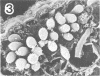
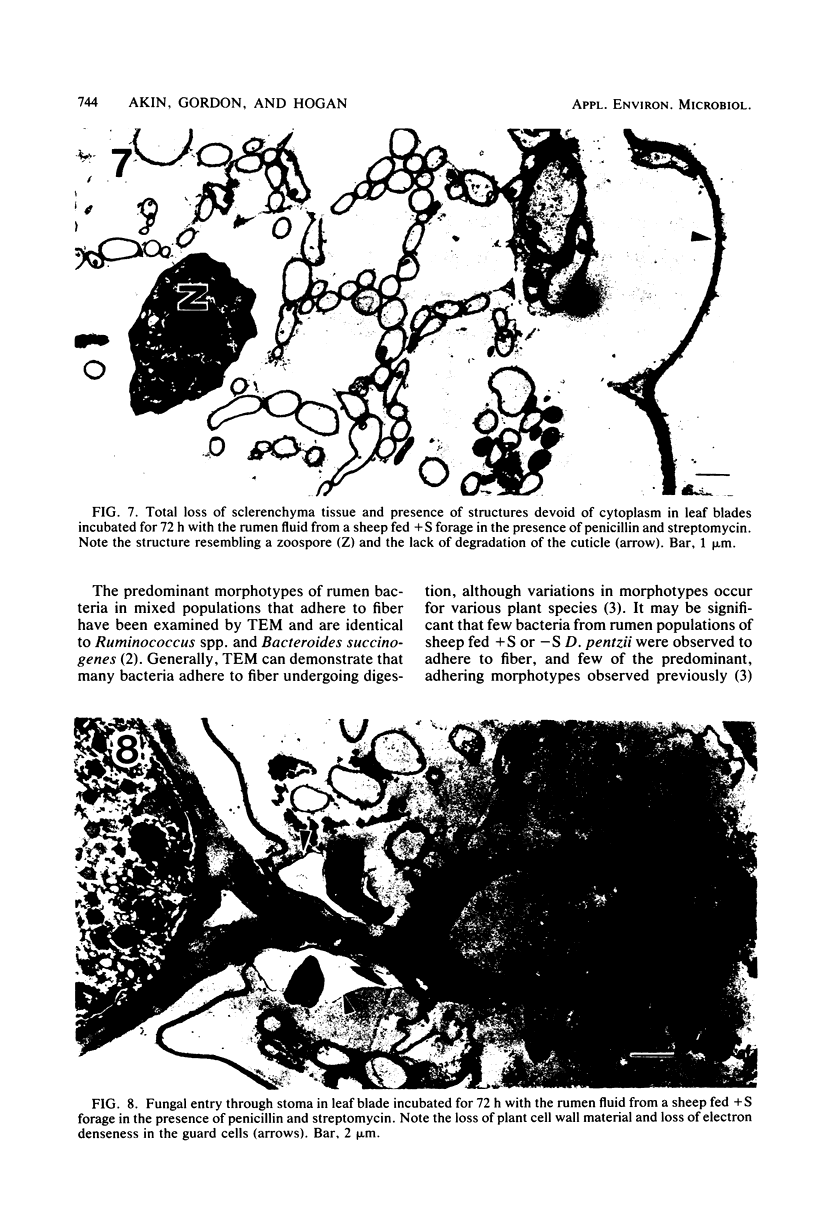
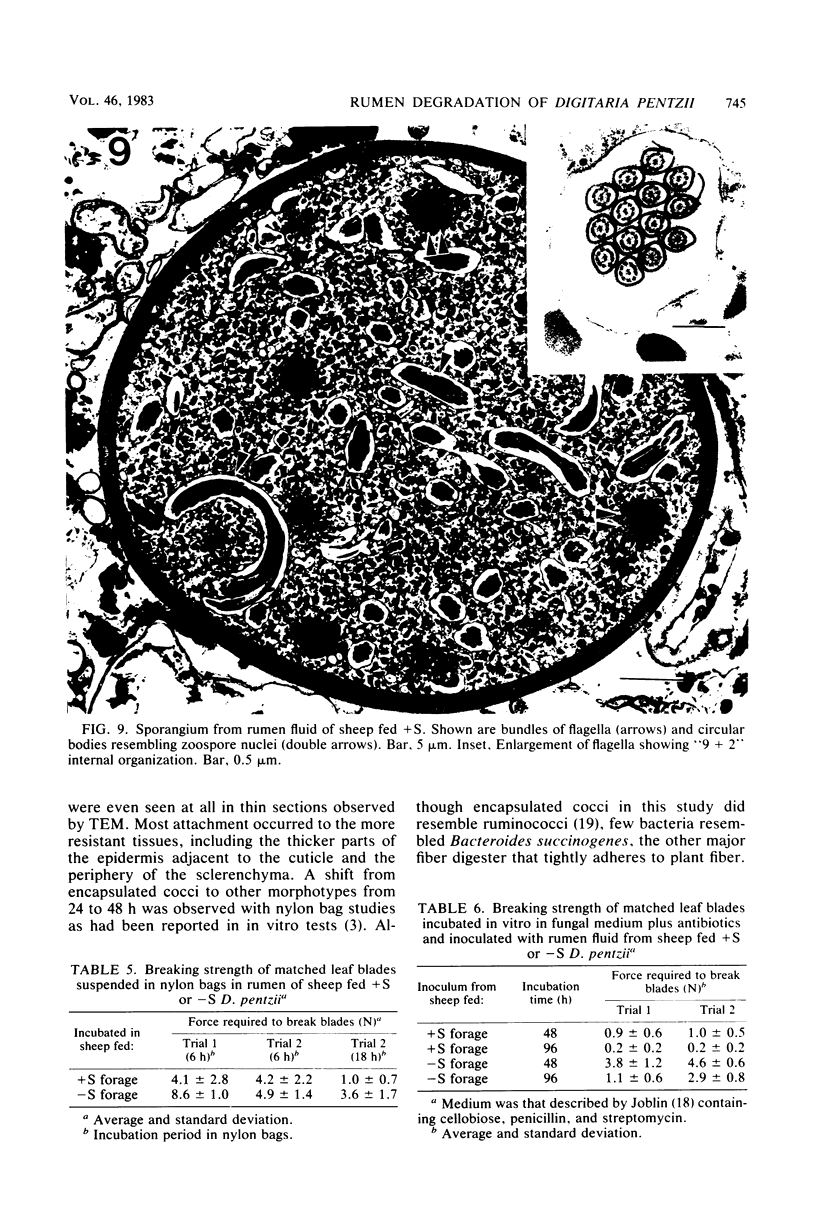
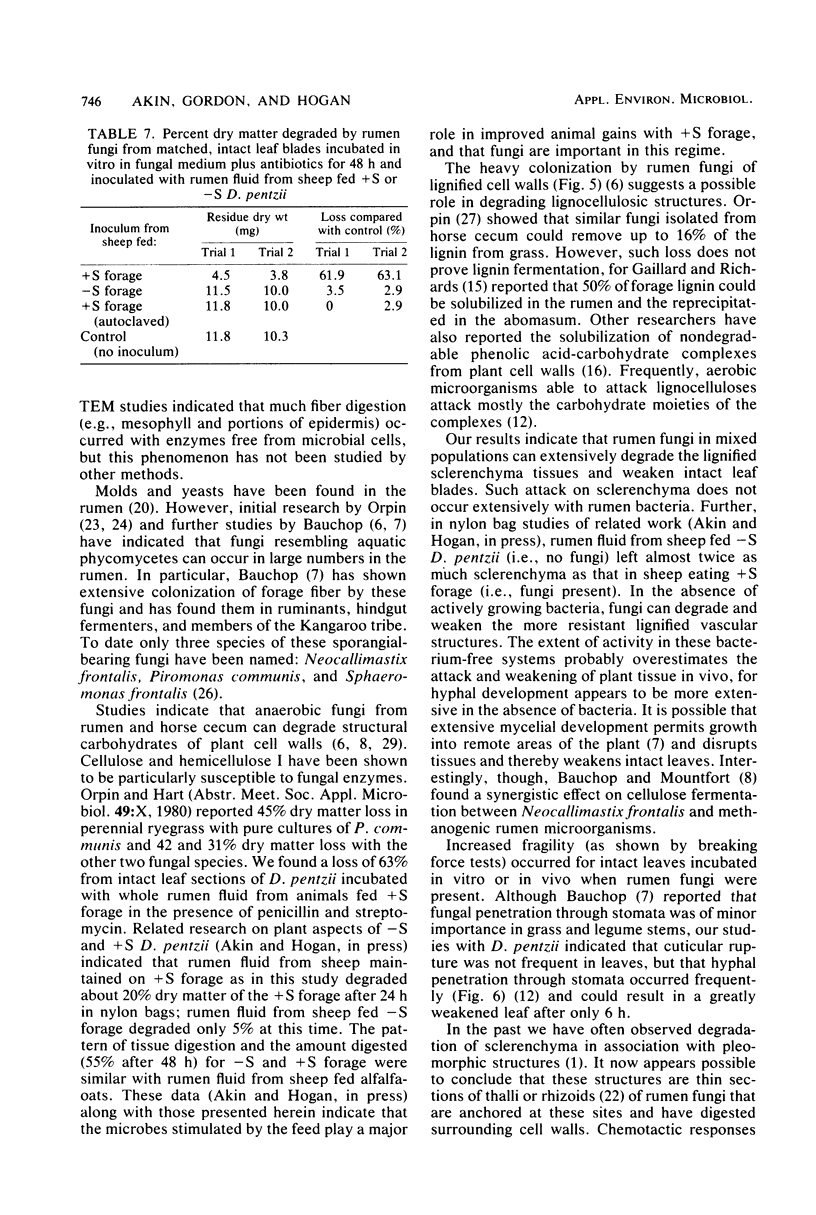
Abstract
Sheep fed the forage Digitaria pentzii fertilized with sulfur were compared with those fed unfertilized forage for the rumen microbial population involved with fiber degradation. No differences were detected in the bacterial population as determined by anaerobic cultures on a habitat-simulating medium, xylan, or pectin, by 35S labeling techniques for microbial protein, or by transmission electron microscopic studies of bacterium-fiber interactions. Rumen volume and water flow from the rumen were not different for sheep fed each of the forages. Rumen fungi were prevalent in sheep fed sulfur-fertilized D. pentzii as shown by sporangia adhering to forage fiber and by colonies developing from zoospores in roll tubes with cellobiose plus streptomycin and penicillin. Fungi were absent or in extremely small numbers in sheep fed unfertilized forage. Nylon bag digestibility studies showed that the fungi preferentially colonized the lignified cells of blade sclerenchyma by 6 h and caused extensive degradation by 24 h. In the absence of bacteria in in vitro studies, extensive hyphal development occurred; other lignified tissues in blades (i.e., mestome sheath and xylem) were attacked, resulting in a residue with partially degraded and weakened cell walls. Nonlignified tissues were also degraded. Breaking force tests of leaf blades incubated in vitro with penicillin and streptomycin and rumen fluid from sheep fed sulfur-fertilized forage or within nylon bags in such sheep showed a residue at least twice as fragile as that from sheep fed unfertilized forage. In vitro tests for dry matter loss showed that rumen