Abstract
Redox-sensitive non-viral delivery systems exploit intracellular reducing environment to improve the efficacy of the delivery of nucleic acids by selectively releasing the cargo in the subcellular space. Bcl-2 overexpression is frequently observed in human cancers and is closely associated with increased resistance to chemotherapy and radiotherapy. One of the biochemical alterations accompanying Bcl-2 overexpression is the increase in cellular glutathione (GSH) levels. In this study, we hypothesize that such increase of GSH concentrations will selectively enhance the transfection activity of redox-sensitive delivery systems in cells overexpressing Bcl-2. Transfection studies were conducted in MCF-7 mammary carcinoma cells and MCF-7 clones overexpressing Bcl-2. It was confirmed that Bcl-2 overexpression resulted in the expected increase in GSH concentrations. Redox-sensitive complexes containing plasmid DNA, mRNA, antisense oligodeoxynucleotides, and siRNA exhibited selectively increased activity in cells overexpressing Bcl-2 compared to non-redox complexes. The effect of Bcl-2 overexpression on the selective enhancement of transfection was highly dependent on the type of the delivered nucleic acid, and was most pronounced for mRNA. This study shows that Bcl-2 overexpression can serve as a proxy redox stimulus to enhance the activity of all major classes of potential nucleic acid therapeutics, when delivered using redox-sensitive vectors.
Keywords: non-viral gene delivery, polyplexes, transfection, Bcl-2 overexpression, glutathione
Introduction
Polyelectrolyte complexes of nucleic acids with polycations (polyplexes) are investigated as promising delivery vectors for a variety of nucleic acid therapeutics [1, 2]. In particular, polyplexes capable of responding to environmental changes or stimuli by altering their properties and behavior seem to promise a significant improvement of the efficacy of the delivery process [3]. One of the several stimuli, which has been utilized for improving the efficiency of nucleic acid delivery, is the redox potential gradient existing between extracellular and intracellular environments [4-10]. The existence of such redox potential gradient has been exploited by incorporating disulfide bonds into the structure of the delivery vectors to provide them with the capability to release the therapeutic nucleic acids selectively in the subcellular reducing space [11-15].
The intracellular reduction of disulfide bonds in polyplexes is most likely mediated by thiol/disulfide exchange reactions with small redox molecules like glutathione (GSH) and thioredoxin; either alone or with the help of redox enzymes. The reduction results in enhanced rates of disassembly of the polycation-nucleic acid complexes, which is believed to increase the biological activity of the nucleic acids [16-18]. Disassembly of the polyplexes also leads to generation of fragments of the reducible polycations with lower molecular weight and thus lower cytotoxicity [14, 19-21]. It is reasonable to hypothesize that changes in intracellular GSH concentrations will alter activity of redox polyplexes. Indeed, available evidence suggests that artificially changing cellular GSH levels leads to changes in biological activity of polyplexes [6, 12, 22, 23]. Modulation of GSH levels using either buthionine sulfoximine (BSO), an inhibitor of cytosolic GSH synthesis or enhancement of GSH levels using GSH monoethyl ester (GSH-Et) demonstrated a small, but significant effect on the transfection activity of plasmid DNA (pDNA) complexes.
Bcl-2 is an anti-apoptotic protein that is overexpressed in multiple human cancers with often serious implications for the disease outcome [24, 25]. Overexpression of Bcl-2 has been shown to contribute to enhanced resistance to chemotherapy [26] and radiotherapy [27]. It is also correlated with increased levels of intracellular GSH [28-35]. It has been demonstrated that the increase in GSH levels resulting from overexpression of Bcl-2 is also accompanied by partial redistribution of GSH to the nucleus [36, 37]. The increased concentration and changes in subcellular distribution of GSH in cancer cells overexpressing Bcl-2 provide an exciting opportunity for redox-sensitive delivery platforms to selectively enhance the efficiency of cancer gene therapy.
The existing evidence about the important role of GSH levels on the activity of redox-sensitive gene delivery systems motivated us to explore overexpression of Bcl-2 as a proxy redox stimulus. We hypothesized that the increase in intracellular GSH levels due to overexpression of Bcl-2 will result in enhanced selectivity of transfection in tumors overexpressing Bcl-2 and the partial redistribution of GSH into the nucleus will further enhance the activity of pDNA complexes. To test the hypothesis, we created several stable clones of the MCF-7 cells transfected with Bcl-2 [33]. We expected that the effect of the changes in the concentration and subcellular distribution of GSH associated with the overexpression of Bcl-2 will be dependent on both the size and the subcellular site of action of the nucleic acids used. Thus, we investigated transfection activity of high molecular weight (pDNA, mRNA) and low molecular weight nucleic acids (antisense oligodeoxynucleotides (AS ODN), siRNA); which are also nuclearly (pDNA, AS ODN) and cytosolically active nucleic acids (mRNA, siRNA). Our study indicates that increase in cellular GSH levels mediated by overexpression of Bcl-2 can advantageously improve the transfection activity of redox complexes. The magnitude of the enhancement was highly dependent on the subcellular site of disassembly of complexes. For the first time, we have successfully demonstrated the possibility to utilize naturally occurring redox changes in cancer cells overexpressing Bcl-2 to improve the efficacy of nucleic acid delivery using redox-responsive vectors.
Materials and methods
Materials
Poly-L-lysine hydrobromide (MW 15,000-30,000) and DOTAP chloride (N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethylammonium chloride) were purchased from Sigma (St. Louis, MO). CK10C peptide (CKKKKKKKKKKC, Mr 1506) was custom-synthesized by Biopeptide Co. (San Diego, CA) and a previously published method was followed to synthesize rPLL with molecular weight ∼30,000 [38]. PLL obtained from Sigma was further purified using Vivaspin concentrators (Sartorius, Edgewood, NY) with 10,000 MW cut-off and a previously published method was used to confirm the molecular weight using size exclusion chromatography (SEC) [19]. Human mammary carcinoma cell line MCF-7 (HTB-22) was obtained from ATCC (Manassas, VA). Mouse monoclonal anti-Bcl-2 (Ab-1) antibody was obtained from Calbiochem (San Diego, CA). CellTracker™ Green CMFDA (5-chloromethylfluorescein diacetate), Hoechst 33342, YOYO®-1 iodide, Roswell Park Memorial Institute (RPMI) 1640 medium and fetal bovine serum (FBS) were obtained from Invitrogen (Carlsbad, CA). Packed Cell Volume (PCV) tubes were obtained from TPP, Midsci (St. Louis, MO). Plasmid DNA vectors, gWiz high-expression luciferase (gWiz-Luc) containing luciferase reporter gene, and gWiz high-expression GFP (gWiz-GFP) containing enhanced green fluorescent protein reporter gene were from Aldevron (Fargo, ND). A previously published method was followed to synthesize mRNA encoding for luciferase and eGFP [16]. Firefly luciferase (GL3) and control siRNA were obtained from Ambion (Austin, TX). Luciferase AS ODN (5′-AACCGCTTCCCCGACTTCC-3′) and negative control AS ODN (5′-CCAATGTCAAGCACTTCCGTT-3′) with phosphorothioate linkages were custom synthesized by Midland Certified Reagent Company (Midland, TX). All other chemicals were obtained from Fisher Scientific unless otherwise noted.
Preparation of MCF-7 clones
pSFFV-Neo and pSFFV-Bcl-2 plasmids were a kind gift from Prof. Clark W. Distelhorst (Case Western Reserve University). Briefly, the plasmids were amplified in DH5α E.coli and purified using Qiagen EndoFree Plasmid Purification system (Valencia, CA). MCF-7 cells were cultured in RPMI 1640 medium supplemented with 10% FBS at 37°C in 5% CO2 atmosphere. The cells were stably transfected with the plasmids by electroporation and cultured in complete growth medium containing 0.5 mg geneticin/mL. Individual clones from single cells were selected by limiting dilution and Bcl-2 expression was confirmed by western blot.
Western Blot
Protein extracts were prepared in 125 mM Tris-HCl (pH 6.8), denatured using 4% SDS and protein content was quantitated using BCA™ Protein Assay Reagent Kit (PIERCE, Rockford, IL). Extracts were subjected to polyacrylamide gel electrophoresis (PAGE) and electroblotted to nitrocellulose. Blots were probed with mouse monoclonal anti-Bcl-2 antibody (Ab-1) and developed with Millipore* Immobilon* Western Chemiluminescent HRP Substrate. Chemiluminescence was detected by Luminescence Image Analyzer, Fuji LAS-1000 Plus, and bands were analyzed by the Science Lab Image Gauge software (Fuji Photo Film).
GSH Analysis
Cells were harvested either in log or stationary growth phase and cell pellets were prepared in PBS (4×106 cells/mL). A previously described method was used to analyze the samples on reverse-phase ion-exchange Waters HPLC system [39]. Cell volume was determined using PCV tubes using the protocol described by the manufacturer (TPP, Midsci). Protein content in the cell pellets was measured using the BCA™ Protein Assay Reagent kit. Where indicated, GSH content in cells was also determined using an enzymatic recycling method. Briefly, cells were harvested, deproteinated using 5% 5-sulfosalicylic acid and GSH concentration was determined by following the formation of yellow-colored 5-thio-2-nitrobenzoic acid (TNB) produced as a result of the reaction between GSH and DTNB (5,5′-dithiobis-2-nitrobenzoic acid, Ellman's reagent). The assay mixture consisted of potassium phosphate buffer (95 mM, pH 7.0), EDTA (0.95 mM), NADPH (0.038 mg/mL), DTNB (0.031 mg/mL), glutathione reductase (0.115 units/mL), and 5-sulfosalicylic acid containing GSH extracted from cells (0.45%). Absorbance of TNB was measured at 405 nm on a νmax kinetic microplate reader (Molecular Devices, Sunnyvale, CA) equipped with SOFTmax PRO 4.0 software. A calibration curve was constructed using various known concentrations of reduced GSH to determine the unknown concentration of GSH in the samples. GSH content was normalized to cell volume, determined earlier. The experiment was conducted three times on different occasions.
