Abstract
Coral bleaching, caused by heat stress, is accompanied by the light-induced loss of photosynthetic pigments in in situ symbiotic dinoflagellate algae (Symbiodinium spp.). However, the molecular mechanisms responsible for pigment loss are poorly understood. Here, we show that moderate heat stress causes photobleaching through inhibition of the de novo synthesis of intrinsic light-harvesting antennae [chlorophyll a–chlorophyll c2–peridinin–protein complexes (acpPC)] in cultured Symbiodinium algae and that two Clade A Symbiodinium species showing different thermal sensitivities of photobleaching also show differential sensitivity of this key protein synthesis process. Photoinhibition of photosystem II (PSII) and subsequent photobleaching were observed at temperatures of >31°C in cultured Symbiodinium CS-73 cells grown at 25–34°C, but not in cultures of the more thermally tolerant control Symbiodinium species OTcH-1. We found that bleaching in CS-73 is associated with loss of acpPC, which is a major antennae protein in Symbiodinium. In addition, the thermally induced loss of this protein is light-dependent, but does not coincide directly with PSII photoinhibition and is not caused by stimulated degradation of acpPC. In cells treated at 34°C over 24 h, the steady-state acpPC mRNA pool was modestly reduced, by ≈30%, whereas the corresponding synthesis rate of acpPC was diminished by >80%. Our results suggest that photobleaching in Symbiodinium is consequentially linked to the relative susceptibility of PSII to photoinhibition during thermal stress and occurs, at least partially, because of the loss of acpPC via undefined mechanism(s) that hamper the de novo synthesis of acpPC primarily at the translational processing step.
Keywords: bleaching, photoinhibition, zooxanthellae, coral, symbiosis
Photobleaching results from the loss of photosynthetic pigments in photosynthetic organisms as a consequence of exposure to environmental stresses that perturb their capacity to adequately dissipate excess light energy (1). In symbiotic dinoflagellate algae (Symbiodinium spp.) within corals, exposure to physiologically damaging temperatures is a common stress that accelerates algal photobleaching (2–6). Associated with this thermally induced photobleaching response is likely the concomitant expulsion of the in situ Symbiodinium in thermally stressed corals, which can lead to mass coral bleaching (7, 8). Severe coral bleaching has resulted in extensive coral mortality and the destruction of diverse coral ecosystems worldwide, prompting significant effort to understand the mechanisms underpinning mass coral bleaching.
In most symbiotic dinoflagellate species, the major light-harvesting pigments are peridinin and chlorophylls a and c2 that bind to two major antennae proteins, the peridinin-chlorophyll a-binding proteins (PCP) and the chlorophyll a-chlorophyll c2-peridinin-protein complexes (acpPC) (9). PCP is a water-soluble protein unique to many dinoflagellates that binds to the luminal side of the thylakoid membrane and can contain either 15-kDa (monomeric form) or 30- to 35-kDa (dimeric form) polypeptides. In contrast, acpPC is integral to the thylakoid membranes and contains a polypeptide of ≈19 kDa that is related at the N-terminal region to the fucoxanthin-chlorophyll protein and the chlorophyll a/b-binding proteins (10). In Symbiodinium species, the majority of the photosynthetic pigments are associated with acpPC (10, 11).
Photobleaching follows photoinhibition of photosystem II (PSII). The initial process of photoinhibition occurs only when the rate of photodamage to PSII exceeds the rate of its repair (12). In Symbiodinium, moderate heat stress has been demonstrated to accelerate photoinhibition primary through suppression of PSII repair (5, 13). It has been proposed that elevated photoinhibition causes photobleaching through oxidative damage to photosynthetic pigments, proteins, and thylakoid membranes in response to a rise in the levels of harmful singlet oxygen (1O2) produced via O2 reacting with triplet-excited chlorophyll (3Chl*) in the light-harvesting complexes (1, 14).
