Abstract
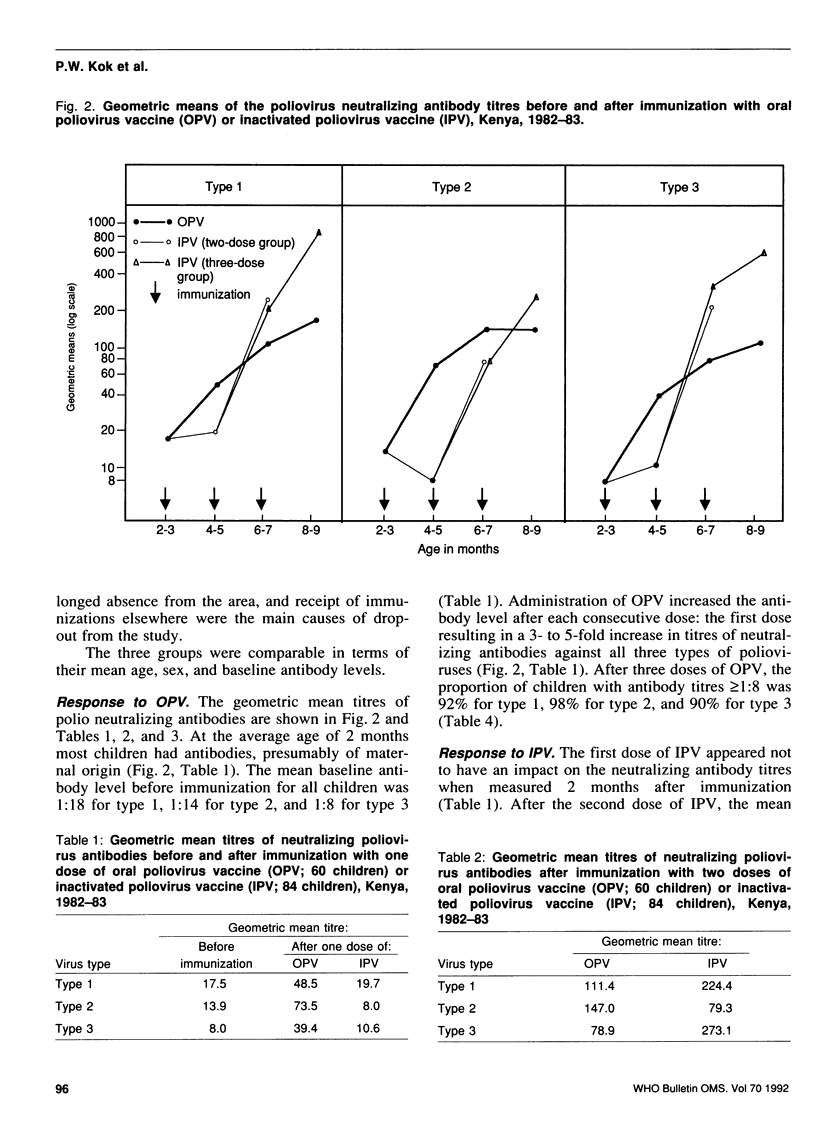
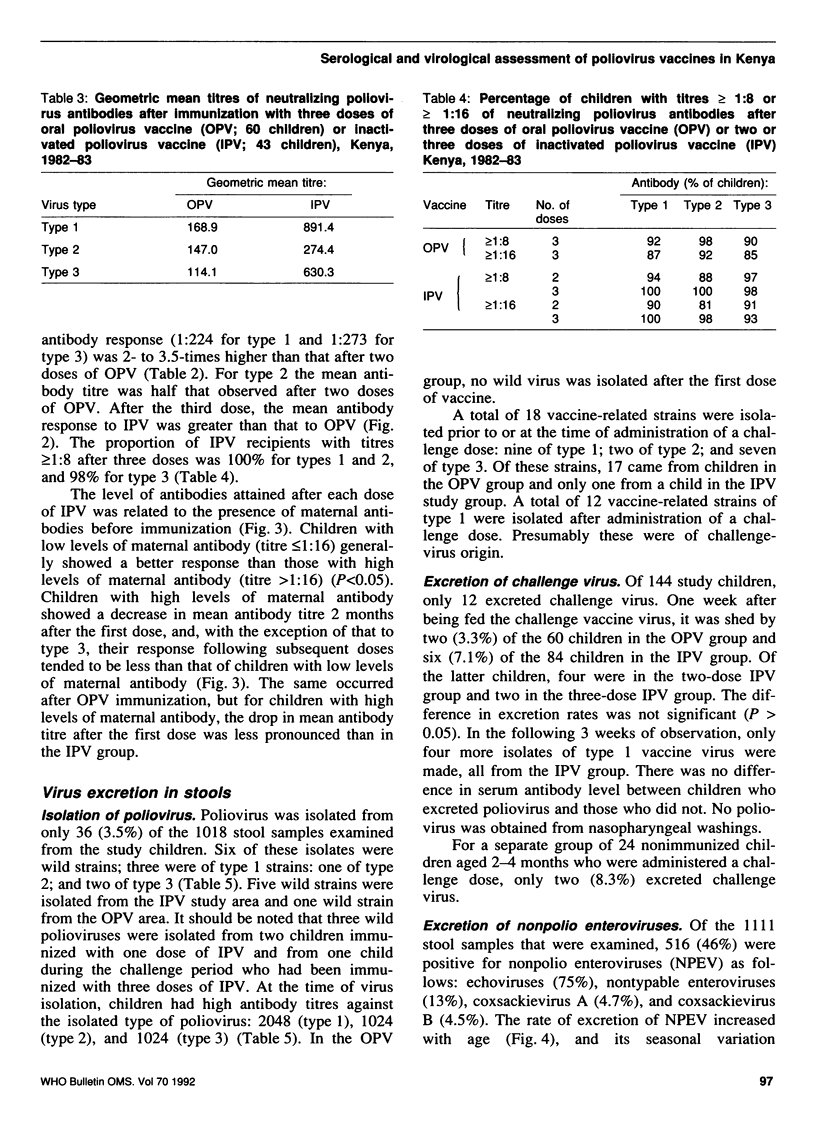
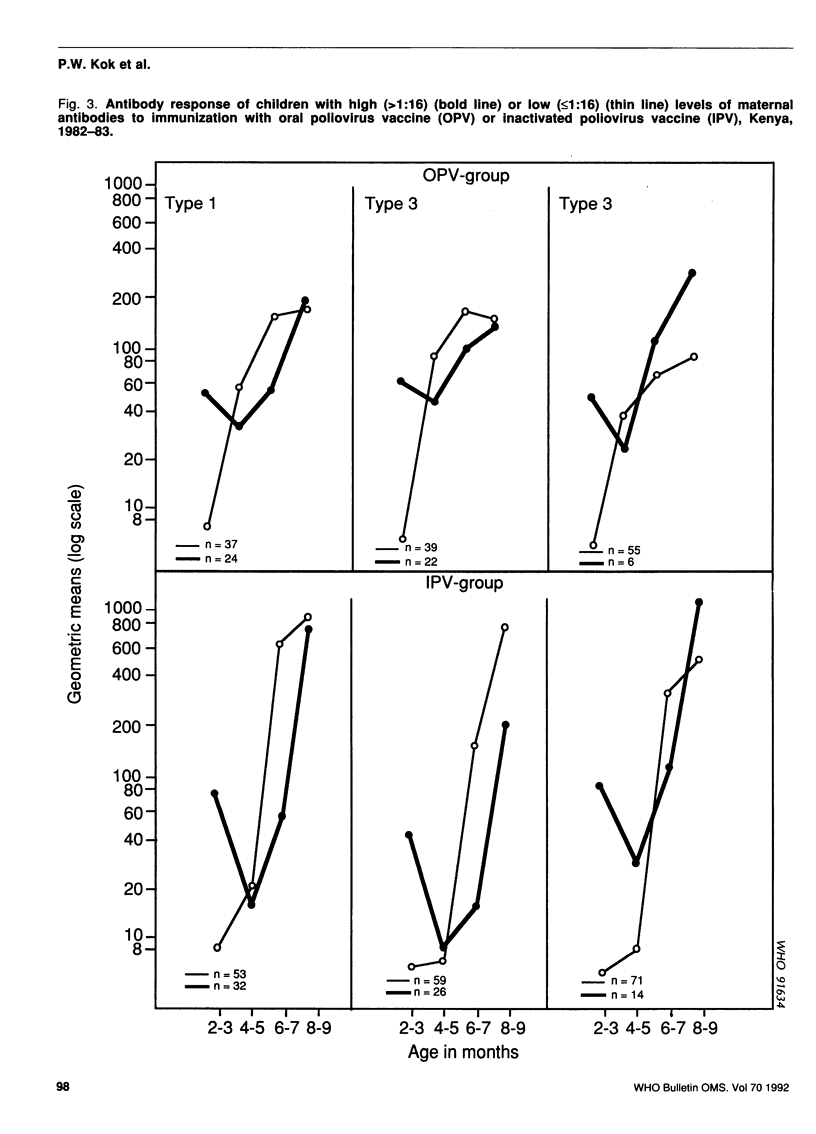
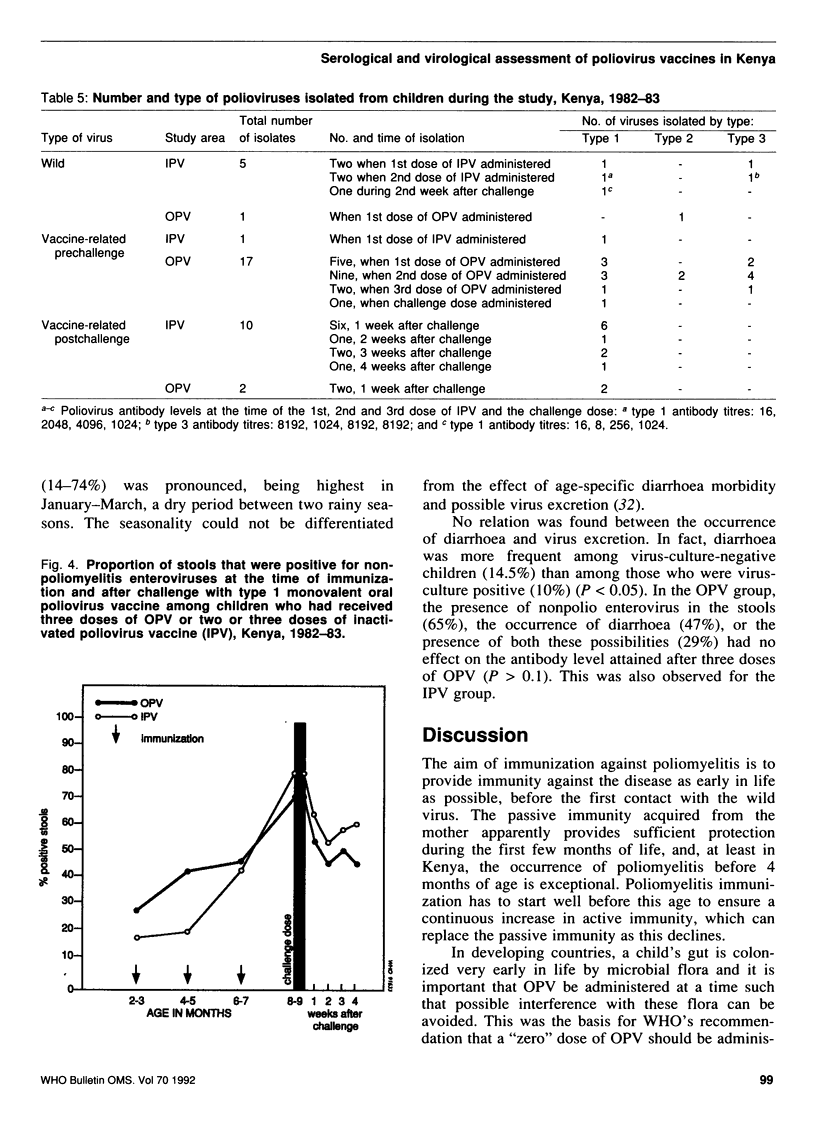
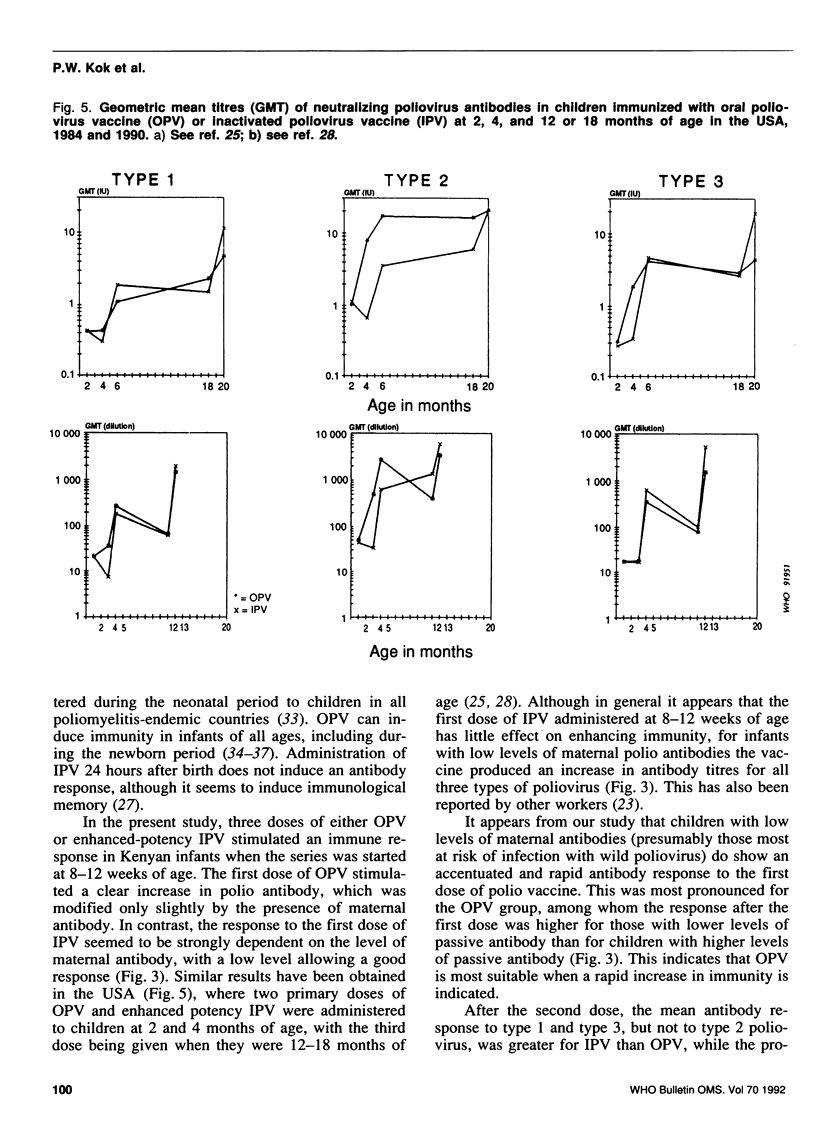
A study was carried out in a rural community in Kenya to compare the humoral and intestinal immunity provided by three doses of oral poliovirus vaccine (OPV) and two or three doses of enhanced-potency inactivated poliovirus vaccine (IPV). The immunization series was started at 8-12 weeks of age and the interval between doses was 2 months. In children with low levels of maternal antibodies (i.e., those most at risk), the first dose of either vaccine stimulated antibody response. Children with high levels of maternal antibodies responded to the first dose of OPV, but not to that of IPV. Subsequent doses led to increases in the mean antibody titres with both vaccines. After three doses of OPV, the proportion of children with antibody titres of greater than or equal to 1:8 was 92% for type 1 virus, 98% for type 2, and 90% for type 3. After two doses of IPV the proportion of children with antibody titres of greater than or equal to 1:8 was 94%, 88%, and 97% for type 1, type 2, and type 3, respectively; after three doses of IPV, 100% of children had antibodies greater