Abstract
The most interesting property of neurons is their long-distance propagation of signals as spiking action potentials. Since 1993, Neurobasal/B27 ™ has been used as a serum-free medium optimized for hippocampal neuron survival. Neurons on microelectrode arrays (MEA) were used as an assay system to increase spontaneous spike rates in media of different compositions. We find spike rates of 0.5/second (Hz) for rat embryonic hippocampal neurons cultured in Neurobasal/B27, lower than cultures in serum-based media and offering an opportunity for improvement. NbActiv4™ was formulated by addition of creatine, cholesterol and estrogen to Neurobasal/B27 that synergistically produced an 8-fold increase in spontaneous spike activity. The increased activity with NbActiv4 correlated with a 2-fold increase in immunoreactive synaptophysin bright puncta and GluR1 total puncta. Characteristic of synaptic scaling, immunoreactive GABAAβ puncta also increased 1.5-fold and NMDA-R1 puncta increased 1.8-fold. Neuron survival in NbActiv4 equaled that in Neurobasal/B27, but with slightly higher astroglia. Resting respiratory demand was decreased and demand capacity was increased in NbActiv4, indicating less stress and higher efficiency. These results show that NbActiv4 is an improvement to Neurobasal/B27 for cultured networks with an increased density of synapses and transmitter receptors which produces higher spontaneous spike rates in neuron networks.
Keywords: neuron culture, serum-free medium, synaptogenesis, electrode array, network
1. Introduction
Neurobasal medium with B27 supplement is a widely-used serum-free medium that was optimized for neuron survival in 1993 (Brewer et al.). Serum-free media offer the advantage of greater lot to lot consistency, known components and inhibition of astroglial proliferation. However, development of synapses in neuron cultures promotes the most interesting aspect of these cells, cell to cell communication by action potentials or spikes. We report here low spontaneous spike rates of <0.5 Hz (spikes/sec) that develop in Neurobasal/B27 medium that can be improved 4 to 8-fold with certain additions to this classic medium. Additions that were optimized include cholesterol, shown to promote synaptogenesis (Pfrieger & Barres, 1997; Goritz et al., 2005), estrogen, to control better handling of the calcium influx (Kumar & Foster, 2002; Brewer et al., 2006), and creatine as an energy precursor for phosphocreatine to empower greater spike rates (Brewer & Wallimann, 2000) and possibly induce more inhibitory synapses (Ducray et al., 2007). The resulting optimized medium, NbActiv4, appears to promote higher spike rates by a mechanism involving greater synaptogenesis.
Materials and Methods
Culture and measures of electrical activity
NbActiv4™ (BrainBitsLLC.com) was formulated at proprietary concentrations by addition to the ingredients in Neurobasal™, B27™ and Glutamax™ (Invitrogen, Carlsbad, CA). Primary hippocampal neurons were isolated from E18 rat embryos and cultured at 37°C in an atmosphere of 5% CO2, 9% O2 on substrates coated with poly-D-lysine (1) (BrainBitsLLC.com). For measurement of electrical activity, neurons were cultured at 500 cells/mm2 on multi-electrode arrays (MEA60 with 10 μm diameter electrodes, Multichannel Systems, Reutlingen, Germany) and signals acquired through an MEA 1060-BC amplifier (gain 1100, filtered at 8-3000Hz, sampled at 25 kHz) and MCRack software from the same company. Signals were also analyzed with PCLAMP 9.0 software (Axon Instruments, Inc., Union City, CA). At 3 weeks after plating, in the same culture conditions, spontaneous spike activity was detected in a one-minute recording period as the number of spikes with amplitude exceeding five times the standard deviation of the baseline noise.
Survival
Hippocampal neurons were cultured at 160 cells/mm2 on polystyrene 24 well plates (Corning 3526, Corning, New York) coated with poly-D-Lysine. Cell media was either Neurobasal/B27 or NbActive4. A 50% medium change was performed at four days without the addition of glutamate. At day eight, cells were rinsed twice in Hanks Balanced Salt Solution (HBSS) without phenol red (Invitrogen 14025-092) and then labeled with 15 μg/ml flourescein diacetate (Sigma F7378) and 4.6 μg/ml propidium iodide (Sigma P4170), both diluted in HBSS without phenol red. Cells were incubated for 1-2 min. at room temperature and then rinsed in HBSS without phenol red. Under fluorescence, live cells were counted using a B1A520 filter (Olympus) and dead cells using a G1B580 filter (three 20X fields/well × 4 wells = n of 12/test).