To further understand the process of photobleaching in Symbiodinium cells caused by thermal stress, we have examined the physiological differences between two cultured Symbiodinium strains (OTcH-1 and CS-73) that show different sensitivities to thermal stress, with CS-73 being more susceptible than OTcH-1 to thermally induced photoinhibition. We find the levels of acpPC and PCP in CS-73, but less so in OTcH-1, decline in response to moderate temperature increases. Here, we focus on acpPC, which has the majority of the light-harvesting pigments associated with it (10, 11). We show that its increased loss in CS-73 can be attributed to limitation in its de novo protein synthesis and, to a lesser extent, a minor decline in the steady-state pool of the acpPC mRNA, rather than accelerated degradation. Our results suggest that variations in the photobleaching sensitivity of different Symbiodinium species to thermal stress is strongly influenced by the differences in the sensitivity of the de novo synthesis of antenna proteins, particularly acpPC, to elevated temperatures.
Results
OTcH-1 and CS-73 Are both Clade A Symbiodinium Species.
Restriction fragment length polymorphism (RFLP) analyses have been used to phylogentically group different Symbiodinium species into eight principal Clades denoted A-G (15). RFLP analysis of the small subunit ribosomal DNAs (srDNAs) with TaqI and DpnII showed that both CS-73 and OTcH-1 had restriction patterns characteristic of Clade A (16), as shown for OTcH-1 [supporting information (SI) Fig. 7] (17).
CS-73 Is More Susceptible than OTcH-1 to Thermally Induced Photoinhibition.
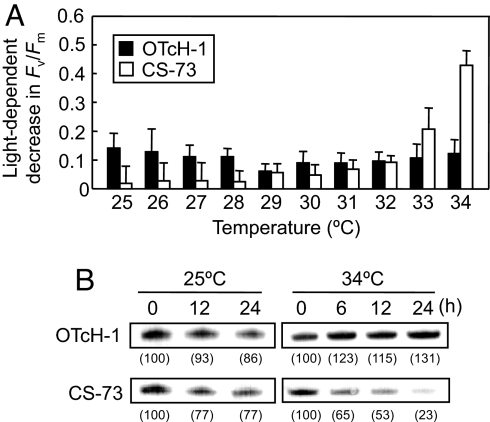
The effect of moderate heat stress on photoinhibition in both Symbiodinium species was examined by measuring the maximum quantum yield of PSII (Fv/Fm). Cultured cells of OTcH-1 and CS-73 grown at 25°C were exposed to temperatures ranging from 25 to 34°C for 3 h with and without illumination. When cells were maintained in darkness, there was little difference in the measured Fv/Fm between the species over the temperature range examined (SI Fig. 8A). However, when illuminated at 200 μmol photons m−2 s−1, the measured Fv/Fm remained unchanged up to 31°C in both species, but declined 95% in the CS-73 cells and 20% in the OTcH-1 cells incubated at 34°C (SI Fig. 8B). Photoinhibition of PSII, measured as the light-dependent decrease in Fv/Fm, was temperature-dependently enhanced in CS-73, but relatively unchanged in OTcH-1 (Fig. 1A). The influence of temperature on the cellular content of the D1 protein, which forms a heterodimer with the D2 protein in the reaction center of PSII, was examined by immunoblot analysis. This showed that in CS-73, but not in OTcH-1, the level of the D1 protein decreased during incubation in light at 34°C (Fig. 1B). These results indicated that CS-73 is much more susceptible to photoinhibition of PSII than OTcH-1 under moderate heat stress.
Fig. 1.