than or equal to 1:8 for types 1 and 3, and 98% for type 2. Intestinal immunity was tested with a challenge dose of type 1 OPV, but the dose used was too small to detect a significant difference between the vaccines.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baptista Risi J., Jr The control of poliomyelitis in Brazil. Rev Infect Dis. 1984 May-Jun;6 (Suppl 2):S400–S403. doi: 10.1093/clinids/6.supplement_2.s400. [DOI] [PubMed] [Google Scholar]
- Bernier R. H. Some observations on poliomyelitis lameness surveys. Rev Infect Dis. 1984 May-Jun;6 (Suppl 2):S371–S375. doi: 10.1093/clinids/6.supplement_2.s371. [DOI] [PubMed] [Google Scholar]
- De Quadros C. A., Andrus J. K., Olivé J. M., Da Silveira C. M., Eikhof R. M., Carrasco P., Fitzsimmons J. W., Pinheiro F. P. Eradication of poliomyelitis: progress in the Americas. Pediatr Infect Dis J. 1991 Mar;10(3):222–229. doi: 10.1097/00006454-199103000-00011. [DOI] [PubMed] [Google Scholar]
- Dong D. X., Hu X. M., Liu W. J., Li J. S., Jin Y. C., Tan S. G., Chen T. Q., Fu J. Z., Niu B. Y., Yu H. M. Immunization of neonates with trivalent oral poliomyelitis vaccine (Sabin). Bull World Health Organ. 1986;64(6):853–860. [PMC free article] [PubMed] [Google Scholar]