Immunocytology
Neurons plated at 20 or 160 cells/mm2 on glass coverslips (Assistent Brand, Carolina Biologicals, Burlington, NC) were fixed in ice-cold methanol for 10 min to detect GABA and NR1 immunoreactive synapses. For synaptophysin and GluR1 (AMPA) immunoreactivity, cells were fixed for 30 min in 4% paraformaldehyde and 0.03% glutaraldehyde in phosphate buffered saline (PBS, Invitrogen, 10010). For all immunocytology, cells were rinsed twice in PBS after fixing. Non-specific sites were blocked and cells were permeabilized for 5 min in 5% normal goat serum, 0.5% Triton X-100 in PBS. Primary and secondary antibodies were diluted in 5% NGS, 0.05% TX-100 in PBS. Cells were incubated with primary antibodies at 4°C as follows: mouse-anti-GABAAβ (1:50, Chemicon, Temecula, CA, MAB341); rabbit-anti-NMDA-R1 (1:100, Sigma, St. Louis, MO, G8913); mouse –anti-synaptophysin (1:1000, Sigma S5768); rabbit-anti-GluR1 (1:3000, Upstate Biotechnology, Charlottesville, VA, 07-660); mouse-anti-MAP2 (1:400, Sigma, M4403); and rabbit-anti-tau (1:2000, Sigma, T6402). After rinsing four times with PBS, cells were incubated 1 hr at 22°C with either Alexa-fluor 568-conjugated affiniPure goat anti-mouse IgG (heavy + light chain, 1:2000, Molecular Probes, Eugene, OR, A11031) together with conjugated Alexa-fluor 488 affiniPure goat anti-rabbit IgG (heavy + light chain, 1:50, Molecular Probes, A11034) or Alexa-fluor 568 conjugated goat anti-rabbit IgG (heavy + light chain, 1:100 Molecular Probes A11036) together with Alexa-fluor 488 conjugated goat anti-mouse (heavy + light chain, 1:300, Molecular Probes A11029). After rinsing four times in PBS, the nuclei of cells were stained for two min. at 22°C with bisbenzamide (final concentration 1 μg/ml, diluted in PBS, Sigma, B2261). After two final rinses, coverslips were mounted with Aquamount and imaged through an Olympus 20X/0.45 or 60X/1.42 objective. Images were recorded with a Retiga Exi CCD camera (QImaging, Surrey, BC, Canada).
Image Analysis
Synaptic puncta were detected at constant threshold using Image-Pro+ 4.5.1 software. Because of the large range of intensity of the fluorescent puncta, digital segmentation required separation into bright and dim classes. A high threshold was used to detect bright objects around the soma and a low threshold was used to detect dimmer objects in the processes. Puncta area limits were set at 0.02-10 μm2 (field size for the 60X objective = 0.016 mm2; minimum area of 0.02 μm2 = 4 pixels). To objectively isolate round, isolated puncta, measurement filters were set to 0-1 holes, to avoid circumferential objects and 0.5-3 roundness, to avoid long strings of unresolved objects. For display, black and white images of the individual stains were colored and combined with the merge function.
For analysis of process length and branching, neurons were plated at 20 cells/mm2, fixed at day 4 for 10 min in 4% paraformaldehyde in PBS, and immunostained for MAP2 and tau. Using Image-Pro, a segmentation threshold for the tau immunostain was set to create a binary mask. A thinning filter was used to create a skeleton. Cells and dendrites were counted.
High Resolution Respirometry
Basal and uncoupled respiration were measured for neurons in situ in Neurobasal/B27 or NbActiv4 medium pre-equilibrated to 37°C, 5% CO2 and 9% O2. Measures of oxygen concentration were acquired over time within the temperature-controlled, continuously stirred, sealed chambers of an Oxygraph-2K (Oroboros, Innsbruck, Austria). DatLab4 software (Oroboros) provided real time measures of oxygen concentration and flux. Although cell suspensions are typically used for measurements in the Oxygraph, we recognized the reliance of neuronal bioenergetics on attachment to a substrate. Therefore, we developed 14.5 mm diameter Teflon star stirbars (Nalgene, Rochester, NY) topped with silicone (World Precision Instruments, Sarasota, FL) discs to accommodate 15 mm glass cover slips (Assistent) on which approximately 100,000 neurons were cultured. Prior to respiration measurements, six fields of cells were photographed with 20x phase optics (Olympus) and counted for normalization purposes. Slips were also photographed following the experiments to confirm the presence of cells. After a basal respiration rate was obtained, 1 μM FCCP (Sigma, C2920) was added from a 100 μM FCCP stock through a 75 mm needle of a 25 μL syringe (Hamilton, Reno, NV).
