In a well known children's tale, the little girl, Goldilocks, nearly gets herself eaten by bears by boldly choosing between the three bears' porridge bowls: one too hot and another too cold. In this issue of PNAS, the article “Size, foraging, and food web structure,” by Petchey et al. (1), tells a similar story of food webs in which predator species choose between prey species that are too large or too small. In doing so, the authors show how an easily observed species trait—body size—can address long-standing ecological questions about who eats whom in complex natural communities. Although previous models explain well the general network properties of food webs, Petchey et al. go further by explaining how the particular links found between species within specific food webs can result from optimal foraging of predators on prey that are neither too large nor too small. Like Goldilocks, optimal predators choose food that is “just right.”
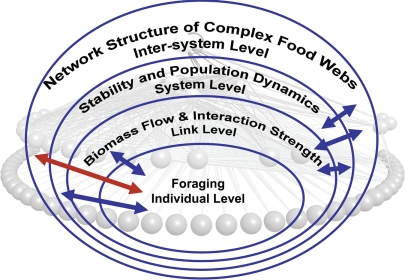
In choosing their food, predators and other consumers create food webs—the feeding networks among species that sustain the life support systems on earth. Ecologists continue to discover fascinating regularities in the structure of this interdependent complexity (2, 3). Elucidating the mechanisms responsible for these regularities is a fundamental step in tackling long-standing questions in ecology that range from “What confers stability to complex ecosystems?” (4) to “How does species loss alter the abundance of other species?” (5). Surprisingly simple rule-based models successfully capture the overall structure of real food webs (2, 6, 7) and enable further theoretical exploration by stochastically generating webs that mimic the overall structure of real webs (6, 8–10). However, such models lack mechanistic explanations for their input parameters and poorly predict the actual links in real food webs. Petchey et al. (1) have synthesized the allometry of body size with optimal foraging theory to address both the first limitation (11), and now the second, by assuming that predators are more efficient at consuming prey that are smaller than themselves, but not too small. This “Goldilocks” model of food choice successfully predicts up to 65% of the feeding links in real food webs, and in doing so helps to solve the notoriously difficult problem of integrating ecology from individuals to ecosystems (Fig. 1).
Fig. 1.
Cross-scale integration from individuals to ecosystems. Petchey et al. (1) model organisms' size-based foraging and integrate it with the network structure of food webs (red arrow). This work contributes to a broad research program that uses body size to integrate different levels of trophic organization and interactions (blue arrows).
The specific idea that the network structure of food webs may emerge from optimally foraging individuals is intuitively appealing but technically challenging. Petchey et al.'s (1) two mathematical foraging functions require that three or four parameters be fitted to each food web. Further work is needed to address the unexplained variability in these parameters (up to 26 orders of magnitude) and to reduce the analytical complexity of formulating and fitting them. Nonetheless, an important contribution is their linking of body size to prey-handling times within the foraging functions, both of which factors set upper bounds to predators' prey size. The more successful of the two functions also sets a lower limit to the body sizes of prey species. This concept of consumers feeding on contiguous ranges of prey body masses within boundaries is deeply rooted in foraging theory (12, 13) and is highly consistent with observed food web structure (14). However, this research has lacked the mechanistic bridge between foraging theory and food web topology. Petchey et al. provide this bridge by integrating both the foraging consequences of body size and the energy content of individual prey.
Between the narrow scale of an individual's foraging and the broad scale of community patterns lie the strengths of interactions between populations (15)—another area of trophic ecology being integrated by the use of body size (Fig. 1). Understanding interaction strength involves the critical ecological challenge of predicting when the extinction, or overharvesting, of one species might cause dramatic changes in the abundances of other species. Recent work (16–20) has shown how the body sizes of predators and their prey can determine feeding rates and biomass flows in food webs. Although large flows of biomass from prey to predator may not indicate strong predator control of prey populations (21), such flows can still drive population dynamics (22). Thus, the coupling of foraging theory with allometric considerations of metabolic theory may unite the structure of food webs with biomass flows to predict species' population dynamics, which in turn can elucidate the strengths of dynamic coupling between species (8). In this case, species' influence on each other and on the outcomes of species loss are seen as emergent properties of population dynamics, driven by foraging behavior and biomass flows that are constrained by species' body size (15).