- Faden H., Modlin J. F., Thoms M. L., McBean A. M., Ferdon M. B., Ogra P. L. Comparative evaluation of immunization with live attenuated and enhanced-potency inactivated trivalent poliovirus vaccines in childhood: systemic and local immune responses. J Infect Dis. 1990 Dec;162(6):1291–1297. doi: 10.1093/infdis/162.6.1291. [DOI] [PubMed] [Google Scholar]
- Halsey N., Galazka A. The efficacy of DPT and oral poliomyelitis immunization schedules initiated from birth to 12 weeks of age. Bull World Health Organ. 1985;63(6):1151–1169. [PMC free article] [PubMed] [Google Scholar]
- Henderson R. H. The World Health Organization's plan of action for global eradication of poliomyelitis by the year 2000. Ann N Y Acad Sci. 1989;569:69–85. doi: 10.1111/j.1749-6632.1989.tb27359.x. [DOI] [PubMed] [Google Scholar]
- Heymann D. L., Murphy K., Brigaud M., Aymard M., Tembon A., Maben G. K. Oral poliovirus vaccine in tropical Africa: greater impact on incidence of paralytic disease than expected from coverage surveys and seroconversion rates. Bull World Health Organ. 1987;65(4):495–501. [PMC free article] [PubMed] [Google Scholar]
- LaForce F. M., Lichnevski M. S., Keja J., Henderson R. H. Clinical survey techniques to estimate prevalence and annual incidence of poliomyelitis in developing countries. Bull World Health Organ. 1980;58(4):609–620. [PMC free article] [PubMed] [Google Scholar]