Statistics
Means and S.E. were calculated using Plot-It software (Scientific Programming Enterprises, Hazlet, MI). Statistical comparisons between groups were analyzed by student's t-test with significance set at 0.05.
Results
Growth and viability
Figure 1 shows the multi-electrode array (MEA) used to record spontaneous electrical activity in the central 1 mm2 of neuronal cultures. Culture medium components were individually varied to obtain maximal spontaneous activity after three weeks in culture, when electrode array measurements were recorded. The combination of these components, NbActiv4, produced cultures that were indistinguishable from cultures in Neurobasal/B27 (Figure 1 C,D). However, at higher magnification and lower plating density, cultures in NbActiv4 produced 30% greater branching of processes (Fig. 1 E, F, G). The means of manual measures of longest axon length were not significantly different. Measures of survival at 160 cells/mm2 were indistinguishable at 8 DIV between Neurobasal/B27 and NbActiv4 (77 ± 2% vs. 78 ± 2%, n.s.). At the more difficult density of 20 cells/mm2, with greater dilution of paracrine trophic factors, survival was also similar (75 ± 3% vs. 74 ± 3%).
Fig. 1.
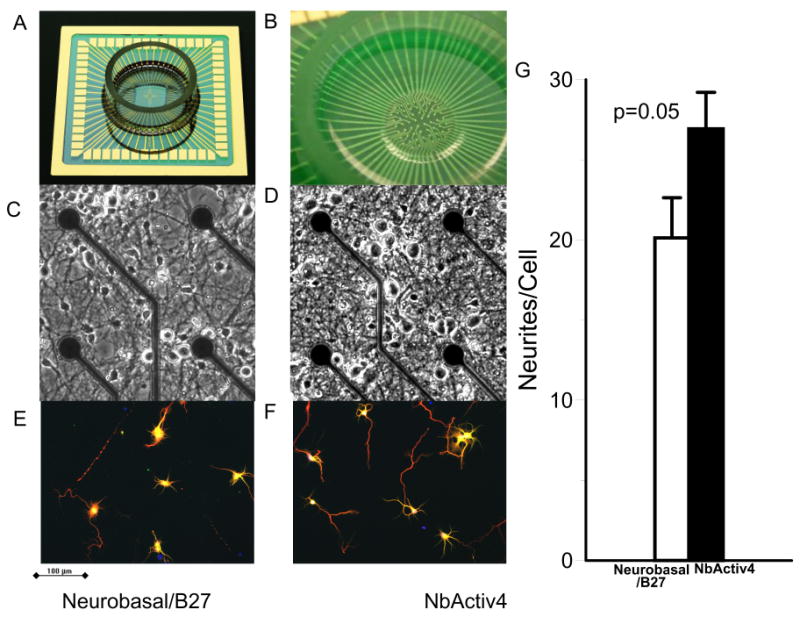
Comparison of neuronal cultures in Neurobasal/B27 to NbActiv4 on both arrays and glass cover slips for differences in electrical activity and immunocytology. (A) 5 cm square MEA with gold leads and attached ring to contain culture medium. (B) 60 electrodes in the central 1 mm2 sense spike activity. (C) and (D) Similar phase contrast images of Neurobasal/B27 and NbActiv4 cultures after three weeks in vitro. Dark circular electrodes are 10 μm diameter electrodes on 200 μm spacing. (E) and (F) Immunofluorescent images (tau-red, MAP2-green, bisbenzamide-blue) to compare neurite branching. (G) Tau neurite branching per cell is higher in NbActiv4 (n=16 fields from 2 cultures).