γ-glutamyl-cysteine-synthetase (γGCS) assay
Enzyme activity was determined either during log or stationary phase of cell growth by following a previously described method [40]. Briefly, the assay mixture consisted of Tris.HCl buffer (100 mM, pH 8.0), sodium-L-glutamate (10 mM), L-α-aminobutyrate (10 mM), MgCl2 (20 mM), Na2ATP (5 mM), sodium phosphoenol pyruvate (2 mM), KCl (150 mM), NADH (0.2 mM), pyruvate kinase (5 units), lactate dehydrogenase (10 units) and the cell lysate. The decrease in absorbance over a minute was followed spectrophotometrically at 340 nm and 37°C. One unit of enzyme activity was defined as the amount that resulted in oxidation of 1 μmol of NADH per minute at 37°C. Specific activity was expressed in terms of units (U)/mg cellular protein. The experiment was conducted three times on different occasions.
Population doubling time
Growth curves were constructed for each of the clones. Briefly, cells were seeded in 24 well plates at a density of 60,000 cells/well. At the indicated time point, cells were washed once with warm PBS, trypsinized and resuspended in complete growth medium prior to counting using a hemacytometer. The total number of cells were determined every 12 h. Growth curves were constructed depicting total number of cells vs. time since seeding. The doubling period was calculated from a linear portion of the log phase. Triplicate wells were counted at each time point.
Subcellular distribution of GSH
Subcellular distribution of GSH was investigated by fluorescence microscopy. Cells in log phase were stained with 7.5 μM CMFDA to label intracellular GSH and 10 μM Hoechst 33342 to label the nucleus. A published method [41] was used to stain the cells and analyze the images. The cells were visualized under a Nikon TE2000-U microscope equipped with a heated stage and the images were acquired at 37°C using 30× magnification. The excitation/emission wavelengths used were 465–495/515–555 nm (CMFDA) and 340–380/435–485 nm (Hoechst 33342). MetaVue software (Molecular Devices, Sunnyvale, CA) was used to analyze the images. The average fluorescence intensity of the nuclear and cytoplasmic GSH staining was quantitated by creating distinct regions encompassing the nucleus and entire intracellular space excluding the nucleus (cytoplasm). Results are expressed as a ratio of average fluorescence intensity in the nuclear to the cytoplasmic regions. At least 100 cells were counted per data point.
Transfection experiments
All transfections were conducted in 48 well plates with cells seeded at a density of 60,000 cells/well (pDNA, AS ODN and siRNA) and 100,000 cells/well (mRNA) 24 h before transfection. A previously published protocol was followed to conduct all the transfections [6, 16]. For AS ODN and siRNA transfections, the cells were co-transfected with complexes containing 0.5 μg pDNA and the indicated amounts of AS ODN or siRNA. Complexes were diluted either in medium lacking FBS (-FBS) or medium supplemented with 10% FBS (+FBS). All transfection experiments were repeated at least twice on different occasions. Raw transfection activity of pDNA and mRNA complexes was expressed as RLU/mg cellular protein ± SD of triplicate samples. Transfection activity of AS ODN and siRNA complexes were expressed as % luciferase knockdown, relative to control pDNA complexes. Effect of Bcl-2 levels on transfection activity was expressed as relative transfection activity, defined as the ratio of levels of gene expression mediated by redox (rPLL-D) complexes to that mediated by control, non-redox (PLL-D) complexes. Standard deviation (SD), denoted as error bars were calculated for the ratios. In GSH depletion experiments, cells were incubated with 1 mM diethylmaleate (DEM) for 1 h in medium lacking serum, washed once with warm PBS prior to adding complexes and transfection was carried out as described earlier.
Polyplex stability in serum
Stability of polyplexes in serum was determined by measuring the fluorescence of pDNA non-covalently labeled with YOYO-1 (Molecular Probes, Invitrogen, Carlsbad, CA) following previously published method [42]. Briefly, complexes containing 1 μg of labeled pDNA were prepared as described earlier and incubated with the indicated concentrations of FBS in phenol red-free growth medium (RPMI). The samples were incubated at 37°C for 4 h, YOYO-1 was excited at 485 nm and the emitted fluorescence was detected using a 538 nm filter on a SpectraMax M5 plate reader (Molecular Devices). Fluorescence recovery was normalized to fluorescence of YOYO-1/pDNA measured in the presence of the same FBS concentration. Results are expressed as mean ± SD of triplicate samples. The stability of polyplexes in serum was also studied using agarose gel electrophoresis assay. Complexes prepared using unlabeled pDNA was subjected to treatment described above and samples containing 0.2 μg pDNA were run on an agarose gel as previously described [8].
Statistical analysis
Significant differences between two groups were determined using Student's t-test. A P-value < 0.05 was considered statistically significant in all cases.
Results
Preparation and characterization of MCF-7 clones overexpressing Bcl-2
MCF-7 cells were transfected with pSFFV-Bcl-2 plasmid containing the human Bcl-2 gene and the levels of Bcl-2 in stable clones were determined by western blot (Fig. 1a). Clones with two different Bcl-2 levels (Bcl-2_1, Bcl-2_2) were selected for further studies and cells transfected with pSFFV vector (Neo clone) were used as a control (Table 1).
Figure 1. Characterization of MCF-7 clones.
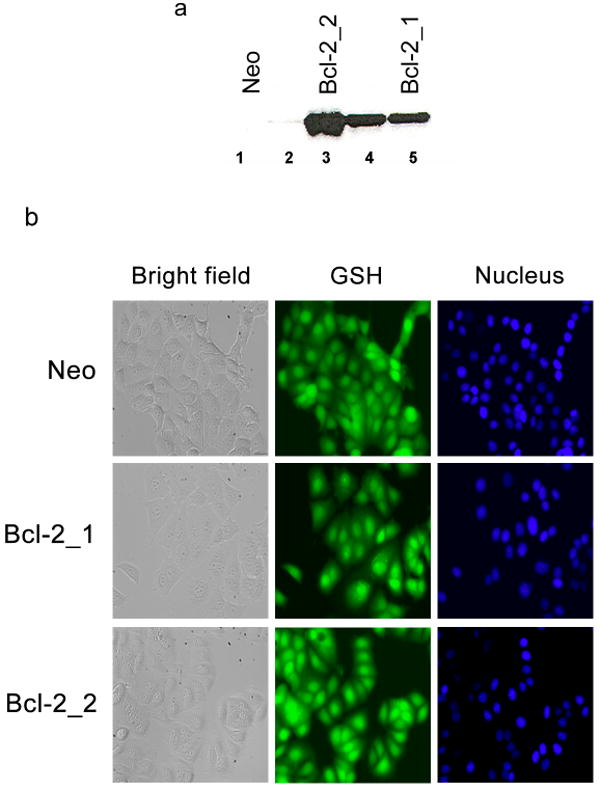
(a) Western blot of MCF-7 clones. Lane 1 and 2, Bcl-2 expression levels in control clones transfected with empty Neo plasmid; lanes 3–5, Bcl-2 expression levels in clones transfected with a human Bcl-2 plasmid. (b) Subcellular distribution of GSH in MCF-7 clones. Cellular GSH stained with CMFDA and nuclei with Hoechst 33342. Size bar = 25 μm.
Table 1. Characterization of MCF-7 clones.
| Bcl-2 content | GSH concentration | γGCS | GSH distribution | Doubling time | |||||
|---|---|---|---|---|---|---|---|---|---|
| Clone | (a.u.)a | (nmol/mg)c | (mM)d | (U/mg protein) | nuclear/cytoplasmice | (h) | |||
| log | stationary | log | stationary | log | stationary | ||||
| Neo | NDb | 52 ± 5 | 57 ± 18 | 2.2 ± 0.1 | 2.9 ± 0.6 | 6.3 ± 1.7 | 17.3 ± 2.9 | 1.6 ± 0.1 | 33 |
| Bcl-2_1 | 2435 | 64 ± 23 | 87 ± 20 | 2.9 ± 0.3 | 4.0 ± 0.2 | 12.2 ± 2.0 | 9.4 ± 4.3 | 1.9 ± 0.2 | 38 |
| Bcl-2_2 | 4864 | 104 ± 38 | 90 ± 55 | 3.7 ± 0.3 | 3.3 ± 0.8 | 32.6 ± 2.1 | 22.9 ± 2.0 | 2.1 ± 0.3 | 47 |
Values indicate mean ± SD
arbitrary units (a.u.) determined from western blot
not detectable
total cellular concentration of GSH in nmol/mg cellular protein
total cellular concentration of GSH in mM
from average fluorescence intensities in nuclear and cytoplasmic compartments of cells stained for GSH
GSH content in the clones was determined and expressed as nmol/mg cellular protein and mM (Table 1). The Bcl-2 clones showed 1.5- to 2-fold increase in cellular GSH levels, compared to the Neo clone. To determine subcellular distribution of GSH in the Bcl-2 clones and control Neo cells, the total cellular GSH pool was fluorescently labeled with CMFDA (Fig. 1b). A previously described method [41] was used to determine the ratio of GSH in the nucleus and cytoplasm (Nuc:Cyt). An increase was observed in the Nuc:Cyt ratios ranging from 1.6 to 2.1, with increasing amounts of Bcl-2. The increase in ratios in the Bcl-2 clones was statistically significant when compared to the Neo clone (P < 0.0001).