Body size also allows integration of the overall dynamic persistence of food webs with the diversity and complexity of food web structure (10). The mathematical improbability of large, complex networks persisting dynamically was recognized early on (4). More recent theory finds that body size ratios between predators and their prey occupy a Goldilocks-type “sweet spot” that enables diversity to enhance both system-level (9, 16) and subsystem-level (22) stability. These stabilizing predator–prey body mass ratios are remarkably consistent with those found in the most comprehensive empirical data available (23). Buffering by larger organisms' low metabolic rate (9, 22) and higher mobility among variably productive patches (24) appear to underlie such stability. Petchey et al. (1) provide more mechanistic insight into such allometric integration of link-level biomass flows with population dynamics and system-level stability by showing that these parametric sweet spots are consistent with optimal foraging.
Leaving dynamics aside, the connection between body size and comparative analyses of the network structure of ecosystems also benefits from the mechanistic detail that Petchey et al. (1) provide. Seminal early work found that species' diets within a food web can form an unbroken segment, or interval, along a fixed sequence of all species within the food web (25). Body size helps explain the hierarchy in one of the earliest structural food web models, which proposed that larger ordered species can eat smaller ordered species, but not vice versa (26). These two ideas were then synthesized by the “niche model” that assigned consumers unbroken feeding ranges on a niche dimension that tends to be located below the consumer (27). Although this model has been shown to successfully predict overall food web structure (6, 7, 28), Petchey et al. provide a testable mechanistic interpretation of the niche axis in terms of body mass [see also Stouffer et al. (14)]. Such mechanisms are also relevant to empirical analyses of food web data that increasingly consider body size (29). Even the issue of parasites—arguably the most numerous of metazoan species that turn predator–prey body size relationships on their head (30)—still supports the strong effect of body size on the network structure of food webs (31). Such empirical analyses have, until now, remained primarily statistical rather than mechanistic in approach.
Petchey et al.'s (1) model is interesting not only in terms of where it fits the data but also where it fails. Their model successfully predicts a substantial number of links, but <50% of the observed links were predicted correctly in 12 of the 15 webs. In two webs, <10% were correct. These failures offer important insights into the limits of body size alone as an explanatory panacea. Predaceous and aquatic herbivorous links were, as expected, much better predicted than parasitic, parasitoid, and pathogenic interactions. Similarly, terrestrial herbivorous interactions were not as well predicted as aquatic ones, and the three nondesert terrestrial webs were fit rather poorly. All of this suggests that some foraging modes fit size-based optimal foraging models better than others. Body size may be most important to gape-limited or visual predators and perhaps unimportant to insect herbivores that choose plants on the basis on leaf chemistry or plant defenses. Similarly, energy maximization may be balanced by avoiding the risk of being consumed (32) or by stoichiometric preferences for particular nutrients (33). To better interpret such success and failure, it would help to know whether a random model with fewer adjustable parameters that simply constrains big things to eat small things does as well, or even better, than Petchey et al.'s foraging models.
These many mutually reinforcing discoveries fuel an increasingly exciting synthetic research program focused on body size and trophic ecology (34, 35). Body size is also key to “ecology's big, hot idea” (36) that successfully explains a broader array of ecological patterns, including species' relative abundances, spatial distributions, and diversity (37). Petchey et al. (1) contribute to this broad agenda by articulating how body size affects not only the energy content of an organism, but also how difficult that organism is to subdue and digest. Still, ecological interactions involve much more than just feeding (e.g., mutualisms, ecosystem engineering) (38), and feeding involves more than just prey body size. But body size and feeding links seem to be the “low-hanging fruit” of ecological complexity research. Both are universal and relatively easy to measure for many organisms. All species are made up of organisms that have mass, and no species we know of escapes the food web. As such, food webs form an essential core of critical interdependence created by the class of species interactions for which we tend to have the most comprehensive data (35). Similarly, body size elegantly links energy content, feeding behavior, and metabolic needs. Does this mean that we have chosen to search for our lost coins under the lamppost simply because that is where the light is? We don't think so. Rather, we may be first picking up the coins that are easily visible before crawling under the car to get the rest. Although examples of nontrophic interactions and exceptions to foraging based on body size are obvious and abundant, the key unanswered questions are when and under what conditions these other factors are important in predicting food web structure and dynamics. Answering these questions requires scientists to tease out the variation in feeding patterns and biomass flows that can be simply explained by body size. In doing so, we can help clarify the importance of fascinating, and often critical, nontrophic ecological interactions and processes.