- LaForce F. M. Poliomyelitis vaccines. Success and controversy. Infect Dis Clin North Am. 1990 Mar;4(1):75–83. [PubMed] [Google Scholar]
- Lahrech M. T., Caudrelier P. Immunological response of Moroccan children and newborns to oral poliovirus vaccine prepared on Vero cells. Vaccine. 1990 Aug;8(4):306–307. doi: 10.1016/0264-410x(90)90085-z. [DOI] [PubMed] [Google Scholar]
- McBean A. M., Thoms M. L., Albrecht P., Cuthie J. C., Bernier R. Serologic response to oral polio vaccine and enhanced-potency inactivated polio vaccines. Am J Epidemiol. 1988 Sep;128(3):615–628. doi: 10.1093/oxfordjournals.aje.a115009. [DOI] [PubMed] [Google Scholar]
- Patriarca P. A., Laender F., Palmeira G., Oliveira M. J., Lima Filho J., Dantes M. C., Cordeiro M. T., Risi J. B., Jr, Orenstein W. A. Randomised trial of alternative formulations of oral poliovaccine in Brazil. Lancet. 1988 Feb 27;1(8583):429–433. doi: 10.1016/s0140-6736(88)91229-9. [DOI] [PubMed] [Google Scholar]
- Patriarca P. A., Wright P. F., John T. J. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis. 1991 Sep-Oct;13(5):926–939. doi: 10.1093/clinids/13.5.926. [DOI] [PubMed] [Google Scholar]