Measures of electrical activity
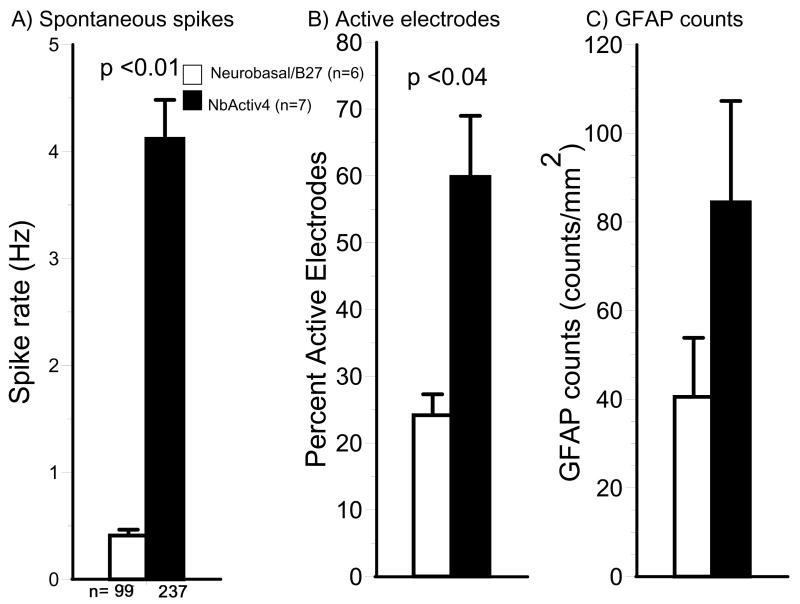
Figure 2 shows traces of electrical activity on three of the active electrodes for cultures in either Neurobasal/B27 or NbActiv 4. In general, neurons in NbActiv4 produced more bursts of electrical activity than in Neurobasal/B27 (12 ± 1 vs. 7 ± 1 bursts/min, p =0.001) with more spikes per burst (18 ± 1 vs. 11 ± 1 spikes/burst, p<0.0001). Activity measures for each added component were optimized for component concentration. The combination of components, NbActiv4, produced higher spike rates than any single component (data not shown). Figure 3A shows spike rates of 0.5 Hz for neurons grown in Neurobasal/B27 compared to 4 Hz for neurons grown in NbActiv4, an eight-fold increase in spike rate. This increase in spike activity also manifested as more than a two-fold increase in percent active electrodes (Fig. 3B). The higher spike rate was also associated with a higher area of astroglial GFAP immunoreactivity (Fig. 3C). But, overall, cultures were not overgrown with astroglia, as occurs with serum-containing media. Other measures of astroglial counts/mm2 of substrate (84 ± 23 vs. 41 ± 13, n=12) and astroglial area/count were not significantly different between NbActiv4 and Neurobasal/B27 (data not shown), indicating 2-4 neurons/astroglia.
Fig. 2.
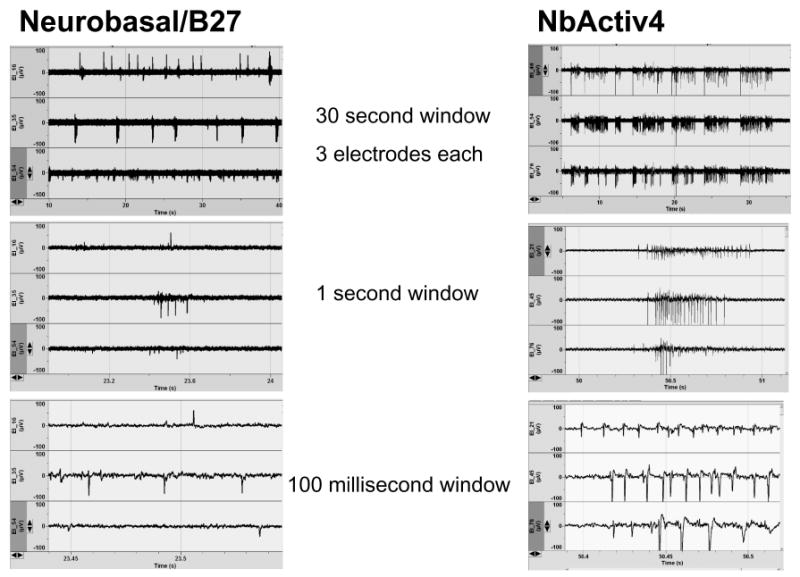
NbActiv4 increases spontaneous spike rates and bursting compared to neurons cultured in Neurobasal/B27. Activity (μV) is compared over the indicated times.
Fig. 3.
NbActiv4 increases electrical activity, the percentage of active electrodes, but not astroglial density compared to cultures in Neurobasal/B27. (A) Mean spontaneous spike rates of electrodes with activity above 0.03 Hz (n=6-7 cultures). (B) Percentage active electrodes with activity above 0.03 Hz (n=6-7 cultures). C) Mean astroglia counts are not significantly different (n = 12 fields of 0.145 mm2 from 2 cultures).
