Abstract
Background
Depressive symptoms have been associated with increased risk of coronary artery disease (CAD) and poor prognosis among patients with existing CAD, but whether depressive symptoms specifically influence atherosclerotic progression among such patients is uncertain.
Methods and Results
The Post-CABG Trial randomized patients with a history of CABG surgery to either an aggressive or moderate lipid lowering strategy and to either warfarin or placebo. Coronary angiography was conducted at enrollment and after a median follow up of 4.2 years. Depressive symptoms were assessed at enrollment using the Centers for Epidemiologic Studies Depression scale (CES-D) in 1319 patients with 2496 grafts. In models adjusting for age, gender, race, treatment assignment and years since CABG surgery, a CES-D score ≥ 16 was positively associated with risk of substantial graft disease progression (odds ratio: 1.50; 95% CI: 1.08, 2.10; p=0.02) and marginally associated with a 0.11 mm (95% CI: −0.22, 0.01 mm; p=0.07) decrease in minimum lumen diameter, but not with risk of graft occlusion (p=0.30). Additional adjustment for past medical history, blood pressure, and renal function did not materially alter these results. This association was virtually absent among participants randomly assigned to aggressive lipid lowering therapy.
Conclusions
These findings suggest that depressive symptoms are associated with a higher risk of atherosclerotic progression among patients with saphenous vein grafts, and that aggressive lipid lowering can minimize this increased risk. Whether depressive symptoms increase progression in other types of coronary atherosclerosis, and whether aggressive lipid lowering attenuates such progression, will require additional study.
Keywords: Depressive symptoms, Coronary disease, atherosclerosis, coronary artery bypass surgery, saphenous vein grafts
Depression and depressive symptoms are highly prevalent among patients with coronary artery disease (CAD) and independently predict adverse cardiovascular events.1-3 In patients with CAD, depression has been associated with recurrent MI, angina, ventricular arrhythmias, and mortality.4 Depression is also specifically associated with a higher risk of cardiac events after coronary artery bypass graft (CABG) surgery.5 Patients with depression before CABG are more often depressed after the surgery and have worse physical function and higher co-morbidity.6 Surprisingly, whether treatment for depression improves clinical outcomes among patients with CAD remains uncertain.7
Several mechanisms may mediate the observed association of depression with adverse outcomes among patients with CAD. Depression has been associated with decreased adherence to treatment,8, 9 with a variety of potentially unfavorable lifestyle characteristics such as obesity and smoking,10, 11 and with higher levels of inflammatory and prothrombotic markers.12 There have been few studies investigating the potential link between depressive symptoms and progression of atherosclerosis per se,13, 14 despite the association between depression and clinical outcomes. Accordingly, we evaluated the hypothesis that depressive symptoms are associated with progression of atherosclerosis among individuals with previous coronary artery bypass graft (CABG) surgery and saphenous vein grafts enrolled in the Post-CABG Trial.15
METHODS
Study population and design
The Post-CABG Study is a completed clinical trial designed to compare the effects of 2 lipid lowering strategies and low-dose anticoagulation versus placebo on the progression of atherosclerosis in saphenous vein grafts, as documented by assessment of angiograms obtained at baseline and 4 to 5 years after study entry. Participants were aged 21−74 years, had undergone CABG surgery 1 to 11 years prior to enrollment, and had ≥ 2 patent saphenous vein grafts in men and ≥ 1 in women. Participants also had a low-density lipoprotein cholesterol (LDL-C) of 130−175 mg/dL, plasma triglycerides <300 mg/dL, and left ventricular ejection fraction ≥ 30%. Specific exclusion criteria included unstable angina, decompensated heart failure, New York Heart Association class III and IV heart failure, life threatening arrhythmias, large left ventricular aneurysm, life-threatening cerebrovascular disease, systemic hypertension refractory to drug therapy, and severe renal or hepatic dysfunction. Subjects with myocardial infarction within the previous 3 months, percutaneous coronary intervention within the previous 6 months, gastrointestinal hemorrhage or diagnosis of an active gastrointestinal ulcer within 2 years, or absolute contraindications to treatment with any of the study medications were excluded. A total of 1351 patients were enrolled between March, 1989, and August, 1991. All participants provided written informed consent.
Participants were randomly assigned in a 2×2 factorial design to either aggressive LDL-C lowering with lovastatin 40−80 mg/day to achieve an LDL-C of 60−85 mg/dl or moderate LDLC lowering with lovastatin 2.5−5 mg/day to achieve a LDL-C of 130−140 mg/dl and either warfarin 1−4 mg/day to achieve an international normalized ratio (INR) of 1.8−2.0 or warfarin-placebo. All prospective participants received active warfarin treatment for a month prior to randomization. Only participants who consumed over 90% of the prescribed medication were randomized. Participants’ adherence to prescribed treatment with lovastatin during the trial was excellent; 85−90% took the medication as prescribed.