fungi, in the absence of actively growing bacteria, could remove about 62% of the forage material. The response of rumen fungi in sheep fed sulfur-fertilized D. pentzii afforded a useful in vivo test to study the role of these microbes in fiber degradation. Our data establish that rumen fungi can be significant degraders of fiber and further establish a unique role for them in attacking and weakening lignocellulosic tissues. The more fragile residues resulting from attack by fungi could explain the greater intake consistently observed by sheep eating sulfur-fertilized compared with unfertilized D. pentzii forage.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E. Attack on lignified grass cell walls by a facultatively anaerobic bacterium. Appl Environ Microbiol. 1980 Oct;40(4):809–820. doi: 10.1128/aem.40.4.809-820.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin D. E. Evaluation by electron microscopy and anaerobic culture of types of rumen bacteria associated with digestion of forage cell walls. Appl Environ Microbiol. 1980 Jan;39(1):242–252. doi: 10.1128/aem.39.1.242-252.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin D. E. Microscopic evaluation of forage digestion by rumen microorganisms--a review. J Anim Sci. 1979 Mar;48(3):701–710. doi: 10.2527/jas1979.483701x. [DOI] [PubMed] [Google Scholar]
- Akin D. E. Ultrastructure of rigid and lignified forage tissue degradation by a filamentous rumen microorganism. J Bacteriol. 1976 Mar;125(3):1156–1162. doi: 10.1128/jb.125.3.1156-1162.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchop T., Mountfort D. O. Cellulose fermentation by a rumen anaerobic fungus in both the absence and the presence of rumen methanogens. Appl Environ Microbiol. 1981 Dec;42(6):1103–1110. doi: 10.1128/aem.42.6.1103-1110.