Abstract
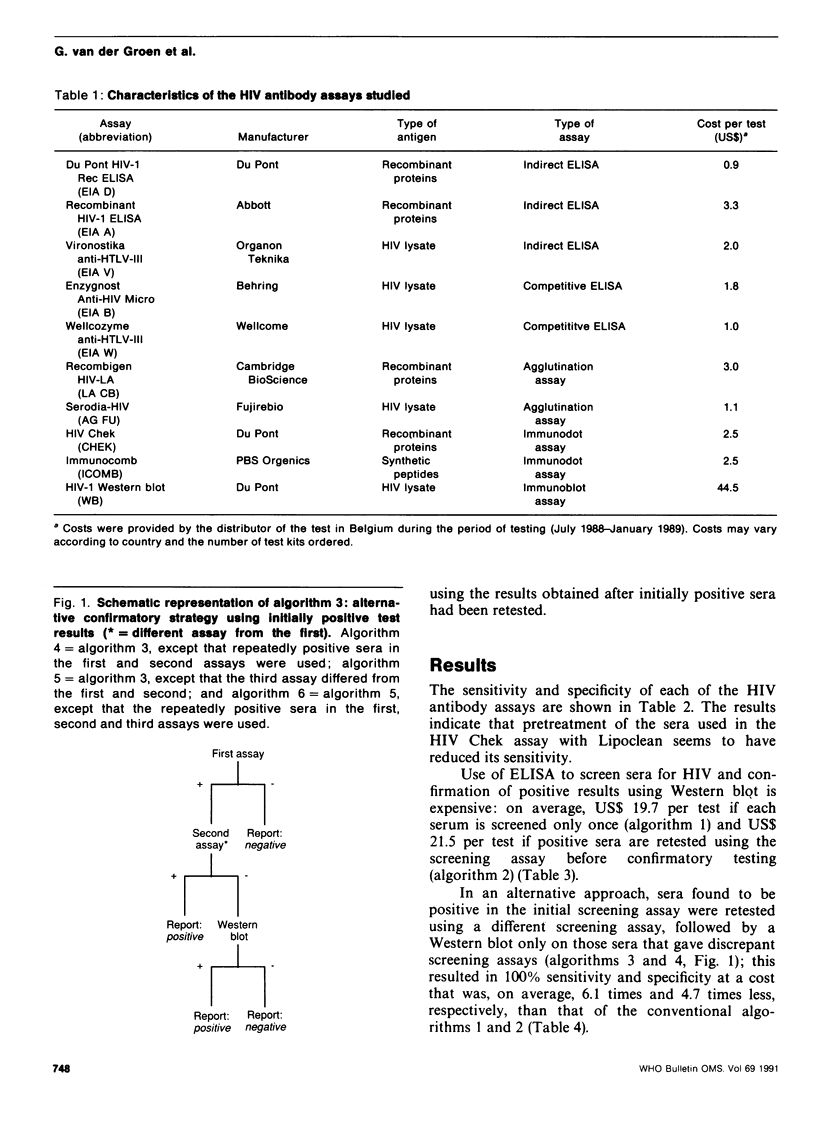
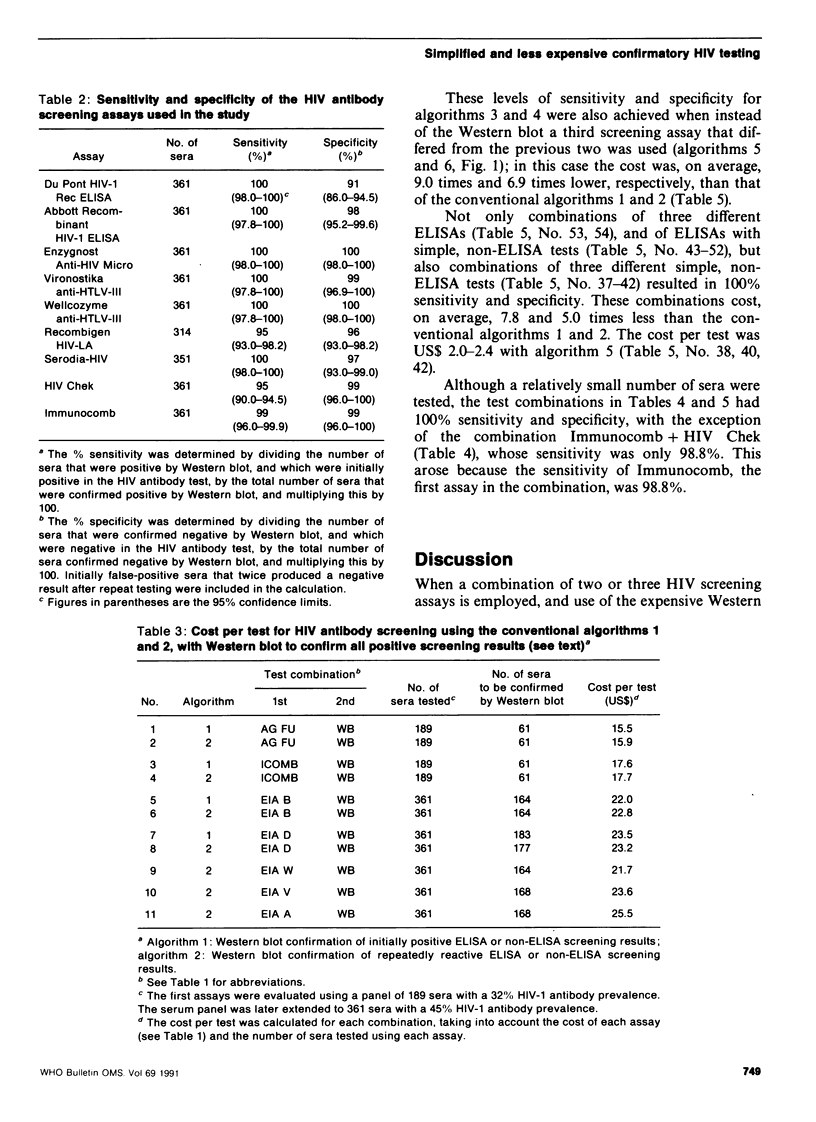
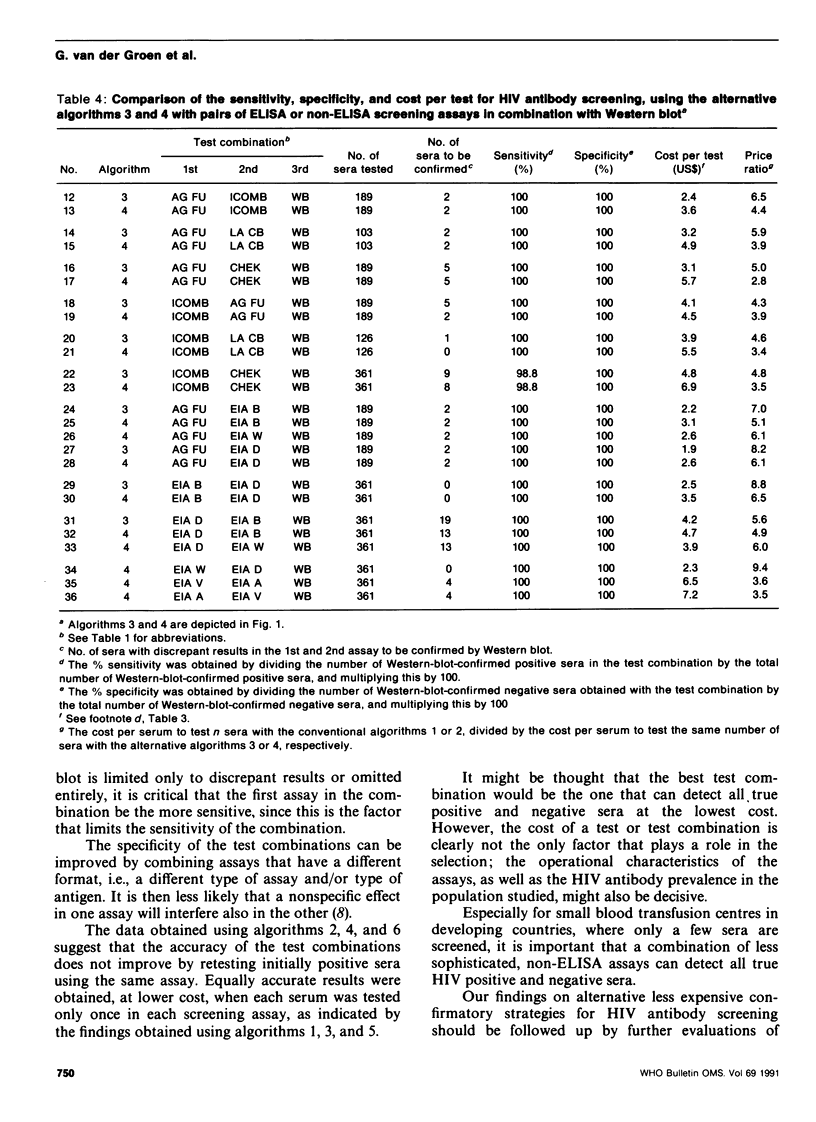
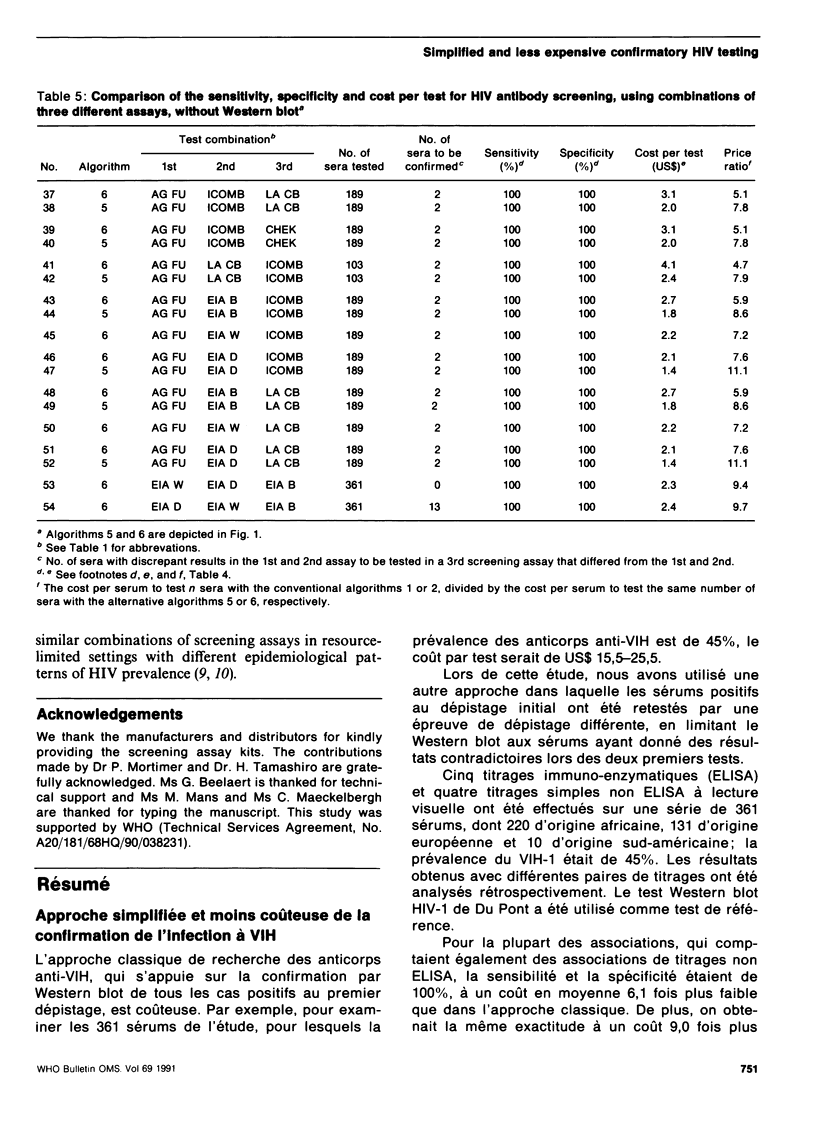
The conventional approach to human immunodeficiency virus (HIV) antibody testing, which relies on confirmation of all initially positive screening results using a Western blot assay, is expensive. In an alternative approach, we retested sera that were positive in an initial screening assay using a second screening assay, which differed from the first, and limited the use of Western blot to those sera that gave discrepant results in the two screening assays. This resulted in 100% sensitivity and specificity at a cost that was, on average, 6.1 times less than that of the conventional approach. This level of sensitivity and specificity was