- Robertson S. E., Traverso H. P., Drucker J. A., Rovira E. Z., Fabre-Teste B., Sow A., N'Diaye M., Sy M. T., Diouf F. Clinical efficacy of a new, enhanced-potency, inactivated poliovirus vaccine. Lancet. 1988 Apr 23;1(8591):897–899. doi: 10.1016/s0140-6736(88)91711-4. [DOI] [PubMed] [Google Scholar]
- Rodríguez Cruz R. Cuba: mass polio vaccination program, 1962-1982. Rev Infect Dis. 1984 May-Jun;6 (Suppl 2):S408–S412. [PubMed] [Google Scholar]
- Sabin A. B. Oral poliovirus vaccine: history of its development and use and current challenge to eliminate poliomyelitis from the world. J Infect Dis. 1985 Mar;151(3):420–436. doi: 10.1093/infdis/151.3.420. [DOI] [PubMed] [Google Scholar]
- Schatzmayr H. G., Maurice Y., Fujita M., de Fillipis A. M. Serological evaluation of poliomyelitis oral and inactivated vaccines in an urban low-income population at Rio de Janeiro, Brazil. Vaccine. 1986 Jun;4(2):111–113. doi: 10.1016/0264-410x(86)90048-4. [DOI] [PubMed] [Google Scholar]
- Schoub B. D., Johnson S., McAnerney J., Gilbertson L., Klaassen K. I., Reinach S. G. Monovalent neonatal polio immunization--a strategy for the developing world. J Infect Dis. 1988 Apr;157(4):836–839. doi: 10.1093/infdis/157.4.836. [DOI] [PubMed] [Google Scholar]