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchop T. Rumen anaerobic fungi of cattle and sheep. Appl Environ Microbiol. 1979 Jul;38(1):148–158. doi: 10.1128/aem.38.1.148-158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beever D. E., Harrison D. G., Thomson D. J., Cammell S. B., Osbourn D. F. A method for the estimation of dietary and microbial protein in duodenal digesta of ruminants. Br J Nutr. 1974 Jul;32(1):99–112. doi: 10.1079/bjn19740061. [DOI] [PubMed] [Google Scholar]
- Bull L. S., Vandersall J. H. Sulfur source for in vitro cellulose digestion and in vivo ration utilization, nitrogen metabolism, and sulfur balance. J Dairy Sci. 1973 Jan;56(1):106–112. doi: 10.3168/jds.S0022-0302(73)85122-7. [DOI] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford D. L. Growth of Thermomonospora fusca on lignocellulosic pulps of varying lignin content. Can J Microbiol. 1974 Jul;20(7):1069–1072. doi: 10.1139/m74-167. [DOI] [PubMed] [Google Scholar]
- DOWNES A. M., MCDONALD I. W. THE CHROMIUM-51 COMPLEX OF ETHYLENEDIAMINE TETRAACETIC ACID AS A SOLUBLE RUMEN MARKER. Br J Nutr. 1964;18:153–162. doi: 10.1079/bjn19640015. [DOI] [PubMed] [Google Scholar]
- Gaillard B. D., Richards G. N. Presence of soluble lignin-carbohydrate complexes in the bovine rumen. Carbohydr Res. 1975 Jun;42(1):135–145. doi: 10.1016/s0008-6215(00)84106-3. [DOI] [PubMed] [Google Scholar]
- Henning P. A., van der Walt A. E. Inclusion of xylan in a medium for the enumeration of total culturable rumen bacteria. Appl Environ Microbiol. 1978 Jun;35(6):1008–1011. doi: 10.1128/aem.35.6.1008-1011.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham M. J., Brooker B. E., Pettipher G. L., Harris P. J. Ruminococcus flavefaciens Cell Coat and Adhesion to Cotton Cellulose and to Cell Walls in Leaves of Perennial Ryegrass (Lolium perenne). Appl Environ Microbiol. 