Depressive Symptoms
Depressive symptoms were assessed at study enrollment using the Centers for Epidemiologic Studies Depression scale (CES-D).16 The CES-D is a 20-item self-administered instrument designed to measure the presence of depressive symptoms over the previous week in community studies. Note that the CES-D does not capture information on a patient's clinical or treatment history and is not useful as a diagnostic tool for depression . The CES-D has been widely used and extensively validated.10, 17-19 As in previous work,20 we chose a priori to use scores of <16 to indicate no or minimal depressive symptoms and ≥16 to indicate the presence of moderate or severe symptoms. In sensitivity analyses, we also considered scores between 8 and 15 to determine whether a dose-response relationship existed among those with no or mild symptoms.
Outcome Measurements
Baseline and follow-up angiograms were obtained with catheterization techniques that permitted computer-assisted quantitative measurement (CAAS system, PIE Medical Maastrecht).21 The primary end point of the trial was significant worsening of initially patent grafts, defined as a decrease of ≥0.6 mm in lumen diameter at the site of greatest change at follow-up. It included worsening of pre-existing lesions, new lesions in previously intact grafts, and occlusion. All initially patent grafts were considered to have developed graft worsening in patients who died before follow up angiography. Surviving participants who did not have follow-up or interim angiograms and who did not undergo repeated bypass surgery or angioplasties were excluded from analyses. Additional pre-specified angiographic trial endpoints included complete occlusion of grafts patent at baseline and change in minimum lumen diameter.
In addition, participants were followed for a prespecified composite endpoint of death, myocardial infarction, stroke, recurrent bypass surgery, or angioplasty. As previously,22, 23 we also examined an endpoint of death or myocardial infarction alone (i.e., excluding revascularizations).
Other covariates
At enrollment, participants reported their smoking history (grouped as never, former, <20 cigarettes per day, and ≥20 per day), physical activity level relative to others of their age and sex (grouped to create 3 levels of activity), and alcohol consumption (grouped as none, 1−6 drinks/week, 7−13 drinks/week, and ≥14 drinks/week). Body mass index was measured at enrollment and grouped into 3 categories (<25, 25−29.9, and ≥30.0 kg/m2). Serum creatinine was measured at enrollment and we estimated glomerular filtration rate (eGFR) using the abbreviated Modified Diet and Renal Disease study (MDRD) equation.24 Participants were grouped according to eGFR in three categories (<60.0, 60.0−74.9, and ≥75.0 ml/min/1.73m2).
Statistical methods
As described by the Post-CABG investigators, we analyzed graft progression on a per-graft basis using generalized estimating equations (GEE) to account for the clustering of grafts within participants.25 We used a logit link function for binary outcomes and an identity link function for change in minimal lumen diameter, and assumed an exchangeable correlation matrix in all models. We created sequentially adjusted models to examine the effects of confounding and potentially mediating factors. We first examined the association of CES-D scores with outcome in models adjusted for age (in quartiles), years since CABG (as a continuous variable), gender, race (white versus other), and treatment assignment (four categories). We then developed additional models that further adjusted for systolic blood pressure, use of anti-hypertensive medication, eGFR, history of myocardial infarction and stroke, and type 2 diabetes mellitus requiring pharmacologic treatment with sulfonylureas or insulin. We lastly adjusted for behavioral determinants that may be on a causal pathway between depressive symptoms and graft disease progression, including body-mass index, former or current smoking, alcohol intake, physical activity, total cholesterol, HDL-C, and triglycerides.
In stratified analyses, we evaluated whether the effect of depressive symptoms varied by age (<65 vs. ≥65) using GEE models including an age-by-CES-D interaction term and controlling for lipid-lowering strategy, warfarin assignment, gender, race, and years since CABG. Similarly, we evaluated whether the effect of depressive symptoms varied by lipid lowering strategy (moderate vs. aggressive) using GEE models including a strategy-by-CES-D interaction term and controlling for warfarin assignment, age, gender, race, and years since CABG . Note that these models assume no interaction between the warfarin and lipid-lowering strategy treatment arms. This assumption is supported by the published primary analysis of the Post-CABG trial.15
Cox proportional hazards models were used to evaluate the association between depressive symptoms and risk of clinical events. We controlled for similar covariates as in GEE models and assessed the validity of the proportional hazards assumption graphically using plots of Schoenfeld residuals for each covariate.26 The number of clinical events was insufficient to support robust stratified analyses by age or treatment assignment.
CES-D scores were available in 1319 (97.6%) of patients. Excluding subjects with missing values from analyses can lead to biased results. Multiple imputation is an effective method for dealing with the missing data under certain assumptions. This method replaces each missing value with a set of plausible values that represent the uncertainty about the right value to impute. The imputed data sets are then analyzed with standard regression techniques and the overall results are obtained by combining the results from analysis on each imputed data set.
We used Markov chain Monte Carlo multiple imputation methods27 to create 5 simulated datasets in which CES-D scores were available in all subjects. All analyses on angiographic and event endpoints were carried out on each simulated dataset. Results for each analysis from the imputed datasets were combined accounting for both the within-imputation and between-imputation variances. This procedure yields standard errors, confidence intervals, and p-values that reflect the uncertainty in the imputed values.