Effect of moderate heat stress on photoinhibition of PSII in OTcH-1 and CS-73. (A) Light-dependent decrease in the maximum quantum yield of PSII (Fv/Fm). The decrease in the Fv/Fm (measured after 15 min of dark adaptation) is shown in the cells illuminated at 200 μmol photons m−2 s−1 for 3 h relative to those maintained in darkness at the indicated temperatures. The values are mean ± SD of three independent experiments. (B) Immunodetection of the D1 protein content in isolated thylakoids from cells illuminated for 0, 12, or 24 h at 25°C or for 0, 6, 12, or 24 h at 34°C. The cellular levels of the D1 protein relative to the 0-h sample are shown in brackets.
CS-73 Is More Susceptible than OTcH-1 to Thermally Induced Photobleaching.
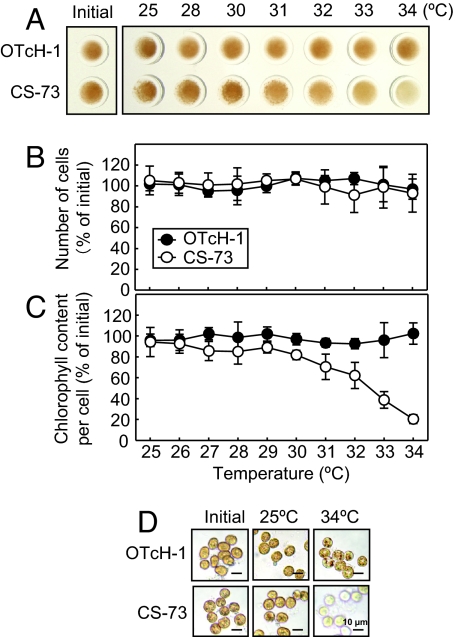
To demonstrate whether moderate heat stress causes photobleaching in OTcH-1 and CS-73, cell number and the chlorophyll content per cell were measured after incubation in light or darkness at temperatures ranging from 25°C to 34°C for 24 h. In the light, increasing levels of photobleaching in the CS-73 cells were visibly evident at a temperature of >31°C, but not for the OTcH-1 cells (Fig. 2A). Cell numbers did not change during the course of the experiments (Fig. 2B). However, the cellular content of chlorophyll a and c2 progressively decreased in CS-73 at temperatures of >31°C while remaining unchanged in OTcH-1 (Fig. 2C). Microscopic examination of the cells before and after incubation at 25°C and 34°C showed little difference in the pigmentation in OTcH-1, whereas significantly less pigmentation was evident in the CS-73 cells treated at 34°C (Fig. 2D). In cells maintained in darkness, there was no thermally induced loss of photosynthetic pigment in CS-73 or OTcH-1 (SI Fig. 9), confirming the temperature-dependent bleaching in CS-73 was light-dependent.
Fig. 2.
Effect of moderate heat stress on photobleaching in the light in OTcH-1 and CS-73. (A–C) Equivalent cell samples (2.5 μg of Chl ml−1) were incubated for 24 h at 200 μmol photons m−2 s−1 at the temperatures shown, and differences in their visual coloration (A), cell number (B), and chlorophyll content measured (C). Initial cell numbers and chlorophyll content per cell in OTcH-1 and CS-73 were 7.9 ± 0.8 × 105 and 14.1 ± 1.3 × 105 cells ml−1 and 3.29 ± 0.41 and 1.76 ± 0.32 pg of Chl a and c2 cell−1, respectively. In B and C, the values are means ± SD of three independent experiments. (D) Light microscope (Axioskop; Zeiss) examination of the OTcH-1 and CS-73 cells after treatment.
Loss of acpPC in CS-73 During Thermal Stress.