also achieved at a cost that was 9.0 times less than the conventional approach if the Western blot was replaced by a third screening assay that differed from the previous two. Retesting positive sera using the same assay did not increase the accuracy of the results obtained by testing the sera only once.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blomberg J., Klasse P. J. Specificities and sensitivities of three systems for determination of antibodies to human immunodeficiency virus by electrophoretic immunoblotting. J Clin Microbiol. 1988 Jan;26(1):106–110. doi: 10.1128/jcm.26.1.106-110.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S. W., Mboup S., Mingle J., Sambe D., Tukei P., Milenge K., Nyamongo J., Mubarak O. K., Sankale J. L., Hanson D. S. Field evaluation of alternative HIV testing strategy with a rapid immunobinding assay and an agglutination assay. Lancet. 1991 Jun 1;337(8753):1328–1331. doi: 10.1016/0140-6736(91)92991-a. [DOI] [PubMed] [Google Scholar]
- Riggin C. H., Thorn R. M. Visually read HIV immunoassays. Lancet. 1989 Mar 25;1(8639):671–672. doi: 10.1016/s0140-6736(89)92175-2. [DOI] [PubMed] [Google Scholar]
- Spielberg F., Kabeya C. M., Ryder R. W., Kifuani N. K., Harris J., Bender T. R., Heyward W. L., Quinn T. C. Field testing and comparative evaluation of rapid, visually read screening assays for antibody to human immunodeficiency virus. Lancet. 1989 Mar 18;1(8638):580–584. doi: 10.1016/s0140-6736(89)91610-3. [DOI] [PubMed] [Google Scholar]
- Tosswill J. H., Parry J. V., Mortimer P. P. Sensitivity of the newer HIV assays. AIDS. 1988 Jun;2(3):230–230. [PubMed] [Google Scholar]