All p values are two-tailed and all confidence intervals (CIs) computed at the 95% level. Analyses were carried out using SAS 9.1 (SAS Institute, Cary, NC). This analysis was approved by the Beth Israel Deaconess Medical Center Committee on Clinical Investigations. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
Baseline characteristics
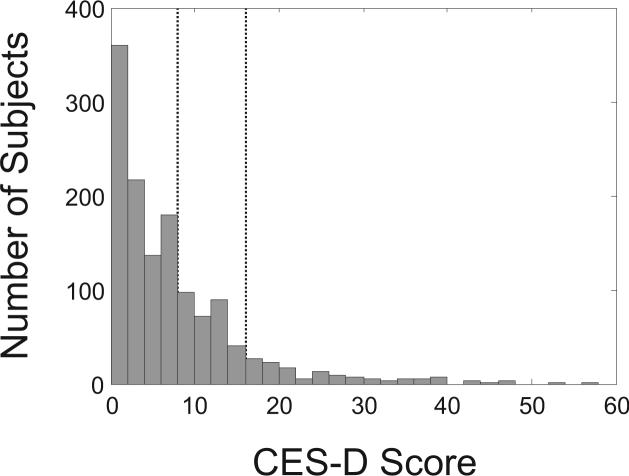
Participants had a mean age of 61.5 years, and were predominantly white (94%) and male (92%). CES-D scores were available in 1319 (97.6%) of patients and ranged from 0 to 56 with a median score of 5 (Figure 1). The 127 (9.6%) participants with CES-D scores ≥16 tended to be younger, more often women, and more likely to have cardiovascular risk factors (Table 1).
Figure 1.
Distribution of CES-D scores among 1319 study participants where CES-D was available at enrollment. Among participants with a CES-D score, 9.6% had a score ≥16 and 32.3% had a score ≥8. Dashed vertical lines denote CES-D scores of 8 and 16.
Table 1.
Baseline characteristics of Post-CABG participants according to CES-D score.
| CES-D Score |
|||
|---|---|---|---|
| >=16 (n=127) | < 16 (n=1192) | Missing (n=32) | |
| Age (y, mean ± SD) | 60.4 ± 7.2 | 61.7 ± 7.3 | 60.5 ± 7.5 |
| Male Gender (%) | 87.4 | 93.2 | 84.4 |
| White Race (%) | 91.3 | 94.6 | 96.9 |
| Time Since CABG (y) | 4.3 ± 2.3 | 4.9 ± 2.6 | 5.3 ± 2.5 |
| Systolic Blood pressure (mmHg) | 135.5 ± 21.3 | 134.2 ± 17.3 | 134.8 ± 13.4 |
| Diastolic Blood Pressure (mmHg) | 79.5 ± 11.0 | 79.8 ± 8.8 | 81.5 ± 8.3 |
| Estimated Glomerular Filtration Rate (ml/min/1.73m2) | 74.1 ± 14.6 | 73.0 ± 14.9 | 75.5 ± 14.4 |
| Past Medical History (%) | |||
| Myocardial Infarction | 55.9 | 48.0 | 59.4 |
| Stroke | 1.6 | 2.9 | 3.1 |
| Hypertension | 39.4 | 35.2 | 28.1 |
| Diabetes Mellitus | 13.4 | 8.2 | 3.1 |
| Smoking History (%) | |||
| Current | 19.7 | 10.3 | 3.1 |
| Former | 62.2 | 63.3 | 81.3 |
| Body Mass Index (kg/m2) | 28.2 ± 4.6 | 27.7 ± 4.5 | 28.0 ± 4.3 |
| Alcohol consumption (drinks/week) | 2.3 ± 5.0 | 2.9 ± 4.9 | 2.5 ± 7.7 |
| White Blood Cell Count (×10^9/L) | 6.9 ± 2.0 | 6.5 ± 1.8 | 6.3 ± 1.2 |
| Total Cholesterol (mg/dl) | 229.6 ± 25.5 | 231.1 ± 25.4 | 232.8 ± 29.9 |
| Triglycerides (mg/dl) | 177.0 ± 79.7 | 161.2 ± 73.4 | 164.0 ± 60.2 |
| LDL Cholesterol (mg/dl) | 155.8 ± 21.0 | 159.0 ± 20.6 | 158.6 ± 20.4 |
| HDL Cholesterol (mg/dl) | 38.5 ± 9.3 | 40.0 ± 9.9 | 41.3 ± 12.1 |
| Number of SVGs (mean, range) | 2.5 (1−5) | 2.6 (1−8) | 2.5 (1−5) |
Angiographic outcomes
Data on substantial progression of atherosclerosis and graft occlusion were available for 1192 (88%) participants (Table 2). Among 213 grafts in participants with depressive symptoms, 34.3% developed substantial progression of atherosclerosis and 9.4% became occluded. Data on change in minimum lumen diameter were available for 1091 (81%) participants.
Table 2.