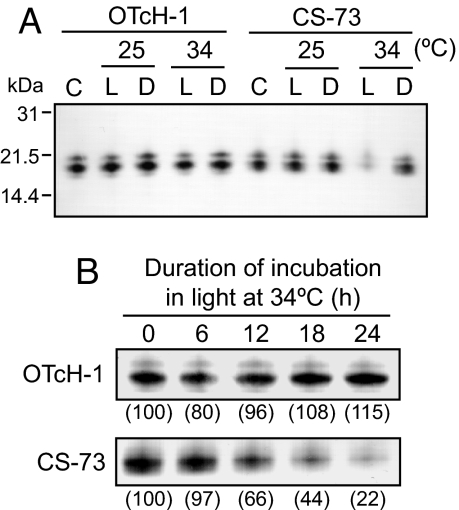
To examine whether photobleaching in CS-73 under moderate heat stress is associated with the loss of the antenna proteins, we examined the influence of temperature on acpPC and PCP levels. When stained with Coomassie blue, both PCP and acpPC were the prominent proteins in the total cellular protein extracts separated by SDS gels, with OTcH-1 producing dimeric-PCP and CS-73 monomeric-PCP (SI Fig. 10). acpPC also was detected by immunoblot analysis by using an antibody against acpPC that recognized three different bands in both OTcH-1 (≈21.5, 19.4, and 17.9 kDa) and CS-73 (≈21.0, 19.9, and 18.9 kDa), suggesting that both Symbiodinium strains have at least three acpPC isoforms, the most prominent being the 19.4- and 19.9-kDa peptides in OTcH-1 and CS-73 (Fig. 3A), respectively, on which we focused. When incubated in light or darkness at 25°C or 34°C for 24 h, there was no change in the level of acpPC protein bands in OTcH-1 cells, but they drastically declined in CS-73 cells incubated in light at 34°C (Fig. 3A). This decline in acpPC became particularly evident after 12 h at 34°C, with <25% of the initial acpPC levels present after 24 h (Fig. 3B). Notably, the variation in the levels of PCP in both CS-73 and OTcH-1 in response to light and 34°C treatment mirrored that of their acpPC (SI Fig. 10), indicating that the thermally induced photobleaching in CS-73 resulted from loss of both acpPC and PCP.
Fig. 3.
Effect of moderate heat stress and light on the level of acpPC. OTcH-1 and CS-73 cells (10 μg of Chl ml−1) were incubated with 200 μmol photons m−2 s−1 (L) or in darkness (D) at 25°C or 34°C for 24 h, and the total cellular protein was separated by SDS/PAGE. (A) Immunodetection of acpPC using antisera against Amphidinium carterae acpPC (loading equivalent to 0.05 μg of Chl of initial cell suspension, C). (B) Comparative levels of acpPC in cells incubated at 34°C, 200 μmol photons m−2 s−1 over time. The cellular levels of acpPC relative to the 0-h sample are shown in brackets.
Degradation of acpPC Is Not Accelerated at 34°C.
Radioactive pulse–chase labeling was used to investigate whether moderate heat stress accelerates the degradation of acpPC in CS-73. OTcH-1 and CS-73 cells were incubated with [35S]methionine/cysteine in the light for 90 min at 25°C and then chased with unlabeled amino acids at 25°C or 34°C (Fig. 4). Over a 24-h period, the level of [35S] label incorporated into acpPC in both OTcH-1 and CS-73 gradually declined to ≈50% of the initial level at both 25°C and 34°C. This indicated that the loss of acpPC at 34°C (Fig. 3) was not attributed to an acceleration of its degradation.
Fig. 4.
Effect of moderate heat stress on the rate of acpPC degradation. The acpPC was pulse-radiolabeled with [35S]methionine/cysteine (10 μCi ml−1) as described in Materials and Methods, and the radiolabel acpPC was chased in the presence of unlabeled 5 mM methionine and 5 mM cysteine during incubation in light at 200 μmol photons m−2 s−1at 25°C or 34°C. (A) Representative autoradiograph signal of acpPC in isolated thylakoid membrane proteins (corresponding to 1.5 μg of Chl in 0-h sample) after SDS/PAGE. (B) Percentage of [35S]acpPC signal relative to 0-h samples from two independent experiments.
Synthesis of acpPC Is Suppressed in CS-73 but Not OTcH-1 at 34°C.