γ-Glutamyl-cysteine synthetase (γGCS) is the rate limiting enzyme in GSH biosynthesis. To understand the cause of the elevated GSH levels in the Bcl-2 clones, the γGCS activity was measured. In the log phase, statistically significant 2- and 5-fold increases in the γGCS activity were observed in the Bcl-2 clones (P = 0.02 and P < 0.0001), compared to the Neo clone. Moreover, a 3-fold increase in the γGCS activity was observed in the Bcl-2_2 clone, compared to the Bcl-2_1 clone (P = 0.0003). In the stationary phase, a statistically significant 1.3-fold increase in γGCS activity was observed for the Bcl-2_2 clone, compared to the Neo clone (P = 0.05). The γGCS activity was mostly lower in the stationary phase than in the log phase. Because of the known effect of the growth rate of cells on the transfection activity of pDNA complexes, doubling period of the clones was also determined. Increased doubling time was observed with increasing levels of Bcl-2.
Effect of overexpression of Bcl-2 on transfection activity of pDNA and mRNA
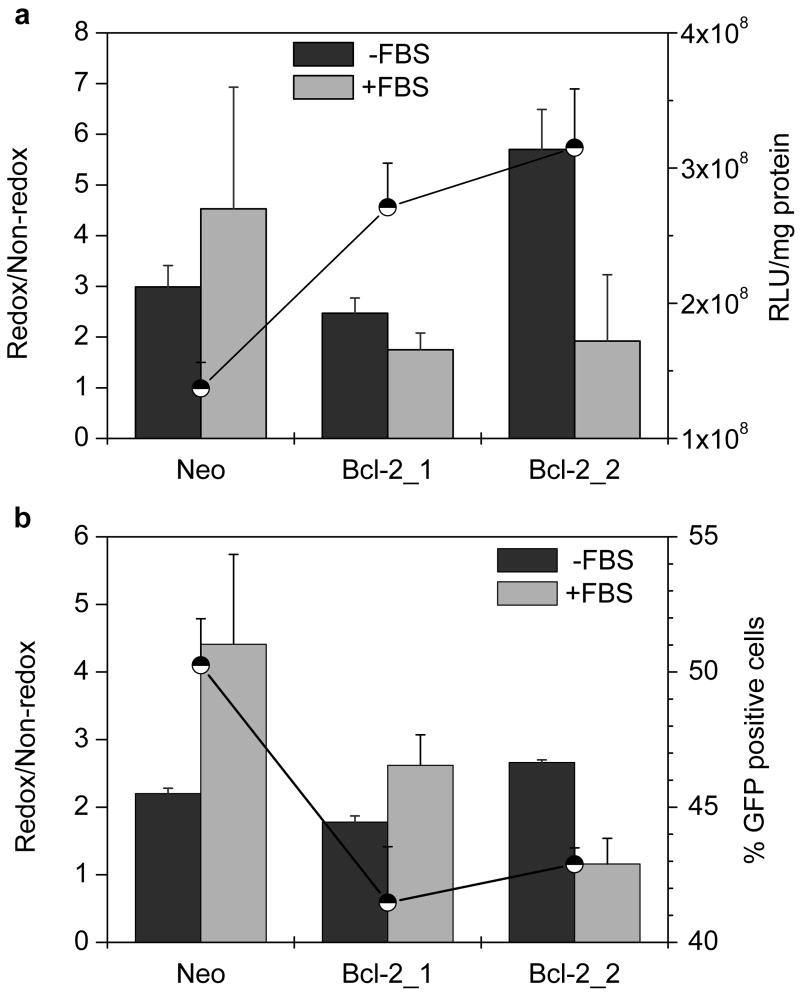
The effect of increased cellular GSH levels in the Bcl-2 clones was investigated using pDNA and mRNA complexes based on reducible PLL (rPLL) and control PLL complexes. To compensate for the differences in transfection activity related to factors other than changes in GSH, all the results were expressed as relative transfection activity. The relative transfections were calculated as a ratio of the transfection mediated by the redox complexes vs. that mediated by the non-redox controls (redox/non-redox). The physicochemical properties of rPLL and PLL complexes used were similar. The transfection activity of redox complexes was always equal or higher than that of the control, non-redox complexes (i.e., relative transfection ≥ 1). The relative transfection of luciferase pDNA complexes significantly increased from 3 to 5.7 (P = 0.006) between Neo and Bcl-2_2 clone, when the transfection was conducted in the absence of serum (Fig. 2a). To provide complete information, secondary axis in Fig. 2a also shows absolute luciferase expression of redox complexes. Statistically significant, 2- and 2.3-fold increase in the luciferase expression was observed for the Bcl-2 clones compared to the Neo clone (P = 0.004 and 0.003, respectively). In the presence of serum, relative transfection decreased from 4.5 to 1.9 with increasing Bcl-2 levels. The decrease in the relative transfection was accompanied by a 3.1-fold decrease in luciferase expression in Bcl-2_2, compared to the Neo clone.
Figure 2. Effect of Bcl-2 levels on transfection activity of pDNA complexes.
(a) Luciferase expression. Cells were transfected for 4 h with 0.5 μg pDNA complexed with PLL or rPLL (N:P 2) to which DOTAP was added to form ternary complexes. Luciferase expression was measured after 24 h as mean RLU/mg cellular protein (n=3). Redox/Non-redox is the ratio (± SD) of transfection activity of rPLL-to-PLL complexes. Secondary axis (●) shows transfection in RLU/mg cellular protein mediated by rPLL complexes in the absence of serum. (b) eGFP expression. Cells were transfected with eGFP pDNA as described above and the gene expression was measured 48 h after transfection as mean % GFP positive cells (n=3). Redox/Non-redox is the ratio (± SD) of transfection activity of rPLL-to-PLL complexes. Secondary axis (●) shows % of GFP positive cells mediated by rPLL complexes in the absence of serum.
Cells were transfected with eGFP pDNA under the above conditions and the data obtained corresponded well to the luciferase data. A small, but significant increase in relative transfection from 2.2 to 2.7 was observed between Neo and Bcl-2_2 in the absence of serum (P = 0.0007). As earlier, a decrease in relative transfection from 4.4 to 1.2 was observed when transfection was conducted in the presence of serum (Fig. 2b). Despite of the decrease in the % of GFP-positive cells for redox complexes in Bcl-2_2, the relative transfection was still enhanced compared to the Neo clone.
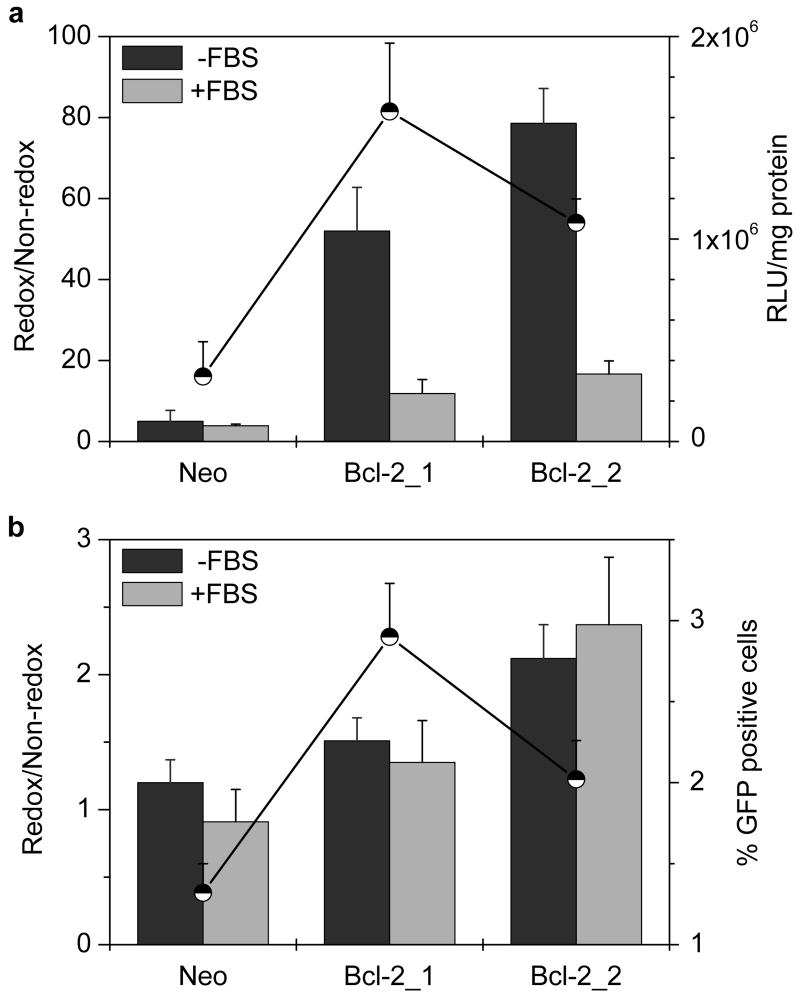
The effect of Bcl-2 overexpression on transfection of mRNA complexes was investigated. In luciferase mRNA transfection, the relative transfection significantly increased from 5 to 79 with increasing Bcl-2 levels in the absence of serum (P = 0.0001) and from 4 to 17 in the presence of serum (P = 0.003) (Fig. 5a). Statistically significant, 5.1- and 3.4-fold increases in the absolute transfection was observed in Bcl-2 clones compared to the Neo clone (P = 0.003). In eGFP mRNA transfection, a significant increase in the relative transfection from 1.2 to 2.1 was observed in the absence of serum (P = 0.006) and 0.9 to 2.4 in the presence of serum (P = 0.01). Similar to the increases observed with luciferase mRNA, 2.2- and 1.5-fold increase in the transfection frequency were observed (P = 0.002 and 0.02, respectively) (Fig. 5b).