Angiographic outcomes among Post-CABG participants according to CES-D score.*
| |
CES-D Score |
||
|---|---|---|---|
| ≥16 | < 16 | Missing | |
| Substantial Progression of Atherosclerosis | |||
| Subjects / grafts, n | 101 / 213 | 1,074 / 2,283 | 17 / 37 |
| Grafts with outcome, n (%) | 73 (34.3) | 662 (29.0) | 15 (40.5) |
| Subjects having ≥1 graft with outcome, n (%) | 53 (52.5) | 473 (44.0) | 8 (47.1) |
| Mean per-patient percent of grafts with outcome | 33.8 | 28.8 | 35.3 |
| Graft Occlusion | |||
| Subjects / grafts, n | 101 / 213 | 1,074 / 2,283 | 17 / 37 |
| Grafts with Outcome, n (%) | 20 (9.4) | 186 (8.2) | 6 (16.2) |
| Subjects having ≥1 graft with outcome, n (%) | 17 (16.8) | 160 (14.9) | 4 (23.5) |
| Mean per-patient percent of grafts with outcome | 8.3 | 7.8 | 13.7 |
| Change in Minimum Lumen Diameter | |||
| Subjects / grafts, n | 89 / 187 | 989 / 2,070 | 13 / 29 |
| Mean Change (mm) ± SD | −0.33 ± 0.73 | −0.28 ± 0.71 | −0.74 ± 1.21 |
| Intraclass correlation coefficient | 0.17 | 0.16 | 0.32 |
We used multiple imputation methods to simulate CES-D scores for subjects with missing values. In regression models controlling for age, gender, race, years since CABG surgery, and treatment assignment, the presence of depressive symptoms was associated with a 50% (95% CI: 8, 210%; p=0.02) greater risk of substantial graft disease progression and marginally associated with a 0.11 mm (95% CI: −0.22, 0.01 mm; p=0.07) greater decrease in minimum lumen diameter (Table 3). Depressive symptoms were not associated with significantly greater risk of graft occlusion, a less common outcome. Additional adjustment for potential confounders did not materially alter these results. Inclusion of potential mediators reduced the odds ratio for significant graft progression from 1.49 to 1.41, a 13.8% decrease in the regression coefficient for depressive symptoms. In sensitivity analyses, no increased risk was observed for any outcome for participants with a CES-D score between 8 and 15.
Table 3.
Association between depressive symptoms (CES-D score ≥16) and angiographic outcomes among Post-CABG participants.*
| Substantial Progression of Atherosclerosis |
Graft Occlusion |
Change in Minimum Lumen Diameter |
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | Δ (95% CI) | P-value | |
| Base Model | 1.50 (1.08, 2.10) | 0.02 | 1.32 (0.78, 2.25) | 0.30 | −0.11 (−0.22, 0.01) | 0.07 |
| Full Model | 1.49 (1.06, 2.09) | 0.02 | 1.31 (0.74, 2.31) | 0.35 | −0.10 (−0.22, 0.02) | 0.09 |
| Full Model + Mediators | 1.41 (1.01, 1.97) | 0.046 | 1.18 (0.70, 1.99) | 0.53 | −0.09 (−0.20, 0.02) | 0.12 |
Referent group is patients with CES-D score <16. Base model is adjusted for age, gender, race, treatment assignment, and years since CABG surgery. Full model is additionally adjusted for systolic blood pressure, estimated glomerular filtration rate, history of MI, stroke, hypertension, and diabetes mellitus. Adjustment for mediators further included body mass index, smoking history, alcohol intake, physical activity, total cholesterol, HDL cholesterol, and triglycerides. Multiple imputation methods were used in all analyses to account for participants in whom CES-D scores were not available. OR: odds ratio; CI: confidence interval, Δ: mean change in mm.
We repeated these analyses excluding subjects in which CES-D score was missing (rather than imputing the missing values). Using the complete-case analysis did not materially change the results, although the p-value for the association between CES-D score and change in minimum lumen diameter from the base model became statistically significant (p=0.05).
Table 4 shows the effect of depressive symptoms on progression of graft disease among participants randomized to aggressive or moderate lipid lowering strategies. The association of depressive symptoms with progression was consistently more apparent among subjects randomized to the moderate lipid lowering strategy, with essentially completely null associations among those assigned to aggressive lipid lowering, although formal tests of interaction were not statistically significant. When subjects with missing CES-D scores were excluded the test for interaction was statistically significant for change in minimum lumen diameter (p=0.04). We found no evidence of effect modification by age.
Table 4.
Association between depressive symptoms (CES-D score ≥16) and angiographic outcomes according to randomized treatment assignment.*
| Odds Ratio | 95% CI | PHomogeneity | ||
|---|---|---|---|---|
| Substantial Progression of Atherosclerosis | ||||
| Moderate lipid lowering strategy | 1.9 | 1.2, 3.0 | 0.23 |
|
| Aggressive lipid lowering strategy |
1.2 |
0.8, 2.0 |
||
| Graft Occlusion | ||||
| Moderate lipid lowering strategy | 1.5 | 0.8, 2.9 | 0.44 |
|
| Aggressive lipid lowering strategy |
1.0 |
0.4, 2.5 |
||
| Change in Minimum Lumen Diameter | Mean Δ (mm) | |||
| Moderate lipid lowering strategy | −0.25 | −0.48, −0.02 | 0.06 | |
| Aggressive lipid lowering strategy | −0.01 | −0.12, 0.10 |
Odds ratios and mean change (Δ) adjusted for warfarin assignment, age, gender, race, and years since CABG surgery. Multiple imputation methods were used in all analyses to account for participants in whom CES-D scores were not available.