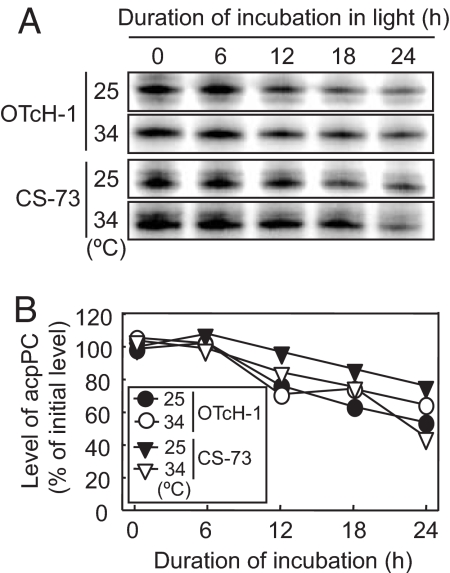
The effect of moderate heat stress on the de novo synthesis of acpPC in OTcH-1 and CS-73 cells was examined by growing the cells at 25°C or 34°C under illumination for 0, 6, 12, or 24 h before incubating with [35S]methionine/cysteine and the relative levels of acpPC incorporated into the thylakoid membranes compared (Fig. 5A). In OTcH-1 and CS-73 cells incubated at 25°C, there was no suppression of acpPC synthesis over the 24-h exposure period. At 34°C, the level of radiolabel incorporation into acpPC at 0 h in both OTcH-1 and CS-73 was greater than at 25°C, indicating that higher temperatures greatly accelerate the de novo synthesis of acpPC in both species. After 6, 12, or 24 h at 34°C, the relative synthesis rate of acpPC in OTcH-1 cells were reduced to ≈70% of the 0-h level, whereas the relative acpPC synthesis rate in CS-73 was greater after 6 h, but was suppressed after 12 h and was almost completely inhibited after 24 h. These results indicate that light-dependent suppression in the de novo synthesis of acpPC occurs under moderate heat stress in CS-73, but to a much lesser extent in OTcH-1.
Fig. 5.
Effect of moderate heat stress on the de novo synthesis of acpPC protein and the relative steady-state level of acpPC mRNA. OTcH-1 and CS-73 cells (10 μg of Chl per 1 ml in 0-h sample) were preincubated at 25°C or 34°C under 200 μmol photons m−2 s−1 for the times shown before incubating with [35S]methionine/cysteine (10 μCi ml−1) for 90 and 30 min at 25°C and 34°C, respectively. (A) Autoradiograph signal of [35S]-labeled acpPC in isolated thylakoid membrane proteins (corresponding to 1.5 μg of Chl in 0-h sample) separated by SDS/PAGE. The levels of acpPC relative to the 0-h sample from two independent experiments are shown in brackets. (B) The level of acpPC transcript level was measured before and after incubation in light at 25°C and 34°C for 24 h and normalized to the ub52 transcript level that remained relatively constant (18). The values are means ± SD (bars) of results from three independent experiments.
The influence of temperature on the steady-state pool of acpPC mRNA was measured by quantitative RT-PCR and normalized to the level of the housekeeping gene ub52 transcript (18). No difference in the initial level of acpPC transcript was found between OTcH-1 and CS-73 (data not shown). Incubating in the light a further 24 h at either 25°C or 34°C resulted in an ≈25% increase in acpPC transcript levels for both temperature treatments in OTcH-1. In CS-73, however, the same treatments produced an ≈10% increase in the acpPC mRNA pool in cells incubated at 25°C, whereas at 34°C it declined by 30% (Fig. 5B). This indicated that there were thermally induced perturbations to acpPC transcript abundance in CS-73, but not in OTcH1.
