.Susman R L, Stern J T, Jungers W L. Folia Primatol. 1984;443:113–156. doi: 10.1159/000156176. [DOI] [PubMed] [Google Scholar]
- 48.Stern J T, Susman R L. In: Origines de la Bipedie chez les Hominides. Coppens Y, Senut B, editors. Paris: Editions du Centre National de la Recherch Scientifique; 1991. pp. 99–111. [Google Scholar]
- 49.Rak Y. J Hum Evol. 1991;20:283–290. [Google Scholar]
- 50.Cartmill M, Schmitt D. Am. J. Phys. Anthropol. 1997. , Suppl. 24, 49 (abstr.). [DOI] [PubMed] [Google Scholar]
- 51.Dart R A. Am J Phys Anthropol. 1949;7:301–334. doi: 10.1002/ajpa.1330070302. [DOI] [PubMed] [Google Scholar]
- 52.Frankel V H, Burstein A, Brooks D B. J Bone Jt Surg Am Vol. 1971;53:945–962. [PubMed] [Google Scholar]
- 53.Burstein A H, Wright T M. Fundamentals of Orthopaedic Biomechanics. Baltimore: Williams & Wilkins; 1994. pp. 1–226. [Google Scholar]
- 54.Blacharski P A, Somerset J H, Murray D G. J Biomech. 1975;8:375–384. doi: 10.1016/0021-9290(75)90073-1. [DOI] [PubMed] [Google Scholar]
- 55.Murray P D F. Bones: A Study of the Development and Structure of the Vertebrate Skeleton. Cambridge, U.K.: Cambridge Univ. Press; 1936. [Google Scholar]
- 56.Fell H B. Embryologia. 1968;10:205–225. [Google Scholar]
- 57.Urban J P G. Brit J Rheumatol. 1994;33:901–908. doi: 10.1093/rheumatology/33.10.901. [DOI] [PubMed] [Google Scholar]
- 58.Duren D L, Lovejoy C O. Am. J. Phys. Anthropol. 1997. , Suppl. 24, 104–105. [Google Scholar]
- 59.Muller G B, Streicher J. Anat Embryol. 1989;179:327–339. doi: 10.1007/BF00305059. [DOI] [PubMed] [Google Scholar]
- 60.Muller G B. J Evol Biol. 1989;2:31–47. [Google Scholar]
- 61.Muller G B. In: Developmental Patterning of the Vertebrate Limb. Hinchliffe J R, Hurle J M, Summerbell D, editors. New York: Plenum; 1991. pp. 395–405. [Google Scholar]
- 62.Blanco M, Misof B, Wagner G. Dev Genes Evol. 1998;208:175–187. doi: 10.1007/s004270050172. [DOI] [PubMed] [Google Scholar]
- 63.Kirschner M, Gerhart J. Proc Natl Acad Sci USA. 1998;95:8420–8427. doi: 10.1073/pnas.95.15.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Washburn S L. Anat Rec. 1947;99:239–248. doi: 10.1002/ar.1090990303. [DOI] [PubMed] [Google Scholar]
- 65.Fisher R A. The Genetical Theory of Natural Selection. London: Oxford Univ. Press; 1930. [Google Scholar]
- 66.Shea B T. Evol Anthropol. 1992;1:125–134. [Google Scholar]
- 67.Nilsson A, Ohlsson C, Issaksson O G P, Lindahl A, Isgaard J. Eur J Clin Nutr. 1998;48, Suppl. 1:S150–S160. doi: 10.1007/BF02558817. [DOI] [PubMed] [Google Scholar]
- 68.Shea B T, Hammer R E, Brinster R L, Ravosa M R. Genet Res. 1990;56:21–34. doi: 10.1017/s0016672300028846. [DOI] [PubMed] [Google Scholar]
- 69.Van der Meulen M C H, Carter D R. J Theor Biol. 1995;172:323–327. doi: 10.1006/jtbi.1995.0029. [DOI] [PubMed] [Google Scholar]