Clinical events
During a median follow-up of 4.2 years, 208 participants developed a recurrent clinical event. We used Markov chain Monte Carlo multiple imputation methods to simulate CES-D scores for subjects with missing values. In the base model, depressive symptoms were associated with a hazard ratio of recurrent clinical events of 1.50 (95% CI: 0.96, 2.36; p=0.08). Additional adjustment for potential confounders reduced the hazard ratio to 1.37 (95% CI: 0.87, 2.17; p=0.18). In a sensitivity analysis, no increased risk was observed for participants with a CES-D score between 8 and 15.
Using an endpoint of death or myocardial infarction with 132 events, depressive symptoms were associated with a hazard ratio of 1.69 (95% CI: 1.00, 2.87; p=0.05) in the base model and 1.50 (0.88, 2.56; p=0.13) in the full model. In a sensitivity analysis, the hazard ratio was linearly associated with CES-D score (pTrend=0.01). Specifically, the hazard ratios were 1.46 (95% CI: 0.94, 2.27) for participants with a CES-D score between 8 and 15 and 1.91 (1.10, 3.29) for participants with a CES-D score ≥16.
We repeated these analyses using excluding subjects in which CES-D score was missing. In all cases the results based on the complete-case analysis were not materially different from the original results.
DISCUSSION
In this study of clinical trial participants with previous CABG surgery, the presence of depressive symptoms was associated with a higher risk of substantial progression of graft atherosclerosis and decrease in minimum lumen diameter over 4−5 years of follow-up. This association was virtually eliminated by random assignment to aggressive lipid lowering with high dose lovastatin.
Several pathophysiological mechanisms have been proposed to underlie the associations of depression and depressive symptoms with CAD and its prognosis. The association of depressive symptoms with graft atherosclerosis observed here suggests that progression of atherosclerosis per se may be at least partly responsible for the observed effects of depression on clinical outcomes, rather than just differences in plaque vulnerability or predisposition to thrombosis or arrhythmias.
Depression is associated with an increase in sympathetic nervous system activity reflected in decreased heart rate variability,28, 29 which may itself increase activation of the renin-angiotensin and corticosteroid axes and subsequently atherosclerosis. Depression is clearly associated with markers of systemic inflammation in population-based studies,12, 30 but the direction of this association and whether it exists among patients with CAD who already have elevated levels is less certain.31-33 Endothelial function is also impaired during and after episodes of depression,34-37 even among patients with CAD,38 perhaps via effects of depression on cortisol release.39 Depression could indirectly alter control of other atherosclerotic risk factors through its effects on weight, smoking, alcohol use, and exercise, and our results suggested that addition of these factors might indeed account for a portion of the observed association of depressive symptoms with atherosclerosis.
In this study, the association between depressive symptoms and graft atherosclerosis was virtually eliminated by random assignment to intensive lipid lowering, with a goal LDL-C of 60−85 mg/dl. This finding suggests that depression and statins may have opposing actions on common pathways in their influence on atherosclerosis. For example, as noted above, depression could influence atherosclerosis by increasing levels of systemic inflammation, while statins have pleiotropic anti-inflammatory effects that could ameliorate this.40-42
Our work may have specific clinical implications. First, depressive symptoms appear to be an important and easily measured risk factor for progression of graft disease, and hence their measurement may contribute to risk stratification following CABG. Second, the association of depressive symptoms with subsequent atherosclerotic progression appears to be substantially attenuated by high-dose statin therapy, and this provides further impetus to ensure all such patients are appropriately and intensively treated. Third, our results provide continued support for efforts to identify effective methods to address depression and depressive symptoms in patients with CAD. Although cognitive-behavioral therapy for depression following acute myocardial infarction has not specifically been shown to reduce clinical events in randomized trials to date,43 limited data suggests it may have promise following CABG,44 and substantial enthusiasm remains for use of serotonin-specific reuptake inhibitors and other antidepressants among patients with CAD and depression.7, 43, 45, 46 At the same time, at least for atherosclerosis, our results suggest that intensive lipid lowering may be sufficient to minimize the risk associated with depressive symptoms.
Specific strengths and limitations of the Post-CABG trial warrant discussion. The trial was large and based in multiple, geographically disparate centers, and the assessment of graft progression was uniform and systematic with prespecified angiographic end points. Robust data on potential confounders was available, and both angiographic and clinical outcomes were assessed. Adherence to medication was a prerequisite for entry, so the effects of depressive symptoms independent of this potential pathway could be studied. Importantly, the study included randomized assignment to lipid-lowering therapy, and hence we could evaluate whether lipid-lowering therapy alters the effects of depressive symptoms without confounding by clinical factors normally associated with the decision to prescribe such medication. Finally, the CES-D is a widely accepted instrument with well established reliability and validity for use in general populations, elderly individuals, and patients with co-morbid medical conditions.