Synaptic basis of higher activity in NbActiv 4
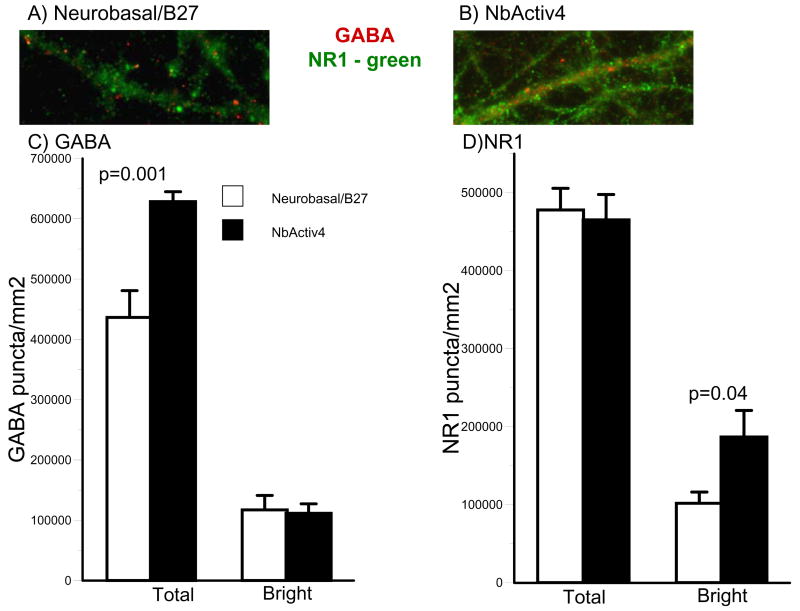
We evaluated the effect of culture medium on synaptic density as puncta of immunoreactivity. Figure 4 shows puncta of bright and dull immunoreactivity for the excitatory NMDA receptor, NR1, and the inhibitory GABAAβ receptor. Puncta were measured at a high threshold for bright densities, suggestive of synaptic receptor clustering (Craig et al., 1994; Elmariah et al., 2004; Boehler et al., 2007), as well as a lower density for less clustered synapses. Clustered receptors admit higher ion fluxes per quantum of transmitter. While total NR1 puncta per square millimeter of culture were not affected by the culture medium, bright puncta were increased nearly two-fold in cultures in NbActiv4 compared to Neurobasal/B27.
Fig. 4.
NbActiv4 increases total GABA puncta 40% and bright NR1 puncta 100% compared to neurons cultured in Neurobasal/B27. (A) and (B) Immunoreactive red GABA and green NR1 synaptic puncta. (C) and (D) Bright and dim segmentation thresholds for GABA and NR1 show effect of medium on stained puncta (n = 16 fields of 0.016 mm2 from 2 cultures).
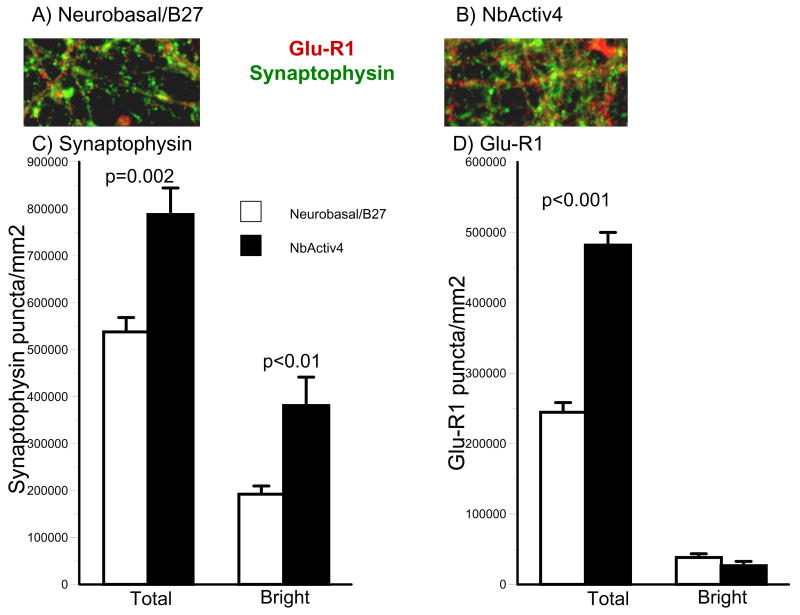
Neuron networks grown in Neurobasal/B27 and NbActiv4 were also immunostained for the presynaptic vesicle marker synaptophysin and the postsynaptic AMPA receptor GluR1 (Figure 5). Both total and bright synaptophysin puncta were higher in cultures in NbActiv4 than in Neurobasal/B27. A similar two-fold increase in total GluR1 puncta was also observed in NbActiv4 over Neurobasal/B27.
Fig. 5.