Figure 5. Effect of Bcl-2 levels on transfection activity of mRNA complexes.
(a) Luciferase expression. Cells were transfected for 4 h with 1 μg Luc-mRNA complexed with PLL or rPLL (N:P 2) to which DOTAP was added to form ternary complexes. Luciferase expression was measured after 6 h as mean RLU/mg cellular protein (n=3). Redox/Non-redox is the ratio (± SD) of transfection activity of rPLL-to-PLL complexes. Secondary axis (●) shows transfection in RLU/mg cellular protein mediated by rPLL complexes in the absence of serum. (b) eGFP expression. Cells were transfected with eGFP mRNA as described above and the gene expression was measured 24 h after transfection as mean % GFP positive cells (n=3). Redox/Non-redox is the ratio (± SD) of transfection activity of rPLL-to-PLL complexes. Secondary axis (●) shows % of GFP positive cells mediated by rPLL complexes in the absence of serum.
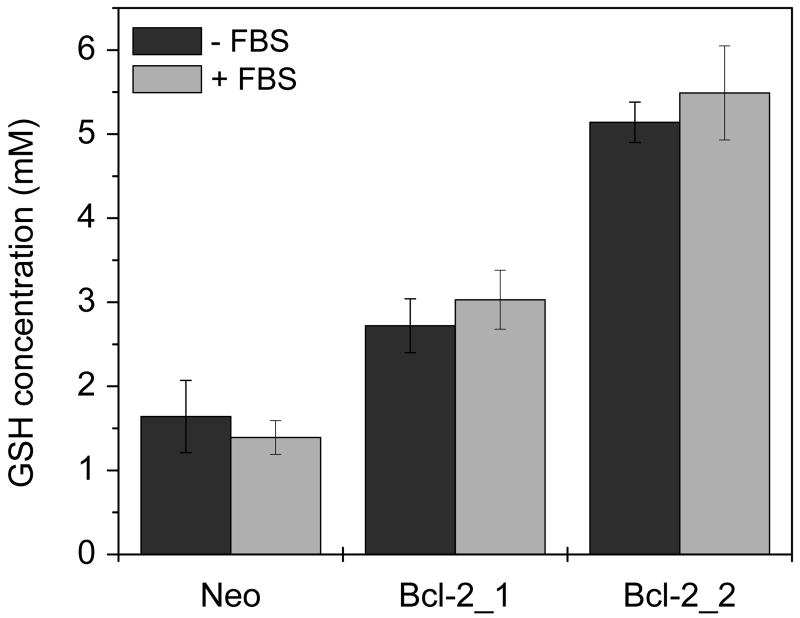
Understanding the effect of serum on activity of pDNA complexes
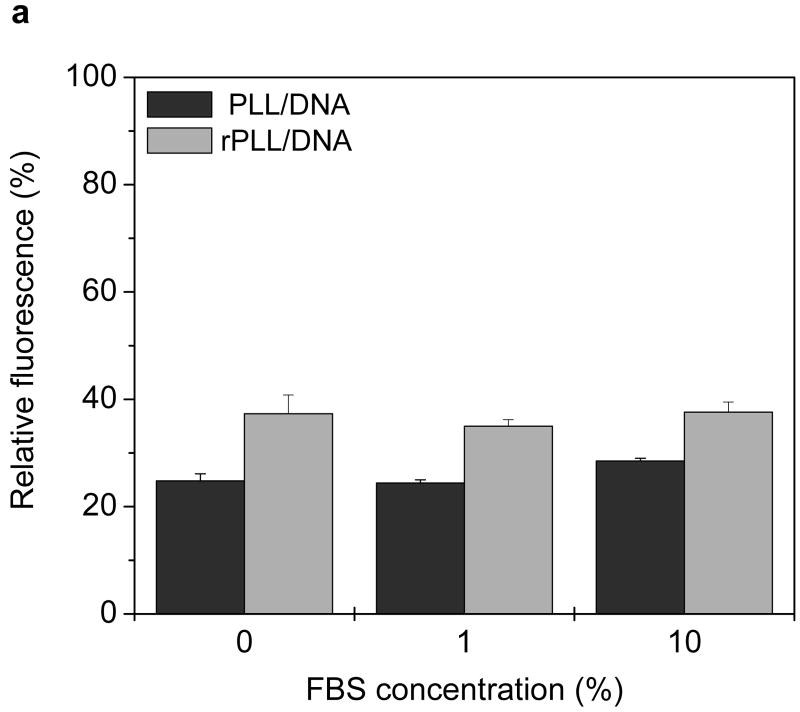
Cells were incubated either in the presence or absence of 10% FBS for 4 h to study possible effects of serum starvation on cellular GSH levels. The GSH levels were similar irrespective of the presence or absence of serum (Fig. 3). The possibility of premature release of pDNA from redox complexes as a result of thiol-disulfide reactions mediated by free thiols was studied then. pDNA was labeled by YOYO-1 and complexed with PLL or rPLL. The complexes were incubated with increasing concentrations of serum for 4 h at 37°C and fluorescence intensities were measured and normalized to free pDNA (Fig. 4a). Similar fluorescence was observed for rPLL and PLL complexes in both the presence and absence of serum, suggesting that no significant reduction of disulfides and no significant pDNA release from the redox complexes occurred. These findings were further validated by agarose gel electrophoresis (Fig. 4b). No free pDNA was detected when PLL and rPLL complexes were incubated with 10% FBS either in RPMI or water. The presence of serum, however, increased the fluorescence in the starting wells, probably indicating loosening of the complexes by serum. The faint smear observed in lanes 7, 9, 11 and 13 is due to the presence of the serum (lane 1) and is not indicative of free pDNA.
Figure 3. Effect of serum deprivation on GSH levels.
Cells were incubated 4 h either in the absence or presence of 10% FBS. Results are expressed as mean ± SD of triplicate samples. Data is representative of three independent experiments.
Figure 4. Effect of serum on the stability of pDNA complexes.
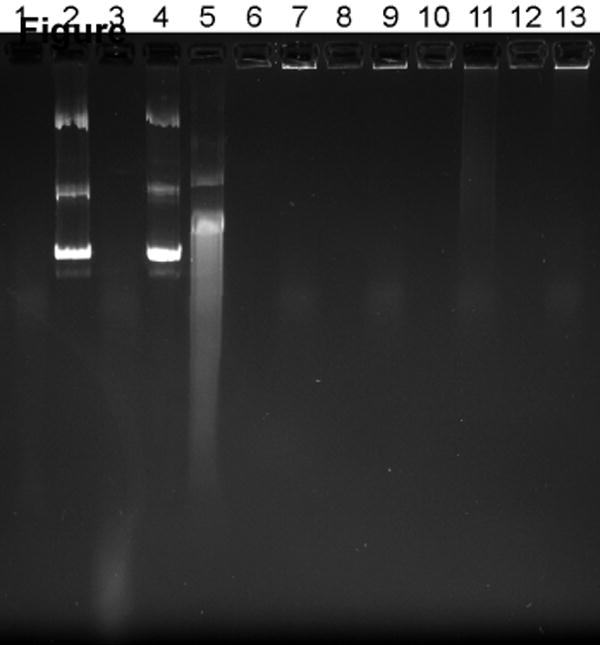
(a) Fluorescence recovery assay. Twenty μL of complexes containing YOYO-1 labeled pDNA were incubated with 80 μL of RPMI containing 0, 1, and 10% FBS for 4 h at 37°C. Fluorescence of YOYO-1 labeled pDNA was measured and normalized to fluorescence intensity of free pDNA. Results are expressed as mean ± SD of triplicate samples. (b) Agarose gel electrophoresis assay. Complexes were prepared using non-labeled pDNA and subjected to treatment as in 4a. Samples containing 0.2 μg pDNA were run on a 0.8% agarose gel and pDNA was stained with ethidium bromide and visualized under UV illumination. Lane 1, RPMI + 10% FBS; lane 2, pDNA + deionized water (DW); lane 3, pDNA + DW + 10% FBS; lane 4, pDNA + RPMI; lane 5, pDNA + RPMI + 10% FBS; lane 6, PLL/DNA + DW; lane 7, PLL/DNA + DW + 10% FBS; lane 8, PLL/DNA + RPMI; lane 9, PLL/DNA + RPMI + 10% FBS; lanes 10-13, rPLL/DNA with respective treatments indicated in lanes 6-9.