Footnotes
The authors declare no conflict of interest.
See companion article on page 4191.
References
- 1.Petchey OL, Beckerman AP, Riede JO, Warren PH. Size, foraging, and food web structure. Proc Natl Acad Sci USA. 2008;105:4191–4196. doi: 10.1073/pnas.0710672105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunne JA. Institute Studies in the Sciences of Complexity. The network structure of food webs. In: Pascual M, Dunne JA, Santa Fe, editors. Ecological Networks: Linking Structure to Dynamics in Food Webs. New York: Oxford Univ Press; 2006. pp. 27–86. [Google Scholar]
- 3.May RM. Network structure and the biology of populations. Trends Ecol Evol. 2006;21:394–399. doi: 10.1016/j.tree.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 4.May RM. Stability and Complexity in Model Ecosystems. Princeton: Princeton Univ Press; 1973. [PubMed] [Google Scholar]
- 5.Paine RT. Food web complexity and species diversity. Am Nat. 1966;100:65–75. [Google Scholar]
- 6.Stouffer DB, Camacho J, Jiang W, Amaral LAN. Evidence for the existence of a robust pattern of prey selection in food webs. Proc R Soc London Ser B. 2007;274:1931–1940. doi: 10.1098/rspb.2007.0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams RJ, Martinez ND. Success and its limits among structural models of complex food webs. J Anim Ecol. 2008 doi: 10.1111/j.1365-2656.2008.01362.x. [DOI] [PubMed] [Google Scholar]
- 8.Brose U, Berlow EL, Martinez ND. Scaling up keystone effects from simple to complex ecological networks. Ecol Lett. 2005;8:1317–1325. [Google Scholar]
- 9.Brose U, Williams RJ, Martinez ND. Allometric scaling enhances stability in complex food webs. Ecol Lett. 2006;9:1228–1236. doi: 10.1111/j.1461-0248.2006.00978.x. [DOI] [PubMed] [Google Scholar]
- 10.Martinez ND, Williams RJ, Dunne JA. Diversity, complexity, and persistence in large model ecosystems. In: Pascual M, Dunne JA, Santa Fe, editors. Ecological Networks: Linking Structure to Dynamics in Food Webs. New York: Oxford Univ Press; 2006. pp. 163–185. Institute Studies in the Sciences of Complexity. [Google Scholar]
- 11.Beckerman AP, Petchey OL, Warren PH. Foraging biology predicts food web complexity. Proc Natl Acad Sci USA. 2006;103:13745–13749. doi: 10.1073/pnas.0603039103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson DS. Adequacy of body size as a niche difference. Am Nat. 1975;109:769–784. [Google Scholar]
- 13.Persson L, Leonardsson K, de Roos AM, Gyllenberg M, Christensen B. Ontogenetic scaling of foraging rates and the dynamics of a size-structured consumer-resource model. Theor Popul Biol. 1998;54:270–293. doi: 10.1006/tpbi.1998.1380. [DOI] [PubMed] [Google Scholar]
- 14.Stouffer DB, Camacho J, Amaral LAN. A robust measure of food web intervality. Proc Natl Acad Sci USA. 2006;103:19015–19020. doi: 10.1073/pnas.0603844103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berlow EL, et al. Interaction strengths in food webs: Issues and opportunities. J Anim Ecol. 2004;73:585–598. [Google Scholar]
- 16.Emmerson MC, Raffaelli D. Predator-prey body size, interaction strength and the stability of a real food web. J Anim Ecol. 2004;73:399–409. [Google Scholar]
- 17.Woodward G, et al. Body size in ecological networks. Trends Ecol Evol. 2005;20:402–409. doi: 10.1016/j.tree.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Wootton JT, Emmerson M. Measurement of interaction strength in nature. Annu Rev Ecol Evol Syst. 2005;36:419–444. [Google Scholar]