NbActiv4 increases bright synaptophysin puncta 2-fold and total Glu-R1 puncta 2-fold compared to neurons cultured in Neurobasal/B27. (A) and (B) Immunoreactive red Glu-R1 and green synaptophysin puncta. (C) and (D) Bright and dim segmentation thresholds for synaptophysin and Glu-R1 show effect of medium on puncta (n = 16 fields of 0.016 mm2 from 2 cultures).
Respiratory efficiency and capacity
We investigated whether the extra ingredients in NbActiv4 could lower resting respiration by sensitive measures of oxygen consumption. A lower resting energy demand indicates less energy needed for energy-demanding processes such as the plasma membrane Na, K and Ca ATPases. To ensure that lower demand is not due to respiratory inhibition, the mitochondrial proton gradient can be uncoupled by the protonophore FCCP to stimulate maximum respiration. Figure 6 shows a resting respiration in NbActiv4 less than half that in Neurobasal/B27. This suggests that the maintenance level of energy demand met by oxidative phosphorylation is lower in NbActiv4. By uncoupling mitochondria with FCCP, maximal rates of respiration are similar in the two media. However, the ratio of uncoupled to coupled respiration was 1.7 ± 0.2 in Neurobasal/B27 and 3.5 ± 0.8 in NbActiv4 (p=0.04). This suggests more efficient oxidative phosphorylation in NbActiv4.
Fig. 6.
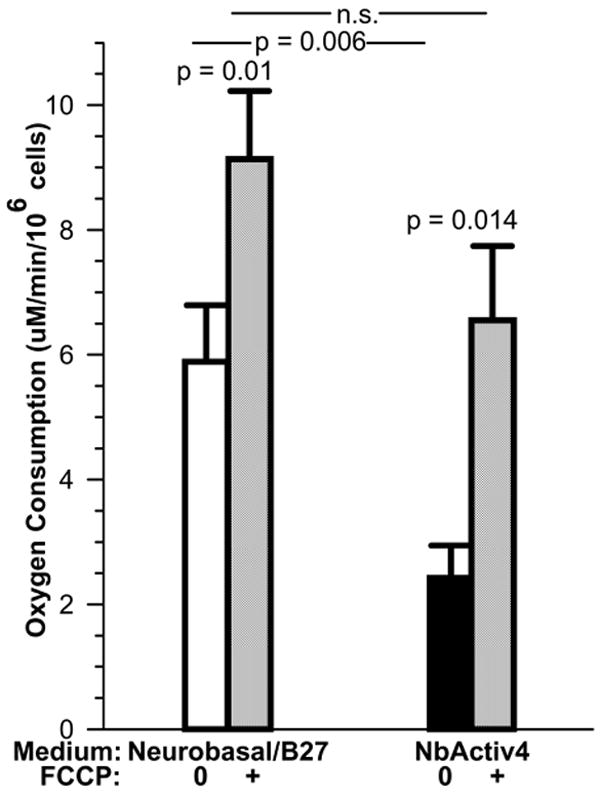
Under resting conditions, neurons consume oxygen at a significantly lower rate in NbActiv4 than in Neurobasal/B27, yet remain capable of higher respiration after uncoupling with FCCP (n=6-7 cultures). This suggests greater mitochondrial efficiency of neurons in NbActiv4.
Discussion
These results show that neuron networks cultured in NbActiv4 are able to sustain up to eight times higher rates of spontaneous spike rates than networks cultured in Neurobasal/B27. Several components of a mechanism for higher spike rates were identified. First, axonal branching was higher in NbActiv4 as judged by immunostaining for tau. Based on the same cell survival, this could allow for higher numbers of synapses per cell. Second, higher synaptic densities were measured as NR1 (NMDA) and GluR1 (AMPA) excitatory puncta. In NbActiv4, the observation of a similar increase in at least one inhibitory transmitter receptor, GABAAβ, suggests synaptic scaling to terminate excitatory signals more rapidly (Turrigiano & Nelson, 2004; Turrigiano et al., 1998; London & Segev, 2001; Buckby et al., 2006). Faster termination of excitatory signals likely enables faster spike rates with shorter refractory periods. One of the NbActiv4 components, estrogen, promotes better handling of calcium signals (Kumar & Foster, 2002; Brewer et al., 2006). Estrogen also limits voltage-gated calcium channel conductances (Kumar & Foster, 2002) and inhibits the mitochondrial permeability transition by increasing Bcl-2 in mitochondria (Brinton et al., 1997; Nilsen & Brinton, 2003; Nilsen et al., 2002).