At the same time, this study has some important limitations. First, because the CES-D specifically assesses the presence of depressive symptoms over the past week, it does not indicate the presence of major affective disorders, nor does it reflect longer-term history of depressive symptoms, clinical diagnoses, or treatment. However, the CES-D has been shown to have excellent test-retest reliability (intraclass correlation coefficient >0.5) in community studies. Second, patients were recruited into this study up to 11 years after CABG surgery, resulting in large variation in the degree of graft atherosclerosis at baseline and likely obscuring the effects of depressive symptoms on atherosclerotic progression. Third, the results of this study may not be generalizable to different patient populations. For example, whether depressive symptoms alter atherosclerotic progression in arterial grafts or native coronary arteries will require further study. The generalizability of the trial is further limited by its restriction to predominantly white and male clinical trial participants. Although this restriction limited the variability among participants and hence reduced potential confounding, similar longitudinal angiographic studies are needed in broader populations. Fourth, as expected from the entry criteria for the trial, the prevalence of depressive symptoms among Post-CABG participants was low, limiting the precision of our estimates. Fifth, although robust data on a number of potential confounders was available, the possibility of residual confounding by lifestyle or other risk factors remains.
In summary, among patients who had undergone previous CABG surgery, depressive symptoms were associated with higher risk of atherosclerotic progression in saphenous vein grafts. Our analysis provides prospective evidence for a direct association between depressive symptoms and atherosclerotic progression as a potential mechanism for the corresponding association of depressive symptoms with clinical prognosis. Despite advances in surgical and medical management of patients after CABG, the prognostic import of depressive symptoms on atherosclerotic progression provides a potentially valuable opportunity to elucidate mechanisms of graft atherosclerosis and to reduce adverse outcomes among patients with saphenous vein grafts. We encourage further studies to determine whether interventions that target depression and related symptoms will impact the progression of graft atherosclerosis or clinical prognosis of such patients.
Footnotes
FUNDING SOURCES
The Post-CABG Study was conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI), NIH in collaboration with the Post-CABG Study Investigators. This manuscript was prepared using a limited access dataset obtained from the NHLBI. The project described was supported by grant number F32-ES013804 from the National Institute of Environmental Health Sciences (NIEHS). Dr. Mittleman holds an Established Investigator Grant from the American Heart Association (AHA 0140219N). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NHLBI, NIH, or AHA.
DISCLOSURES
None.
REFERENCES
- 1.Frasure-Smith N, Lesperance F. Reflections on depression as a cardiac risk factor. Psychosom Med. 2005;67(Suppl 1):S19–25. doi: 10.1097/01.psy.0000162253.07959.db. [DOI] [PubMed] [Google Scholar]
- 2.Jiang W, Glassman A, Krishnan R, O'Connor CM, Califf RM. Depression and ischemic heart disease: what have we learned so far and what must we do in the future? Am Heart J. 2005;150(1):54–78. doi: 10.1016/j.ahj.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Shimbo D, Davidson KW, Haas DC, Fuster V, Badimon JJ. Negative impact of depression on outcomes in patients with coronary artery disease: mechanisms, treatment considerations, and future directions. J Thromb Haemost. 2005;3(5):897–908. doi: 10.1111/j.1538-7836.2004.01084.x. [DOI] [PubMed] [Google Scholar]
- 4.Jiang W, Krishnan RR, O'Connor CM. Depression and heart disease: evidence of a link, and its therapeutic implications. CNS Drugs. 2002;16(2):111–127. doi: 10.2165/00023210-200216020-00004. [DOI] [PubMed] [Google Scholar]
- 5.Blumenthal JA, Lett HS, Babyak MA, White W, Smith PK, Mark DB, Jones R, Mathew JP, Newman MF. Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet. 2003;362(9384):604–609. doi: 10.1016/S0140-6736(03)14190-6. [DOI] [PubMed] [Google Scholar]
- 6.Mallik S, Krumholz HM, Lin ZQ, Kasl SV, Mattera JA, Roumains SA, Vaccarino V. Patients with depressive symptoms have lower health status benefits after coronary artery bypass surgery. Circulation. 2005;111(3):271–277. doi: 10.1161/01.CIR.0000152102.29293.D7. [DOI] [PubMed] [Google Scholar]
- 7.Carney RM, Freedland KE. Does treating depression improve survival after acute coronary syndrome? Invited commentary on... Effects of antidepressant treatment following myocardial infarction. Br J Psychiatry. 2007;190:467–468. doi: 10.1192/bjp.bp.107.035360. [DOI] [PubMed] [Google Scholar]