- 19.Bascompte J, Melian CJ, Sala E. Interaction strength combinations and the overfishing of a marine food web. Proc Natl Acad Sci USA. 2005;102:5443–5447. doi: 10.1073/pnas.0501562102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reuman DC, Cohen JE. Estimating relative energy fluxes using the food web, species abundance, and body size. In: Caswell H, editor. Food Webs: From Connectivity to Energetics, Advances in Ecological Research. Vol 36. San Diego: Elsevier; 2005. pp. 137–182. [Google Scholar]
- 21.Paine RT. Food webs: Linkage, interaction strength, and community infrastructure. J Anim Ecol. 1980;49:667–685. [Google Scholar]
- 22.Otto SB, Rall BC, Brose U. Allometric degree distributions facilitate food web stability. Nature. 2007;450:1226–1230. doi: 10.1038/nature06359. [DOI] [PubMed] [Google Scholar]
- 23.Brose U, et al. Consumer-resource body-size relationships in natural food webs. Ecology. 2006;87:2411–2417. doi: 10.1890/0012-9658(2006)87[2411:cbrinf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.McCann KS, Rasmussen JB, Umbanhowar J. The dynamics of spatially coupled food webs. Ecol Lett. 2005;8:513–523. doi: 10.1111/j.1461-0248.2005.00742.x. [DOI] [PubMed] [Google Scholar]
- 25.Cohen JE. Food Webs and Niche Space. Princeton: Princeton Univ Press; 1978. [PubMed] [Google Scholar]
- 26.Cohen JE, Briand F, Newman CM. Community Food Webs: Data and Theory, Lecture Notes in Biomathematics. Vol 20. Berlin: Springer; 1990. [Google Scholar]
- 27.Williams RJ, Martinez ND. Simple rules yield complex food webs. Nature. 2000;404:180–183. doi: 10.1038/35004572. [DOI] [PubMed] [Google Scholar]
- 28.Dunne JA, Williams RJ, Martinez ND. Network structure and robustness of marine food webs. Mar Ecol Progr Ser. 2004;273:291–302. [Google Scholar]
- 29.Cohen JE, Jonsson T, Carpenter SR. Ecological community description using the food web, species abundance, and body size. Proc Natl Acad Sci USA. 2003;100:1781–1786. doi: 10.1073/pnas.232715699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lafferty KD, et al. Parasites in food webs: The ultimate missing links. Ecol Lett. 2008 doi: 10.1111/j.1461-0248.2008.01174.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen JE, Jonsson T, Muller CB, Godfray HCJ, Savage VM. Body sizes of hosts and parasitoids in individual feeding relationships. Proc Natl Acad Sci USA. 2005;102:684–689. doi: 10.1073/pnas.0408780102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitz OJ. Direct and indirect effects of predation and predation risk in old-field interaction webs. Am Nat. 1998;151:327–342. doi: 10.1086/286122. [DOI] [PubMed] [Google Scholar]
- 33.Mayntz D, Raubenheimer D, Salomon M, Toft S, Simpson SJ. Nutrient-specific foraging in invertebrate predators. Science. 2005;307:111–113. doi: 10.1126/science.1105493. [DOI] [PubMed] [Google Scholar]
- 34.Brown JH, Gillooly JF. Ecological food webs: High-quality data facilitate theoretical unification. Proc Natl Acad Sci USA. 2003;100:1467–1468. doi: 10.1073/pnas.0630310100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pascual M, Dunne JA, editors. Ecological Networks: Linking Structure to Dynamics in Food Webs, Santa Fe Institute Studies in the Sciences of Complexity. New York: Oxford Univ Press; 2006. [Google Scholar]
- 36.Whitfield J. Ecology's big, hot idea. PLoS Biol. 2004;2:2023–2027. doi: 10.1371/journal.pbio.0020440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. Toward a metabolic theory of ecology. Ecology. 2004;85:1771–1789. [Google Scholar]
- 38.Montoya JM, Pimm SL, Sole RV. Ecological networks and their fragility. Nature. 2006;442:259–264. doi: 10.1038/nature04927. [DOI] [PubMed] [Google Scholar]