Effect of overexpression of Bcl-2 on transfection activity of AS ODN and siRNA
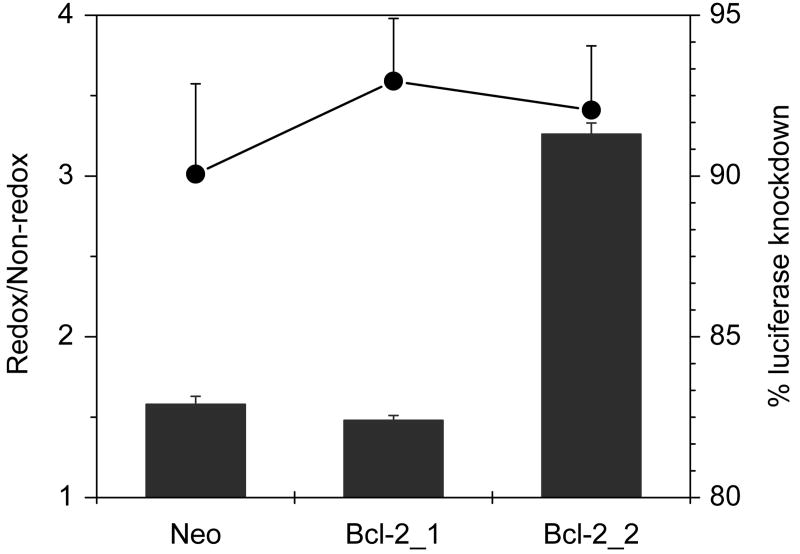
Transfections were conducted with complexes of AS ODN targeted against the luciferase gene. Relative activity of 1.5 was observed in the Neo clone in the absence of serum, whereas the ratio increased significantly (to 3.3) in Bcl-2_2 clone (P < 0.0001) (Fig. 6). The absolute levels of knockdown in the case of redox complexes did not vary significantly. The increase in relative activity in the Bcl-2_2 clone was due to the selective enhancement of the activity of the redox complexes. In the Bcl-2_2 clone, no knockdown of expression was observed for control non-redox complexes, when the experiment was conducted in the presence of serum. Instead, a 4.5-fold increase in the luciferase expression was observed in cells treated with non-redox AS ODN complexes. However, a small increase in the relative activity from 1.7 to 2.0 was observed when comparing the results between Neo and Bcl-2_1 clone (not shown).
Figure 6. Effect of Bcl-2 levels on transfection activity of luciferase AS ODN complexes.
Cells were co-transfected for 4 h in the absence of FBS with 0.5 μg Luc-pDNA and 0.35 μg AS ODN targeted against luciferase, complexed with PLL/rPLL (N:P 2) to which DOTAP was added to form ternary complexes. Luciferase expression was measured after 24 h. Knockdown of luciferase expression was calculated by comparison with cells transfected with pDNA only. Redox/Non-redox is the ratio (± SD, n=3) of knockdown activity of rPLL-to-PLL complexes. Secondary axis (●) shows % knockdown mediated by rPLL complexes.
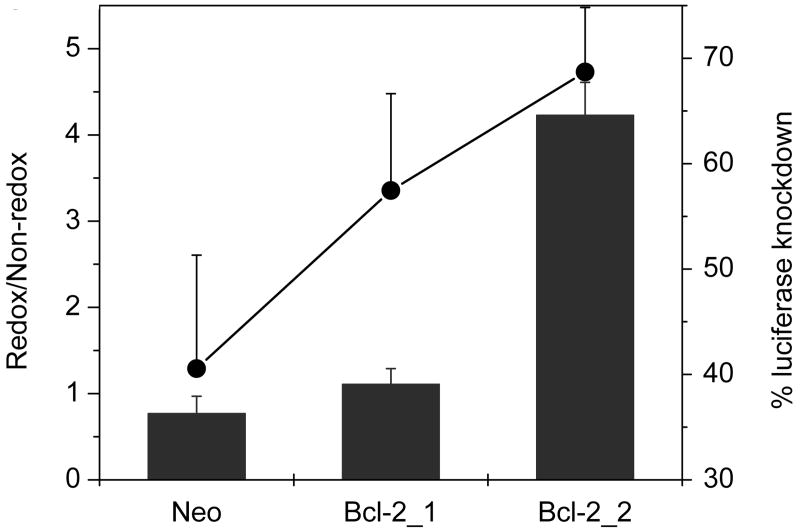
Transfections conducted with siRNA complexes targeted against luciferase mRNA showed a similar trend as AS ODN, wherein the relative activity significantly increased from 0.8 to 4.2 (P = 0.0001) (Fig. 7). A statistically significant 1.7-fold increase in the absolute levels of knockdown was observed in Bcl-2_2 clone, compared to the Neo clone (P = 0.02). As described with AS ODN, no knockdown was observed in neither of the Bcl-2 clones transfected with control non-redox complexes in the presence of serum. In fact, 4.6- and 3.3-fold increases in luciferase expression were observed in Bcl-2 clones treated with non-redox siRNA complexes.
Figure 7. Effect of Bcl-2 levels on transfection activity of luciferase siRNA complexes.
Cells were co-transfected for 4 h in the absence of FBS with 0.5 μg Luc-pDNA and 0.2 μg siRNA targeted against luciferase, complexed with PLL/rPLL (N:P 2) to which DOTAP was added to form ternary complexes. Luciferase expression was measured after 24 h. Knockdown of luciferase expression was calculated by comparison with cells transfected with pDNA only. Redox/Non-redox is the ratio (± SD, n=3) of knockdown activity of rPLL-to-PLL complexes. Secondary axis (●) shows % knockdown mediated by rPLL complexes.
Enhancement of relative transfection is modulated by increased GSH levels
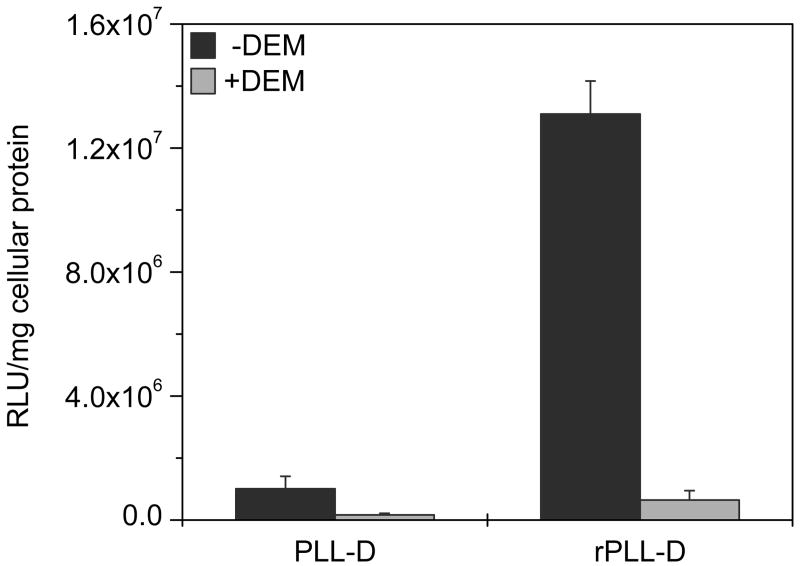
Transfections were conducted in Bcl-2_2 clone after treating cells with diethylmaleate (DEM) to transiently deplete all cellular thiols. A 20-fold decrease in transfection activity was observed for the redox complexes, while only a 6-fold decrease was seen for the control non-redox complexes (Fig. 8).
Figure 8. Effect of GSH depletion on transfection activity.
Cells were treated with 1 mM DEM for 1 h, washed with PBS, and then transfected for 2 h with 0.75 μg Luc-pDNA complexed with PLL or rPLL (N:P 2) to which DOTAP was added to form ternary complexes. Luciferase expression was measured after 4 h as mean RLU/mg cellular protein ± SD (n=3).
Discussion
This study investigates the possibility to utilize changes occurring in pathological states to modulate delivery of therapeutics using redox-sensitive vectors. We studied the effect of increased GSH levels and altered subcellular distribution of GSH resulting from overexpression of Bcl-2, on the transfection activity of complexes based on reducible polycations and four main classes of potential nucleic acid therapeutics. We predicted that the redox changes associated with overexpression of Bcl-2 could be advantageously exploited for improvement of the delivery of nucleic acids by modulating the rates and location of the unpacking of complexes. We also hypothesized that the magnitude of the effects of GSH changes on activity of the complexes will depend on the size of the nucleic acid used. Lastly, changes in subcellular distribution of GSH were expected to differently influence the delivery efficacy of nucleic acids active in the distinct subcellular locations.
We prepared MCF-7 clones stably overexpressing the human Bcl-2 gene and characterized select changes in their properties (Table 1). As expected, we observed significantly increased GSH levels in the Bcl-2 clones compared to the control Neo clone. These findings confirm previously reported changes in GSH concentration in response to Bcl-2 overexpression in MCF-7 cells [33]. The cellular redox state is determined by both the total GSH concentration and the ratio GSH:GSSG. No detectable amount of GSSG was observed in our analysis, which indicates high GSH:GSSG ratio typical for cancer cells. Furthermore, Bcl-2 overexpression is known to increase the GSH:GSSG ratio [32]. Considering the sensitivity of the assay, we can conclude that [GSH]:[GSSG] > 100. Cellular redox state is dependent on the cell cycle as shown previously by decreased GSH levels in mouse melanoma cells in stationary phase compared to log phase [43, 44]. Here, we compared GSH levels of the Bcl-2 clones harvested in two phases of cellular growth. Unlike the previous report, none of the observed differences in GSH levels between the two phases were statistically significant. All transfection experiments described in this study were conducted with cells in the log phase.