To examine whether photoinhibition directly contributed to the suppressed de novo synthesis of acpPC in CS-73, the cells were exposed to photoinhibitory light levels for up to 3 h at 25°C and acpPC synthesis measured after 0, 1, 2, and 3 h. Exposure to high light (1,600 μmol photons m−2 s−1) resulted in concurrent reductions in Fv/Fm and the content of D1 protein indicative of PSII photoinhibition (SI Fig. 11 A and B). In contrast, there was no discernable difference in the de novo synthesis of acpPC in the photoinhibited cells (SI Fig. 11C). This result signified that perturbations to de novo synthesis of acpPC were not directly influenced by photoinhibition.
Discussion
Loss of Antenna Proteins Causes Photobleaching.
Exposure to moderate heat stress at temperatures between 31°C and 34°C resulted in photosynthetic pigmentation loss in Symbiodinium strain CS-73 in an illumination-dependent manner (Fig. 2). In contrast, no photobleaching was evident in the Symbiodinium strain OTcH-1 over the same temperature range (Fig. 2). We showed that photobleaching in CS-73 is attributed to the loss of both acpPC (Fig. 3) and monomeric PCP (SI Fig. 10). Therefore, it is likely that thermal stress-induced photobleaching in Symbiodinium is attributed to loss of antenna proteins, in particular acpPC, which has the majority of the light-harvesting pigments associated with it (10, 11). This finding suggests that during coral bleaching events induced by elevated temperatures, the observed photobleaching of in situ Symbiodinium (2–4, 19) is at least partly attributable to the loss of photosynthetic pigments through the loss of antenna proteins.
Inhibition of the de Novo Protein Synthesis Causes Loss of acpPC.
Fig. 6 shows a hypothetical scheme describing the depletion of acpPC during thermal stress. Light exposure damages PSII proteins, including antennae proteins, and its extent is light intensity-dependent (20, 21). Damaged PSII proteins are proteolytically degraded and then replaced by newly synthesized proteins (22). In higher plants, the antenna proteins are specifically degraded by a Zn2+-dependent metalloprotease FtsH6 (23, 24). In Symbiodinium, much less is known about the processes regulating antenna protein levels. However, for acpPC, the balance between the rate of its synthesis and its degradation (occurring either before or after incorporation into the thylakoid membrane) plainly define its content. By monitoring the depletion of acpPC, we found no difference in the degradation rate of the protein at temperatures between 25°C and 34°C in either the thermal-tolerant Symbiodinium OTcH-1 or thermal-sensitive Symbiodinium CS-73. This finding suggests that thermal stress and even its resultant photoinhibition had no influence on the processes responsible for photodamage of acpPC and its degradation (Fig. 4). In contrast, the de novo synthesis of acpPC showed severe inhibition in CS-73 after exposure to thermal stress at 34°C, but not at 25°C, suggesting that the loss of acpPC caused by elevated temperature in CS-73 is attributed to the inhibition of acpPC synthesis (Fig. 5A). In OTcH-1, elevated temperatures caused neither photobleaching (Fig. 2) nor severe inhibition of the de novo synthesis of acpPC (Fig. 5A), signifying that variations in photobleaching heat sensitivity in Symbiodinium species are strongly influenced by differences in the susceptibility of acpPC de novo synthesis to elevated temperatures. Similarly, there is variation in the thermal tolerance of different corals to the onset of photobleaching (and associated algal expulsion from the corals) of their intracellular Symbiodinium. For example, exposure to 34°C for 24 h increased photobleaching and an associated loss of algal cells for the Symbiodinium within Montastrea annularis, but not Siderastrea radians (4). In some cases, this variation in thermal tolerance of the coral to mass bleaching has been shown to depend on the species of the symbiont, for which there can be multiple Symbiodiunium taxa coexisting within the same coral host and may be spatially distributed according to environmental factors such as irradiance level (25–27). Our present results suggest that these differences in the photobleaching sensitivities of corals may be strongly dependent on the thermal stability of antennae protein synthesis in the Symbiodinium symbiont and thus may play an important role in determining the associated phenomenon of mass coral bleaching.