- 8.Ziegelstein RC, Fauerbach JA, Stevens SS, Romanelli J, Richter DP, Bush DE. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med. 2000;160(12):1818–1823. doi: 10.1001/archinte.160.12.1818. [DOI] [PubMed] [Google Scholar]
- 9.Carney RM, Freedland KE, Eisen SA, Rich MW, Jaffe AS. Major depression and medication adherence in elderly patients with coronary artery disease. Health Psychol. 1995;14(1):88–90. doi: 10.1037//0278-6133.14.1.88. [DOI] [PubMed] [Google Scholar]
- 10.Farmer ME, Locke BZ, Moscicki EK, Dannenberg AL, Larson DB, Radloff LS. Physical activity and depressive symptoms: the NHANES I Epidemiologic Follow-up Study. Am J Epidemiol. 1988;128(6):1340–1351. doi: 10.1093/oxfordjournals.aje.a115087. [DOI] [PubMed] [Google Scholar]
- 11.Onyike CU, Crum RM, Lee HB, Lyketsos CG, Eaton WW. Is obesity associated with major depression? Results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2003;158(12):1139–1147. doi: 10.1093/aje/kwg275. [DOI] [PubMed] [Google Scholar]
- 12.Kop WJ, Gottdiener JS, Tangen CM, Fried LP, McBurnie MA, Walston J, Newman A, Hirsch C, Tracy RP. Inflammation and coagulation factors in persons > 65 years of age with symptoms of depression but without evidence of myocardial ischemia. Am J Cardiol. 2002;89(4):419–424. doi: 10.1016/s0002-9149(01)02264-0. [DOI] [PubMed] [Google Scholar]
- 13.Haas DC, Davidson KW, Schwartz DJ, Rieckmann N, Roman MJ, Pickering TG, Gerin W, Schwartz JE. Depressive symptoms are independently predictive of carotid atherosclerosis. Am J Cardiol. 2005;95(4):547–550. doi: 10.1016/j.amjcard.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 14.Stewart JC, Janicki DL, Muldoon MF, Sutton-Tyrrell K, Kamarck TW. Negative emotions and 3-year progression of subclinical atherosclerosis. Arch Gen Psychiatry. 2007;64(2):225–233. doi: 10.1001/archpsyc.64.2.225. [DOI] [PubMed] [Google Scholar]
- 15.The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass grafts The Post Coronary Artery Bypass Graft Trial Investigators. N Engl J Med. 1997;336(3):153–162. doi: 10.1056/NEJM199701163360301. [DOI] [PubMed] [Google Scholar]
- 16.Redloff LS. The CES-D scale: a self-report depressive scale for research in the general population. J Appl Psychol Measurement. 1977;1:385–401. [Google Scholar]
- 17.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106(3):203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 18.Bisschop MI, Kriegsman DM, Deeg DJ, Beekman AT, van Tilburg W. The longitudinal relation between chronic diseases and depression in older persons in the community: the Longitudinal Aging Study Amsterdam. J Clin Epidemiol. 2004;57(2):187–194. doi: 10.1016/j.jclinepi.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Wulsin LR, Evans JC, Vasan RS, Murabito JM, Kelly-Hayes M, Benjamin EJ. Depressive symptoms, coronary heart disease, and overall mortality in the Framingham Heart Study. Psychosom Med. 2005;67(5):697–702. doi: 10.1097/01.psy.0000181274.56785.28. [DOI] [PubMed] [Google Scholar]
- 20.Whang W, Albert CM, Sears SF, Jr., Lampert R, Conti JB, Wang PJ, Singh JP, Ruskin JN, Muller JE, Mittleman MA. Depression as a predictor for appropriate shocks among patients with implantable cardioverter-defibrillators: results from the Triggers of Ventricular Arrhythmias (TOVA) study. J Am Coll Cardiol. 2005;45(7):1090–1095. doi: 10.1016/j.jacc.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 21.Reiber JH, van der Zwet PM, Koning G, von Land CD, van Meurs B, Gerbrands JJ, Buis B, van Voorthuisen AE. Accuracy and precision of quantitative digital coronary arteriography: observer-, short-, and medium-term variabilities. Cathet Cardiovasc Diagn. 1993;28(3):187–198. doi: 10.1002/ccd.1810280301. [DOI] [PubMed] [Google Scholar]
- 22.Mukamal KJ, Girotra S, Mittleman MA. Alcohol consumption, atherosclerotic progression, and prognosis among patients with coronary artery bypass grafts. Am Heart J. 2006;151(2):368–372. doi: 10.1016/j.ahj.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 23.Wellenius GA, Mukamal KJ, Winkelmayer WC, Mittleman MA. Renal dysfunction increases the risk of saphenous vein graft occlusion: results from the Post-CABG trial. Atherosclerosis. 2007;193(2):414–420. doi: 10.1016/j.atherosclerosis.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 25.Domanski MJ, Borkowf CB, Campeau L, Knatterud GL, White C, Hoogwerf B, Rosenberg Y, Geller NL. Prognostic factors for atherosclerosis progression in saphenous vein grafts: the postcoronary artery bypass graft (Post-CABG) trial. Post-CABG Trial Investigators. J Am Coll Cardiol. 2000;36(6):1877–1883. doi: 10.1016/s0735-1097(00)00973-6. [DOI] [PubMed] [Google Scholar]
- 26.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. [Google Scholar]
- 27.Schafer JL. Analysis of Incomplete Multivariate Data. Vol. 72. Chapman & Hall/CRC; New York: 1997. [Google Scholar]