Several mechanisms have been proposed to explain the increased GSH levels associated with Bcl-2 expression. For example, Bcl-2 expressing cells show increased expression of CD53 [45], known to be involved in the formation of a complex with γ-glutamyltransferase [46], which regulates cellular redox potential by recycling extracellular GSH. Increased GSH levels may also come from alterations in genes controlling cellular redox state, which is influenced by localization of Bcl-2 in the nucleus [45]. Elevated levels of NAD(P)H in cells overexpressing Bcl-2 can also increase the levels of the reduced GSH [47]. No differences were observed previously in the concentrations of rate limiting enzymes involved in GSH biosynthesis, such as γGCS and GSH synthetase in mouse lymphomas overexpressing Bcl-2 [45, 48]. However, the significant increase in the activity of γGCS observed here suggests that γGCS is at least partly responsible for the elevated GSH levels in the MCF-7 cells overexpressing Bcl-2. Reports indicate that increase in cellular GSH levels caused by overexpression of Bcl-2 is followed by partial redistribution of the GSH to the nucleus [49]. Fig. 1b clearly demonstrates the increased nuclear localization of GSH in the Bcl-2 clones compared to the Neo clone. The Nuc:Cyt ratios observed provide quantitative support for the partial nuclear sequestration of GSH. Our data analysis revealed that the clones have an estimated 60–67% of the GSH localized in the nucleus. Both the cytosolic and nuclear GSH concentrations were increased in the Bcl-2 clones compared to the control cells. However, combining the nuclear redistribution with the overall increase in total GSH levels indicated that the nuclear environment in the Bcl-2 clones became relatively more reducing than that of the cytoplasm. Another factor known to influence the transfection activity of complexes is the rate of cell growth. Fast-growing cells typically exhibit higher transfection activity when compared to slow-growing or non-dividing cells. Here, we observed increased doubling times with increasing levels of Bcl-2, thus suggesting that the cell growth rates are not responsible for the observed changes in transfections of redox complexes. Decreased cellular proliferation rates with increasing Bcl-2 levels have been reported earlier by several groups [28, 50].
We selected rPLL and PLL as the reducible and non-reducible polycations to investigate the influence of altered GSH levels in the Bcl-2 clones on transfection activity. Because of the well-known effects of chemical structure of polycations on transfection activity, it was crucial that the polycations were structurally as similar as possible. rPLL and PLL satisfy the requirement very well as the only difference between the two polymers are the disulfide bonds separating every 10 lysine residues in the rPLL. The rPLL and PLL polycations had similar average molecular weights (∼30,000), although the PLL was less polydisperse than rPLL. Both polycations formed complexes with similar size [38, 51] and surface charge and thus permitted a fairly direct evaluation of the effects of redox-sensitive properties of complexes in Bcl-2 expressing cells. A limitation of the selected polycations is the requirement for a lysosomotropic agent to improve their transfection activity. We therefore followed a previously published strategy to improve endosomal escape of the complexes [51]. Complexes were first prepared by mixing the polycations with the respective nucleic acid, and that was followed by formation of ternary complexes with DOTAP. Transfection data are reported here as relative transfection to permit the use of the non-redox complexes as an internal control to account for any variations accompanying Bcl-2 overexpression and its resulting influence on transfection activity. We expected that because of the strong dependence of disassembly on the size of nucleic acids, their release from the complexes after GSH-mediated reduction of the disulfide bonds would be most enhanced in the case of pDNA and mRNA. We indeed observed an increase in relative transfection with pDNA complexes with increasing Bcl-2/GSH levels, although the changes were relatively small compared to those seen with mRNA complexes. The increase of GSH content in the cytoplasm of Bcl-2 clones provides direct explanation for the increase in relative transfection of mRNA. The lower magnitude of enhancement of relative transfection of pDNA compared to mRNA is likely due to the differences in subcellular trafficking and disassembly. Previously published evidence suggests that the unpacking of complexes is a more crucial barrier for mRNA delivery than for pDNA delivery [16]. There appears to be a relatively efficient mechanism of nuclear disassembly of pDNA complexes, but no such mechanism is in place for cytoplasmic unpacking of mRNA complexes. As a result, it is reasonable to expect that increasing the GSH levels will have a more pronounced effect on activity of mRNA complexes than on pDNA complexes. It also may be concluded that there is an optimum rate of cytoplasmic disassembly of pDNA complexes, which maximizes nuclear delivery while minimizing the negative effects of cytoplasmic, nuclease degradation of pDNA. However, it needs to be pointed-out that Bcl-2 overexpression is a complex phenomenon and it is conceivable that factors other than GSH changes might contribute to the observed results. Finally, we note that the luciferase expression of the redox complexes increased in the Bcl-2 clones, which may have additional benefits for use of this class of delivery systems in cancer gene therapy.
Additional analysis of the Nuc:Cyt data indicated that ∼32, 42 and 70 nmol/mg cellular protein of the total cellular GSH was localized in the nucleus in the Neo, Bcl-2_1 and Bcl-2_2 clones, respectively. Similarly, 20, 22 and 34 nmol/mg cellular protein of the total cellular GSH was estimated to be localized in the cytoplasm. The above mentioned amounts of GSH were calculated based on information available from the Nuc:Cyt ratios and the absolute GSH levels measured by HPLC. It must be noted that the above quantities represent estimates and are only intended to serve as a guide to demonstrate the increase of GSH in the cytoplasm and nucleus with increasing Bcl-2 levels. Even though our estimate suggests larger increase of nuclear than cytoplasmic GSH concentration in the Bcl-2 clones, this increase was translated into a relatively small improvement of pDNA activity. As noted above, this small increase is most likely a reflection of the relatively lower importance of unpacking of the pDNA complexes in the nucleus compared to that in the cytoplasm.
Transfection activity of redox-responsive complexes typically is more sensitive to the presence of serum than that of non-redox complexes [6, 51]. Here, not only quantitative changes were observed for pDNA complexes but also qualitative changes in the relative transfection were observed with increasing GSH (Fig. 2). Unlike pDNA complexes, the increase in relative transfection was preserved irrespective of the transfection condition in case of the mRNA complexes (Fig. 5). In the presence of serum, an inverse correlation with Bcl-2-induced effects was observed on relative transfection of pDNA complexes. To explain the effect of serum, we first speculated that the overall GSH levels might be lowered in cells deprived of serum during the 4 h transfection period compared to cells maintained continuously in the presence of serum. The results in Fig. 3 disprove this speculation as similar GSH content was observed in the MCF-7 clones regardless of the presence or absence of serum. We then tested the possibility that redox-active serum components may cause reduction of disulfides in the redox complexes and thus premature release of pDNA, which might explain the selective effect of serum on transfection activity of redox complexes. As the results in Fig. 4 clearly show, serum has similar effect on both PLL and rPLL complexes, suggesting that serum does not selectively compromise the stability of redox-sensitive complexes. Based on these findings, we speculate that serum determines subcellular trafficking and uptake mechanism of complexes. Specifically, we propose that binding of serum albumin can have pronounced effect on the cellular uptake pathway and transfection activity of redox complexes [8]. Albumin binds to transmembrane gp60 receptor which is known to induce caveolar uptake as opposed to the traditional clathrin-coated vesicular endocytosis pathway [52-54]. It is known that caveolar endocytosis routes cargo towards the endoplasmic reticulum (ER) [53]. ER is a highly oxidizing subcellular compartment where the redox ratio (GSH:GSSG) ranges from 1:1 to 3:1, compared to the highly reducing environment of the cytosol (GSH:GSSG ranges from 30:1 to >100:1) [55, 56]. Thus, we speculate that the differences in the redox environment during subcellular trafficking might be responsible for the observed disproportionate effect of serum on redox complexes. Studies are under way to test the hypothesis.
The reduction of disulfides in redox complexes is likely to have less significant effect on the activity of small nucleic acids such as AS ODN and siRNA due to a smaller effect of polycation size on unpacking rates of their complexes. We observed a significant increase in relative activity of both AS ODN and siRNA between Neo and Bcl-2_2 clones. This observation is indicative of the enhanced activity of redox complexes compared to control, non-redox complexes (Figs. 6 and 7). As expected, the magnitude of the enhancement in relative activity was smaller owing to a requirement for lower concentrations of GSH for unpacking complexes. As discussed for luciferase pDNA and mRNA, a significant improvement of absolute activity (% knockdown) was also observed for redox complexes of siRNA with increasing Bcl-2 levels. Unexpected increases were observed in the transfection activity of non-redox complexes of AS ODN and siRNA when cells were transfected in the presence of serum. It has been reported that small non-coding RNAs targeted to the noncoding regulatory regions in gene promoters can induce gene expression leading to sequence-specific and long-lasting gene activation [57].
In published studies of redox complexes, transfections were often conducted in the absence of serum and especially the dependence of transfection activity on GSH levels was demonstrated under conditions of relatively high N:P ratios [6]. We have conducted all our experiments in both the presence and absence of serum and used formulation parameters that induced minimal cell stress. To verify that the increases in the relative transfection with increasing Bcl-2 expression were mediated by increased GSH levels, we transiently depleted cellular thiols using DEM and investigated the activity of redox complexes (Fig. 8). It is known that DEM depletes the thiols for ∼2 h, which is followed by recovery to the original thiol levels [58]. The transfection was therefore measured 6 h after DEM treatment. The small decrease that was observed in the control, non-redox complexes might be due to the synergistic effect of dose-induced toxicity of PLL and the general effect of DEM on cellular proteins, whereas the larger decrease observed in the case of redox complexes may be related to a combination of the GSH depletion and the general effect of DEM. It must be noted that cells treated with redox complexes did not display any observable signs of toxicity. Additionally, no measurable changes in total cellular GSH were observed after transfection with the redox or non-redox complexes (data not shown). Another way of verifying that the Bcl-2 induced changes in GSH are responsible for the improvement in activity would be a knockdown of Bcl-2 expression. However, it has been demonstrated that the knockdown of Bcl-2 expression using AS ODN does not significantly alter cellular GSH levels [32]. Reasons for the failure to reverse the changes in cellular redox state induced by Bcl-2 expression are currently unknown. Most published studies that investigated the role of GSH in the activity of redox complexes used BSO, which specifically inhibits GSH biosynthesis in the cytoplasm [6, 12, 22, 23]. Typically, a small decrease in the transfection activity of pDNA complexes was reported. It is known that BSO inhibits γGCS, which is responsible for the synthesis of the cytosolic GSH pool, leaving the nuclear and mitochondrial pools intact [37]. Since the activity of pDNA complexes might be regulated in part by nuclear GSH, we believe that depleting the total cellular GSH pool with DEM is a better alternative to BSO. In line with published evidence on the BSO effect, we also observed a modest 2- and 3.3-fold decrease in transfection activity of pDNA and mRNA redox complexes, respectively (data not shown). The larger decrease in the case of mRNA complexes may be indicative of the dependence of activity of mRNA polyplexes solely on the cytosolic GSH pool.