Thirdly, the increase of synaptophysin immunoreactivity that we see with NbActiv4 may be due to the cholesterol which increases presynaptic release probability by increasing the presynaptic pools of transmitter (Pfrieger & Barres, 1997; Goritz et al., 2005). Fourthly, the increased astroglial area in NbActiv4 could support greater synapse density and higher spike rates by more quickly removing excess transmitter from the synapse (Boehler et al., 2007). Although this increase was 2-fold, many fields had no GFAP-reactive astroglia, nor were cultures overgrown with astroglia. Previously, in Neurobasal/B27 medium, we explicitly added extra astroglia up to 160 cells/mm2, a 1:1 ratio with neurons (Boehler et al., 2007). This also produced higher spike rates, though only double the rate in cultures without added astroglia, much less than the 8-fold increase observed here. For hippocampal neurons on patterned substrates in Neurobasal/B27 medium, we have also seen increased spike rates associated with increased spontaneous generation of astroglia (Nam et al., 2004; 2007). Together with previous observations, the present results suggest that NbActiv4 enables higher spike rates by multiple mechanisms, not solely by promoting the growth of astroglia.
These results show the ease with which neuron electrophysiology can be studied with multielectrode arrays (Thomas et al., 1972; Novak & Wheeler, 1988; Gross, 1979). Multielectrode arrays are efficient at evaluating network activity under different conditions for neurotoxicology and neuropharmacology (Keefer et al., 2001) as well as advancing our understanding of network communication (Chang et al., 2006; Jimbo et al., 1999).
The clustered synaptic NMDA receptors and higher density of GluR1 AMPA receptors observed in networks cultured in NbActiv4 can be expected to transmit a larger calcium signal per bolus of glutamate excitation (Elmariah et al., 2004). The rate at which postsynaptic signaling reaches threshold would certainly enable the higher spike rates observed in NbActiv4 as well as the higher number of spikes per burst.
Besides uses on multielectrode arrays, NbActiv4 medium should be useful in nearly all the applications where Neurobasal/B27 is used. These include sharp electrode and patch clamp neurophysiology, immunocytology, neuron cell signaling, development, excitotoxicity, synaptogenesis, neurotoxicology, neuronal networks, adult rat and mouse neuron culture (Brewer & Torricelli, 2007) and adult human neuron culture (Brewer et al., 2001). Possible limitations might arise for explicit studies of the effects of estrogen, cholesterol or creatine, but these can be controlled by comparisons to formulations of NbActiv4 with these compounds omitted. The increased activity of neurons in NbActiv4 should promote more robust neuron cultures.
Acknowledgments
We thank John Torricelli for assistance in isolating hippocampal neurons and Rose Pearson for data analysis. We are grateful for support from the NIH for AG13435 and NS52233.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests Statement: Authors Boehler, Jones and Wheeler declare no competing interests. Brewer owns BrainBits LLC, which currently manufactures NbActiv4, and receives royalties from Invitrogen through Southern Illinois University.
References
- Boehler M, Wheeler BC, Brewer G. Added astroglia promote greater synapse density and higher activity in neuronal networks. Neuron Glia Biology. 2007 doi: 10.1017/S1740925X07000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–76. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Brewer GJ. Isolation and culture of adult rat hippocampal neurons. J Neurosci Methods. 1997;71:143–55. doi: 10.1016/s0165-0270(96)00136-7. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Wallimann TW. Protective effect of the energy precursor creatine against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. J Neurochem. 2000;74:1968–78. doi: 10.1046/j.1471-4159.2000.0741968.x. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Espinosa J, McIlhaney MP, Pencek TP, Kesslak JP, Cotman C, Viel J, McManus DC. Culture and regeneration of human neurons after brain surgery. J Neurosci Methods. 2001;107:15–23. doi: 10.1016/s0165-0270(01)00342-9. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Reichensperger JD, Brinton RD. Prevention of age-related dysregulation of calcium dynamics by estrogen in neurons. Neurobiol Aging. 2006;27:306–17. doi: 10.1016/j.neurobiolaging.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Tran J, Proffitt P, Montoya M. 17 b-estradiol enhances the outgrowth and survival of neocortical neurons in culture. Neurochem Res. 1997;22:1339–51. doi: 10.1023/a:1022015005508. [DOI] [PubMed] [Google Scholar]