- Simoes E. A., Padmini B., Steinhoff M. C., Jadhav M., John T. J. Antibody response of infants to two doses of inactivated poliovirus vaccine of enhanced potency. Am J Dis Child. 1985 Oct;139(10):977–980. doi: 10.1001/archpedi.1985.02140120023021. [DOI] [PubMed] [Google Scholar]
- Slater P. E., Orenstein W. A., Morag A., Avni A., Handsher R., Green M. S., Costin C., Yarrow A., Rishpon S., Havkin O. Poliomyelitis outbreak in Israel in 1988: a report with two commentaries. Lancet. 1990 May 19;335(8699):1192–1198. doi: 10.1016/0140-6736(90)92705-m. [DOI] [PubMed] [Google Scholar]
- Sutter R. W., Patriarca P. A., Brogan S., Malankar P. G., Pallansch M. A., Kew O. M., Bass A. G., Cochi S. L., Alexander J. P., Hall D. B. Outbreak of paralytic poliomyelitis in Oman: evidence for widespread transmission among fully vaccinated children. Lancet. 1991 Sep 21;338(8769):715–720. doi: 10.1016/0140-6736(91)91442-w. [DOI] [PubMed] [Google Scholar]
- Swartz T. A., Handsher R., Stoeckel P., Drucker J., Caudrelier P., Van Wezel A. L., Cohen H., Salk D., Salk J. Immunologic memory induced at birth by immunization with inactivated polio vaccine in a reduced schedule. Eur J Epidemiol. 1989 Jun;5(2):143–145. doi: 10.1007/BF00156819. [DOI] [PubMed] [Google Scholar]
- Tswana S. A., Berejena C. Sero-conversion of infants to three doses of oral poliomyelitis vaccine. Cent Afr J Med. 1988 Dec;34(12):290–294. [PubMed] [Google Scholar]