Conclusion
We have demonstrated, for the first time, that overexpression of Bcl-2 can be utilized as a proxy redox stimulus to selectively enhance the activity of four major classes of potential nucleic acid therapeutics, when delivered by redox-sensitive vectors. In particular, this stimulus can be advantageously utilized to efficiently deliver mRNA. Our study indicates that the magnitude of enhancement of delivery, achieved by exploiting the overexpression of Bcl-2, greatly depends on the size and subcellular site of action and disassembly of the polyplexes. These findings strongly encourage further exploitation of reducible delivery systems, with the promise of achieving enhanced selectivity of therapeutic efficacy in Bcl-2 overexpressing tumors in vivo.
Acknowledgments
We would like to thank Yezi You for molecular weight analysis of the polycations. This work was supported by the National Institutes of Health (CA 109711) and the Susan G. Komen Breast Cancer Foundation (BCTR 0600711).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walker GF, Fella C, Pelisek J, Fahrmeir J, Boeckle S, Ogris M, et al. Toward Synthetic Viruses: Endosomal pH-Triggered Deshielding of Targeted Polyplexes Greatly Enhances Gene Transfer in vitro and in vivo. Mol Ther. 2005;11(3):418–425. doi: 10.1016/j.ymthe.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Liu X, Howard KA, Dong M, Andersen MO, Rahbek UL, Johnsen MG, et al. The influence of polymeric properties on chitosan/siRNA nanoparticle formulation and gene silencing. Biomaterials. 2007;28(6):1280–1288. doi: 10.1016/j.biomaterials.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Wagner E. Strategies to improve DNA polyplexes for in vivo gene transfer: Will “artificial viruses” be the answer? Pharm Res. 2004;21(1):8–14. doi: 10.1023/b:pham.0000012146.04068.56. [DOI] [PubMed] [Google Scholar]
- 4.Christensen LV, Chang CW, Kim WJ, Kim SW, Zhong Z, Lin C, et al. Reducible poly(amido ethylenimine)s designed for triggered intracellular gene delivery. Bioconjug Chem. 2006;17(5):1233–1240. doi: 10.1021/bc0602026. [DOI] [PubMed] [Google Scholar]
- 5.McKenzie DL, Smiley E, Kwok KY, Rice KG. Low molecular weight disulfide cross-linking peptides as nonviral gene delivery carriers. Bioconjug Chem. 2000;11(6):901–909. doi: 10.1021/bc000056i. [DOI] [PubMed] [Google Scholar]
- 6.Read ML, Singh S, Ahmed Z, Stevenson M, Briggs SS, Oupicky D, et al. A versatile reducible polycation-based system for efficient delivery of a broad range of nucleic acids. Nucleic Acids Res. 2005;33(9):e86. doi: 10.1093/nar/gni085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kloeckner J, Wagner E, Ogris M. Degradable gene carriers based on oligomerized polyamines. Eur J Pharm Sci. 2006;29(5):414–425. doi: 10.1016/j.ejps.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Manickam DS, Oupicky D. Multiblock reducible copolypeptides containing histidine-rich and nuclear localization sequences for gene delivery. Bioconjug Chem. 2006;17(6):1395–1403. doi: 10.1021/bc060104k. [DOI] [PubMed] [Google Scholar]
- 9.Wang X-L, Nguyen T, Gillespie D, Jensen R, Lu Z-R. A multifunctional and reversibly polymerizable carrier for efficient siRNA delivery. Biomaterials. 2008;29(1):15–22. doi: 10.1016/j.biomaterials.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 10.Wang YX, Chen P, Shen JC. The development and characterization of a glutathione-sensitive cross-linked polyethylenimine gene vector. Biomaterials. 2006;27(30):5292–5298. doi: 10.1016/j.biomaterials.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 11.McKenzie DL, Kwok KY, Rice KG. A potent new class of reductively activated peptide gene delivery agents. J Biol Chem. 2000;275(14):9970–9977. doi: 10.1074/jbc.275.14.9970. [DOI] [PubMed] [Google Scholar]
- 12.Christensen LV, Chang CW, Yockman JW, Conners R, Jackson H, Zhong Z, et al. Reducible poly(amido ethylenediamine) for hypoxia-inducible VEGF delivery. J Control Release. 2007;118(2):254–261. doi: 10.1016/j.jconrel.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoon Jeong J, Christensen LV, Yockman JW, Zhong Z, Engbersen JF, Jong Kim W, et al. Reducible poly(amido ethylenimine) directed to enhance RNA interference. Biomaterials. 2007;28(10):1912–1917. doi: 10.1016/j.biomaterials.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Zhong Z, Song Y, Engbersen JF, Lok MC, Hennink WE, Feijen J. A versatile family of degradable non-viral gene carriers based on hyperbranched poly(ester amine)s. J Control Release. 2005;109(13):317–329. doi: 10.1016/j.jconrel.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 15.Kakizawa Y, Harada A, Kataoka K. Glutathione-Sensitive Stabilization of Block Copolymer Micelles Composed of Antisense DNA and Thiolated Poly(ethylene glycol)-block-poly(L-lysine): A Potential Carrier for Systemic Delivery of Antisense DNA. Biomacromolecules. 2001;2(2):491–497. doi: 10.1021/bm000142l. [DOI] [PubMed] [Google Scholar]
- 16.Bettinger T, Carlisle RC, Read ML, Ogris M, Seymour LW. Peptide-mediated RNA delivery: a novel approach for enhanced transfection of primary and post-mitotic cells. Nucleic Acids Res. 2001;29(18):3882–3891. doi: 10.1093/nar/29.18.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaffer DV, Fidelman NA, Dan N, Lauffenburger DA. Vector unpacking as a potential barrier for receptor-mediated polyplex gene delivery. Biotechnol Bioeng. 2000;67(5):598–606. doi: 10.1002/(sici)1097-0290(20000305)67:5<598::aid-bit10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 18.Soundara Manickam D, Oupicky D. Polyplex gene delivery modulated by redox potential gradients. J Drug Target. 2006;14(8):519–526. doi: 10.1080/10611860600834409. [DOI] [PubMed] [Google Scholar]
- 19.Soundara Manickam D, Bisht HS, Wan L, Mao G, Oupicky D. Influence of TAT-peptide polymerization on properties and transfection activity of TAT/DNA polyplexes. J Control Release. 2005;102(1):293–306. doi: 10.1016/j.jconrel.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24(7):1121–1131. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- 21.Godbey WT, Wu KK, Mikos AG. Poly(ethylenimine)-mediated gene delivery affects endothelial cell function and viability. Biomaterials. 2001;22(5):471–480. doi: 10.1016/s0142-9612(00)00203-9. [DOI] [PubMed] [Google Scholar]
- 22.Balakirev M, Schoehn G, Chroboczek J. Lipoic acid-derived amphiphiles for redox-controlled DNA delivery. Chem Biol. 2000;7(10):813–819. doi: 10.1016/s1074-5521(00)00030-2. [DOI] [PubMed] [Google Scholar]
- 23.Neu M, Germershaus O, Mao S, Voigt KH, Behe M, Kissel T. Crosslinked nanocarriers based upon poly(ethylene imine) for systemic plasmid delivery: In vitro characterization and in vivo studies in mice. J Control Release. 2007;118(3):370–380. doi: 10.1016/j.jconrel.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Bhargava V, Kell DL, van de Rijn M, Warnke RA. Bcl-2 immunoreactivity in breast carcinoma correlates with hormone receptor positivity. Am J Pathol. 1994;145(3):535–540. [PMC free article] [PubMed] [Google Scholar]
- 25.Leek RD, Kaklamanis L, Pezzella F, Gatter KC, Harris AL. bcl-2 in normal human breast and carcinoma, association with oestrogen receptor-positive, epidermal growth factor receptor-negative tumours and in situ cancer. Br J Cancer. 1994;69(1):135–139. doi: 10.1038/bjc.1994.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jansen BA, Brouwer J, Reedijk J. Glutathione induces cellular resistance against cationic dinuclear platinum anticancer drugs. J Inorg Biochem. 2002;89(34):197–202. doi: 10.1016/s0162-0134(02)00381-1. [DOI] [PubMed] [Google Scholar]
- 27.Mirkovic N, Voehringer DW, Story MD, McConkey DJ, McDonnell TJ, Meyn RE. Resistance to radiation-induced apoptosis in Bcl-2-expressing cells is reversed by depleting cellular thiols. Oncogene. 1997;15(12):1461–1470. doi: 10.1038/sj.onc.1201310. [DOI] [PubMed] [Google Scholar]