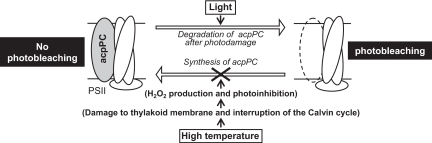
Fig. 6.
A scheme for high temperature-induced photobleaching (loss of acpPC) in heat-sensitive Symbiodinium (see Discussion for details). The depletion of acpPC is primarily caused by an inhibition of protein synthesis that likely follows the elevation of ROS species such as H2O2 in the cell. acpPC, chlorophyll a–chlorophyll c2–peridinin–protein complexes.
How Does Moderate Heat Stress Inhibit the de Novo Synthesis of acpPC in Symbiodinium?
The acpPC is encoded by acpPC gene(s) in the nucleus (28, 29) and synthesized with N-terminal transit peptides directing chloroplast translocation (28–30). Our results demonstrated that the acpPC mRNA pool was reduced by 30% in CS-73, but not OTcH-1, after exposure to thermal stress at 34°C (Fig. 5B), suggesting that thermally induced inhibition of the de novo synthesis of acpPC in CS-73 may be partially attributed to a reduction in acpPC transcript abundance. In the chlorophyte alga, Dunaliella tertiolecta, interruption of photosynthesis by supplying photosynthetic electron transport inhibitors and uncouplers has been shown to stimulate reduction in Lhcb1 mRNA pool (31). Therefore, thermally induced perturbations to acpPC transcript abundance in CS-73 might be attributable to photoinhibition (Fig. 6).
In CS-73, exposure to thermal stress for 24 h reduced acpPC transcript levels by ≈30% (Fig. 5B), whereas the de novo synthesis of acpPC was inhibited by ≈80% (Fig. 5A). This suggests that inhibition of acpPC synthesis primarily occurs at the level of protein translational. However, inhibition of protein synthesis is likely to result from the ensuing effects of temperature on other photosynthetic processes, rather than being the progenitor to temperature sensitivity. In Symbiodinium strains sensitive to thermal stress, enhanced temperature interrupts the Calvin cycle by the inactivation of ribulose-1,5-bisphosphate carboxylase/oxygenase (32, 33) or the impairment of ATP synthesis through damage to the thylakoid membrane (34). Under such conditions, electrons originating from the oxidation of water at PSII are transferred to oxygen at PSII and produce H2O2 via O2− (35, 36). Because H2O2 inhibits the de novo synthesis of PSII proteins at the translation elongation step (37–40), the production of H2O2 might be involved in the inhibition of acpPC mRNA translation in Symbiodinium (Fig. 6). In higher plants, H2O2 has been demonstrated to inhibit the synthesis of chlorophyll at the step of the 5-aminolevulinic acid synthesis (41). Therefore, the inhibition of the synthesis of acpPC also might correlate with inhibition of the synthesis of chlorophylls.
What Determines the Susceptibility of Symbiodinium to Photobleaching Under Thermal Stress?
The present results show that the sensitivity of photobleaching in Symbiodinium to thermal stress differs within the Clade A Symbiodinium strains OTcH-1 and CS-73 (Fig. 2), suggesting that photobleaching susceptibility is independent of Clade association, as was found for the susceptibility to the onset of photoinhibition (34). The photobleaching sensitivity of Symbiodinium species to elevated temperature was consistent with the photoinhibition sensitivity of them to thermal stress (Fig. 1), suggesting that photobleaching and photoinhibition sensitivities are related to a common factor(s). In cultured Symbiodinium, photoinhibition sensitivity to elevated temperature has been demonstrated to be defined by the lipid composition of the thylakoid membrane (34). The differential thermal sensitivities of photoinhibition of Symbiodinium OTcH-1 and CS-73 might be therefore attributed to their thylakoid lipid composition. Thermally sensitive Symbiodinium species, which have a higher content of the major polyunsaturated fatty acid (Δ6,9,12,15-cis-octadecatetrasenoic acid), showed higher photoinhibition sensitivity and H2O2 production under elevated temperature (34). Because photoinhibition and the production of H2O2 have the potential to cause photobleaching through inhibition of the de novo synthesis of antennae proteins, as we have discussed above, the photobleaching sensitivity of Symbiodinium to elevated temperature might be defined by the thermal tolerance of their thylakoid membranes. Our results provide an experimental demonstration that relatively simple measurements of PSII photoinhibition may enable a good prediction of the onset of photobleaching in Symbiodinium in response to thermal stress. Indeed, measuring PSII photoinhibition in Symbiodinium directly in corals in the field might prove useful for gauging the concomitant susceptibility of corals to mass bleaching in response to elevated seawater temperatures.