- Buckby LE, Jensen TP, Smith PJ, Empson RM. Network stability through homeostatic scaling of excitatory and inhibitory synapses following inactivity in CA3 of rat organotypic hippocampal slice cultures. Mol Cell Neurosci. 2006;31:805–16. doi: 10.1016/j.mcn.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Chang JC, Brewer GJ, Wheeler BC. Neuronal network structuring induces greater neuronal activity through enhanced astroglial development. J Neural Eng. 2006;3:217–26. doi: 10.1088/1741-2560/3/3/004. [DOI] [PubMed] [Google Scholar]
- Craig AM, Blackstone CD, Huganir RL, Banker G. Selective clustering of glutamate and gamma-aminobutyric acid receptors opposite terminals releasing the corresponding neurotransmitters. Proc Natl Acad Sci U S A. 1994;91:12373–7. doi: 10.1073/pnas.91.26.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducray AD, Qualls R, Schlattner U, Andres RH, Dreher E, Seiler RW, Wallimann T, Widmer HR. Creatine promotes the GABAergic phenotype in human fetal spinal cord cultures. Brain Res. 2007;1137:50–7. doi: 10.1016/j.brainres.2006.12.038. [DOI] [PubMed] [Google Scholar]
- Elmariah SB, Crumling MA, Parsons TD, Balice-Gordon RJ. Postsynaptic TrkB-mediated signaling modulates excitatory and inhibitory neurotransmitter receptor clustering at hippocampal synapses. J Neurosci. 2004;24:2380–93. doi: 10.1523/JNEUROSCI.4112-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goritz C, Mauch DH, Pfrieger FW. Multiple mechanisms mediate cholesterol-induced synaptogenesis in a CNS neuron. Mol Cell Neurosci. 2005;29:190–201. doi: 10.1016/j.mcn.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Gross GW. Simultaneous single unit recording in vitro with a photoetched laser deinsulated gold multimicroelectrode surface. IEEE Trans Biomed Eng. 1979;26:273–9. doi: 10.1109/tbme.1979.326402. [DOI] [PubMed] [Google Scholar]
- Jimbo Y, Tateno T, Robinson HP. Simultaneous induction of pathway-specific potentiation and depression in networks of cortical neurons. Biophys J. 1999;76:670–8. doi: 10.1016/S0006-3495(99)77234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefer EW, Norton SJ, Boyle NA, Talesa V, Gross GW. Acute toxicity screening of novel AChE inhibitors using neuronal networks on microelectrode arrays. Neurotoxicology. 2001;22:3–12. doi: 10.1016/s0161-813x(00)00014-0. [DOI] [PubMed] [Google Scholar]
- Kumar A, Foster TC. 17beta-estradiol benzoate decreases the AHP amplitude in CA1 pyramidal neurons. J Neurophysiol. 2002;88:621–6. doi: 10.1152/jn.2002.88.2.621. [DOI] [PubMed] [Google Scholar]
- London M, Segev I. Synaptic scaling in vitro and in vivo. Nat Neurosci. 2001;4:853–4. doi: 10.1038/nn0901-853. [DOI] [PubMed] [Google Scholar]
- Nam Y, Brewer GJ, Wheeler BC. Development of astroglial cells in patterned neuronal cultures. J Biomater Sci Polym Ed. 2007;18:1091–1100. doi: 10.1163/156856207781494430. [DOI] [PubMed] [Google Scholar]
- Nam Y, Chang J, Khatami D, Brewer GJ, Wheeler BC. Patterning to enhance activity of cultured neuronal networks. IEE Proc Nanobiotechnol. 2004;151:109–115. doi: 10.1049/ip-nbt:20040706. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Chen S, Brinton RD. Dual action of estrogen on glutamate-induced calcium signaling: mechanisms requiring interaction between estrogen receptors and src/mitogen activated protein kinase pathway. Brain Research. 2002;930:216–34. doi: 10.1016/s0006-8993(02)02254-0. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Mechanism of estrogen-mediated neuroprotection: Regulation of mitochondrial calcium and Bcl-2 expression. Proc Natl Acad Sci U S A. 2003;100:2842–7. doi: 10.1073/pnas.0438041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak JL, Wheeler BC. Multisite hippocampal slice recording and stimulation using a 32 element microelectrode array. J Neurosci Methods. 1988;23:149–59. doi: 10.1016/0165-0270(88)90187-2. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW, Barres BA. Synaptic efficacy enhanced by glial cells in vitro. Science. 1997;277:1684–7. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- Thomas CA, Jr, Springer PA, Loeb GE, Berwald-Netter Y, Okun LM. A miniature microelectrode array to monitor the bioelectric activity of cultured cells. Exp Cell Res. 1972;74:61–6. doi: 10.1016/0014-4827(72)90481-8. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–6. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]