- 28.Hoetelmans RW, Vahrmeijer AL, van Vlierberghe RL, Keijzer R, van de Velde CJ, Mulder GJ, et al. The role of various Bcl-2 domains in the anti-proliferative effect and modulation of cellular glutathione levels: a prominent role for the BH4 domain. Cell Prolif. 2003;36(1):35–44. doi: 10.1046/j.1365-2184.2003.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hour TC, Shiau SY, Lin JK. Suppression of N-methyl-N'-nitro-N-nitrosoguanidine- and S-nitrosoglutathione-induced apoptosis by Bcl-2 through inhibiting glutathione-S-transferase pi in NIH3T3 cells. Toxicol Lett. 1999;110(3):191–202. doi: 10.1016/s0378-4274(99)00158-7. [DOI] [PubMed] [Google Scholar]
- 30.Lee M, Hyun DH, Marshall KA, Ellerby LM, Bredesen DE, Jenner P, et al. Effect of overexpression of BCL-2 on cellular oxidative damage, nitric oxide production, antioxidant defenses, and the proteasome. Free Radic Biol Med. 2001;31(12):1550–1559. doi: 10.1016/s0891-5849(01)00633-5. [DOI] [PubMed] [Google Scholar]
- 31.Meredith MJ, Cusick CL, Soltaninassab S, Sekhar KS, Lu S, Freeman ML. Expression of Bcl-2 increases intracellular glutathione by inhibiting methionine-dependent GSH efflux. Biochem Biophys Res Commun. 1998;248(3):458–463. doi: 10.1006/bbrc.1998.8998. [DOI] [PubMed] [Google Scholar]
- 32.Ortega A, Ferrer P, Carretero J, Obrador E, Asensi M, Pellicer JA, et al. Down-regulation of glutathione and Bcl-2 synthesis in mouse B16 melanoma cells avoids their survival during interaction with the vascular endothelium. J Biol Chem. 2003;278(41):39591–39599. doi: 10.1074/jbc.M303753200. [DOI] [PubMed] [Google Scholar]
- 33.Rudin CM, Yang Z, Schumaker LM, VanderWeele DJ, Newkirk K, Egorin MJ, et al. Inhibition of glutathione synthesis reverses Bcl-2-mediated cisplatin resistance. Cancer Res. 2003;63(2):312–318. [PubMed] [Google Scholar]
- 34.Takahashi M, Saito H, Atsukawa K, Ebinuma H, Okuyama T, Ishii H. Bcl-2 prevents doxorubicin-induced apoptosis of human liver cancer cells. Hepatol Res. 2003;25(2):192–201. doi: 10.1016/s1386-6346(02)00244-9. [DOI] [PubMed] [Google Scholar]
- 35.Vahrmeijer AL, Hoetelmans RW, Mulder GJ, Schutrups J, van Vlierberghe RL, van de Velde CJ, et al. Development of resistance to glutathione depletion-induced cell death in CC531 colon carcinoma cells: association with increased expression of bcl-2. Biochem Pharmacol. 2000;59(12):1557–1562. doi: 10.1016/s0006-2952(00)00286-0. [DOI] [PubMed] [Google Scholar]
- 36.Voehringer DW, Meyn RE. Reversing drug resistance in bcl-2-expressing tumor cells by depleting glutathione. Drug Resist Updat. 1998;1(6):345–351. doi: 10.1016/s1368-7646(98)80010-1. [DOI] [PubMed] [Google Scholar]
- 37.Green RM, Graham M, O'Donovan MR, Chipman JK, Hodges NJ. Subcellular compartmentalization of glutathione: correlations with parameters of oxidative stress related to genotoxicity. Mutagenesis. 2006;21(6):383–390. doi: 10.1093/mutage/gel043. [DOI] [PubMed] [Google Scholar]
- 38.Oupicky D, Parker AL, Seymour LW. Laterally stabilized complexes of DNA with linear reducible polycations: strategy for triggered intracellular activation of DNA delivery vectors. J Am Chem Soc. 2002;124(1):8–9. doi: 10.1021/ja016440n. [DOI] [PubMed] [Google Scholar]
- 39.Fariss MW, Reed DJ. High-performance liquid chromatography of thiols and disulfides: dinitrophenol derivatives. Methods Enzymol. 1987;143:101–109. doi: 10.1016/0076-6879(87)43018-8. [DOI] [PubMed] [Google Scholar]
- 40.Huang CS, Moore WR, Meister A. On the active site thiol of gamma-glutamylcysteine synthetase: relationships to catalysis, inhibition, and regulation. Proc Natl Acad Sci U S A. 1988;85(8):2464–2468. doi: 10.1073/pnas.85.8.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Markovic J, Borras C, Ortega A, Sastre J, Vina J, Pallardo FV. Glutathione is recruited into the cell nucleus in early phases of cell proliferation. J Biol Chem. 2007 doi: 10.1074/jbc.M609582200. [DOI] [PubMed] [Google Scholar]
- 42.Burke RS, Pun SH. Extracellular Barriers to in Vivo PEI and PEGylated PEI Polyplex-Mediated Gene Delivery to the Liver. Bioconjug Chem. 2008 doi: 10.1021/bc700388u. [DOI] [PubMed] [Google Scholar]
- 43.Grande S, Luciani AM, Rosi A, Palma A, Giovannini C, Sapora O, et al. Metabolism of glutathione in tumour cells as evidenced by 1H MRS. FEBS Lett. 2007;581(4):637–643. doi: 10.1016/j.febslet.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 44.Ortega AL, Carretero J, Obrador E, Gambini J, Asensi M, Rodilla V, et al. Tumor cytotoxicity by endothelial cells. Impairment of the mitochondrial system for glutathione uptake in mouse B16 melanoma cells that survive after in vitro interaction with the hepatic sinusoidal endothelium. J Biol Chem. 2003;278(16):13888–13897. doi: 10.1074/jbc.M207140200. [DOI] [PubMed] [Google Scholar]
- 45.Voehringer DW, Hirschberg DL, Xiao J, Lu Q, Roederer M, Lock CB, et al. Gene microarray identification of redox and mitochondrial elements that control resistance or sensitivity to apoptosis. Proc Natl Acad Sci U S A. 2000;97(6):2680–2685. doi: 10.1073/pnas.97.6.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nichols TC, Guthridge JM, Karp DR, Molina H, Fletcher DR, Holers VM. Gamma-glutamyl transpeptidase, an ecto-enzyme regulator of intracellular redox potential, is a component of TM4 signal transduction complexes. Eur J Immunol. 1998;28(12):4123–4129. doi: 10.1002/(SICI)1521-4141(199812)28:12<4123::AID-IMMU4123>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 47.Esposti MD, Hatzinisiriou I, McLennan H, Ralph S. Bcl-2 and mitochondrial oxygen radicals. New approaches with reactive oxygen species-sensitive probes. J Biol Chem. 1999;274(42):29831–29837. doi: 10.1074/jbc.274.42.29831. [DOI] [PubMed] [Google Scholar]
- 48.Voehringer DW, Meyn RE. Redox aspects of Bcl-2 function. Antioxid Redox Signal. 2000;2(3):537–550. doi: 10.1089/15230860050192314. [DOI] [PubMed] [Google Scholar]
- 49.Voehringer DW, McConkey DJ, McDonnell TJ, Brisbay S, Meyn RE. Bcl-2 expression causes redistribution of glutathione to the nucleus. Proc Natl Acad Sci U S A. 1998;95(6):2956–2960. doi: 10.1073/pnas.95.6.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borner C. Diminished cell proliferation associated with the death-protective activity of Bcl-2. J Biol Chem. 1996;271(22):12695–12698. doi: 10.1074/jbc.271.22.12695. [DOI] [PubMed] [Google Scholar]
- 51.Read ML, Bremner KH, Oupicky D, Green NK, Searle PF, Seymour LW. Vectors based on reducible polycations facilitate intracellular release of nucleic acids. J Gene Med. 2003;5(3):232–245. doi: 10.1002/jgm.331. [DOI] [PubMed] [Google Scholar]
- 52.Minshall RD, Tiruppathi C, Vogel SM, Niles WD, Gilchrist A, Hamm HE, et al. Endothelial cell-surface gp60 activates vesicle formation and trafficking via G(i)-coupled Src kinase signaling pathway. J Cell Biol. 2000;150(5):1057–1070. doi: 10.1083/jcb.150.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pelkmans L, Helenius A. Endocytosis via caveolae. Traffic. 2002;3(5):311–320. doi: 10.1034/j.1600-0854.2002.30501.x. [DOI] [PubMed] [Google Scholar]
- 54.Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127(5):1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Braakman I, Helenius J, Helenius A. Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO J. 1992;11(5):1717–1722. doi: 10.1002/j.1460-2075.1992.tb05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hwang C, Sinskey AJ, Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257(5076):1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- 57.Li LC, Okino ST, Zhao H, Pookot D, Place RF, Urakami S, et al. Small dsRNAs induce transcriptional activation in human cells. Proc Natl Acad Sci U S A. 2006;103(46):17337–17342. doi: 10.1073/pnas.0607015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weber CA, Duncan CA, Lyons MJ, Jenkinson SG. Depletion of tissue glutathione with diethyl maleate enhances hyperbaric oxygen toxicity. Am J Physiol. 1990;258(6 Pt 1):L308–312. doi: 10.1152/ajplung.1990.258.6.L308. [DOI] [PubMed] [Google Scholar]