- 28.Horsten M, Ericson M, Perski A, Wamala SP, Schenck-Gustafsson K, Orth-Gomer K. Psychosocial factors and heart rate variability in healthy women. Psychosom Med. 1999;61(1):49–57. doi: 10.1097/00006842-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Carney RM, Freedland KE, Veith RC. Depression, the autonomic nervous system, and coronary heart disease. Psychosom Med. 2005;67(Suppl 1):S29–33. doi: 10.1097/01.psy.0000162254.61556.d5. [DOI] [PubMed] [Google Scholar]
- 30.Ford DE, Erlinger TP. Depression and C-reactive protein in US adults: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2004;164(9):1010–1014. doi: 10.1001/archinte.164.9.1010. [DOI] [PubMed] [Google Scholar]
- 31.Almeida OP, Norman P, Hankey GJ, Jamrozik K, Flicker L. The association between C-reactive protein concentration and depression in later life is due to poor physical health: results from the Health in Men Study (HIMS). Psychol Med. 2007:1–12. doi: 10.1017/S0033291707000827. [DOI] [PubMed] [Google Scholar]
- 32.van den Biggelaar AH, Gussekloo J, de Craen AJ, Frolich M, Stek ML, van der Mast RC, Westendorp RG. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Exp Gerontol. 2007;42(7):693–701. doi: 10.1016/j.exger.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Schins A, Tulner D, Lousberg R, Kenis G, Delanghe J, Crijns HJ, Grauls G, Stassen F, Maes M, Honig A. Inflammatory markers in depressed post-myocardial infarction patients. J Psychiatr Res. 2005;39(2):137–144. doi: 10.1016/j.jpsychires.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Rybakowski JK, Wykretowicz A, Heymann-Szlachcinska A, Wysocki H. Impairment of endothelial function in unipolar and bipolar depression. Biol Psychiatry. 2006;60(8):889–891. doi: 10.1016/j.biopsych.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 35.Wagner JA, Tennen H, Mansoor GA, Abbott G. History of major depressive disorder and endothelial function in postmenopausal women. Psychosom Med. 2006;68(1):80–86. doi: 10.1097/01.psy.0000195868.68122.9e. [DOI] [PubMed] [Google Scholar]
- 36.Broadley AJ, Korszun A, Jones CJ, Frenneaux MP. Arterial endothelial function is impaired in treated depression. Heart. 2002;88(5):521–523. doi: 10.1136/heart.88.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajagopalan S, Brook R, Rubenfire M, Pitt E, Young E, Pitt B. Abnormal brachial artery flow-mediated vasodilation in young adults with major depression. Am J Cardiol. 2001;88(2):196–198, A197. doi: 10.1016/s0002-9149(01)01623-x. [DOI] [PubMed] [Google Scholar]
- 38.Sherwood A, Hinderliter AL, Watkins LL, Waugh RA, Blumenthal JA. Impaired endothelial function in coronary heart disease patients with depressive symptomatology. J Am Coll Cardiol. 2005;46(4):656–659. doi: 10.1016/j.jacc.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 39.Broadley AJ, Korszun A, Abdelaal E, Moskvina V, Deanfield J, Jones CJ, Frenneaux MP. Metyrapone improves endothelial dysfunction in patients with treated depression. J Am Coll Cardiol. 2006;48(1):170–175. doi: 10.1016/j.jacc.2005.12.078. [DOI] [PubMed] [Google Scholar]
- 40.Chang LT, Sun CK, Chiang CH, Wu CJ, Chua S, Yip HK. Impact of simvastatin and losartan on antiinflammatory effect: in vitro study. J Cardiovasc Pharmacol. 2007;49(1):20–26. doi: 10.1097/FJC.0b013e31802ba4ec. [DOI] [PubMed] [Google Scholar]
- 41.Blanco-Colio LM, Martin-Ventura JL, de Teresa E, Farsang C, Gaw A, Gensini G, Leiter LA, Langer A, Martineau P, Hernandez G, Egido J. Increased soluble Fas plasma levels in subjects at high cardiovascular risk: Atorvastatin on Inflammatory Markers (AIM) study, a substudy of ACTFAST. Arterioscler Thromb Vasc Biol. 2007;27(1):168–174. doi: 10.1161/01.ATV.0000250616.26308.d7. [DOI] [PubMed] [Google Scholar]
- 42.Devaraj S, Chan E, Jialal I. Direct demonstration of an antiinflammatory effect of simvastatin in subjects with the metabolic syndrome. J Clin Endocrinol Metab. 2006;91(11):4489–4496. doi: 10.1210/jc.2006-0299. [DOI] [PubMed] [Google Scholar]
- 43.Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, Czajkowski SM, DeBusk R, Hosking J, Jaffe A, Kaufmann PG, Mitchell P, Norman J, Powell LH, Raczynski JM, Schneiderman N. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. Jama. 2003;289(23):3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 44.Doering LV, Cross R, Vredevoe D, Martinez-Maza O, Cowan MJ. Infection, depression, and immunity in women after coronary artery bypass: a pilot study of cognitive behavioral therapy. Altern Ther Health Med. 2007;13(3):18–21. [PubMed] [Google Scholar]
- 45.Joynt KE, O'Connor CM. Lessons from SADHART, ENRICHD, and other trials. Psychosom Med. 2005;67(Suppl 1):S63–66. doi: 10.1097/01.psy.0000163454.25036.fc. [DOI] [PubMed] [Google Scholar]
- 46.Sauer WH, Berlin JA, Kimmel SE. Selective serotonin reuptake inhibitors and myocardial infarction. Circulation. 2001;104(16):1894–1898. doi: 10.1161/hc4101.097519. [DOI] [PubMed] [Google Scholar]