1978 Jan;35(1):156–165. doi: 10.1128/aem.35.1.156-165.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund A. Yeasts and moulds in the bovine rumen. J Gen Microbiol. 1974 Apr;81(2):453–462. doi: 10.1099/00221287-81-2-453. [DOI] [PubMed] [Google Scholar]
- Munn E. A., Orpin C. G., Hall F. J. Ultrastructural studies of the free zoospore of the rumen phycomycete Neocallimastix frontalis. J Gen Microbiol. 1981 Aug;125(2):311–323. doi: 10.1099/00221287-125-2-311. [DOI] [PubMed] [Google Scholar]
- Orpin C. G. Invasion of plant tissue in the rumen by the flagellate Neocallimastix frontalis. J Gen Microbiol. 1977 Feb;98(2):423–430. doi: 10.1099/00221287-98-2-423. [DOI] [PubMed] [Google Scholar]
- Orpin C. G. Isolation of cellulolytic phycomycete fungi from the caecum of the horse. J Gen Microbiol. 1981 Apr;123(2):287–296. doi: 10.1099/00221287-123-2-287. [DOI] [PubMed] [Google Scholar]
- Orpin C. G. On the induction of zoosporogenesis in the rumen phycomycetes Neocallimastix frontalis, Piromonas communis and Sphaeromonas communis. J Gen Microbiol. 1977 Aug;101(2):181–189. doi: 10.1099/00221287-101-2-181. [DOI] [PubMed] [Google Scholar]
- Orpin C. G. Studies on the rumen flagellate Neocallimastix frontalis. J Gen Microbiol. 1975 Dec;91(2):249–262. doi: 10.1099/00221287-91-2-249. [DOI] [PubMed] [Google Scholar]
- Orpin C. G. The rumen flagellate Piromonas communis: its life-history and invasion of plant material in the rumen. J Gen Microbiol. 1977 Mar;99(1):107–117. doi: 10.1099/00221287-99-1-107. [DOI] [PubMed] [Google Scholar]
- Purser D. B., Buechler S. M. Amino acid composition of rumen organisms. J Dairy Sci. 1966 Jan;49(1):81–84. doi: 10.3168/jds.S0022-0302(66)87791-3. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]