Materials and Methods
Cultures and Growth Conditions.
Cultures of Symbiodinium spp., OTcH-1 (MBIC11180) (17), and CS-73 (42) were obtained from the Marine Biotechnology Institute Culture Collection (Kamaishi, Japan) and the Commonwealth Scientific and Industrial Research Organisation's microalgae research center (Clayton, South, Australia), respectively. Cells were grown at 25°C in artificial seawater (sea salts; Sigma–Aldrich) containing Daigo's IMK medium for marine microalgae (Wako) under fluorescent lights at 40 μmol photons m−2 s−1. The cells were collected by filtration (0.22-μm Stericup; Millipore) during their midlogarithmic growth phase (<0.5 μg of Chl ml−1) and suspended in fresh growth medium for experiments.
RFLP Analysis.
Genomic DNA was extracted from Symbiodinium by using the DNeasy plant mini-kit (Qiagen) and used to amplify the srDNA as described by using universal primers ss5 and ss3 (16). The PCR products were purified with the QIAquick PCR purification Kit (Qiagen), digested with TaqI or DpnII, and the DNA fragments resolved by electrophoresis in Tris-Acetate-EDTA buffered 2% (wt/vol) agarose gels and visualized by ethidium bromide staining.
Temperature Treatments.
Freshly harvested cells were diluted to 2.5 or 10 μg of Chl per ml and equal volumes incubated at different temperatures in darkness for 30 min before either being illuminated at 200 μmol photons m−2 s−1 or maintained in darkness.
Photoinhibition, Cell Number, and Chlorophyll Measurements.
Maximum quantum yield of PSII (Fv/Fm) was measured with a PAM-2000 chlorophyll fluorometer (Walz) after the cells had been incubated for 15 min in darkness. Cell numbers were counted by using a hemocytometer. The concentration of Chl a and c2 was measured by treating cells collected by centrifugation (16,000 × g) for 1 min with 80% (vol/vol) methanol at 70°C for 10 min. This chlorophyll extraction method was more accurate in determining chlorophyll content than freeze-thawing the cells after storage at −80°C for 24 h and 48 h (SI Fig. 12). Cell debris was removed by centrifugation (as above), and the absorption spectrum of the supernatant was measured by using a diode-array spectrophotometer (Cary 50 Bio; Varian) and the total Chl a and c2 concentration calculated according to ref. 43.
Pulse–Chase Analysis of Protein Turnover.
Cells (10 μg of Chl in 1 ml) were illuminated with 200 μmol photons m−2 s−1 in the presence of 10 μCi ml−1 [35S]methionine/cysteine (Trans[35S] label; MP Biomedicals) for 90 min at 25°C then pelleted by centrifugation (16,000 × g) for 1 min and suspended in fresh growth medium containing unlabeled 5 mM methionine and 5 mM cysteine. The cells were incubated in the light a further 3 h at 25°C to complete protein labeling before incubating at 25°C or 34°C for a further 24 h, during which time equal volumes of cells were collected at different times by centrifugation (16,000 × g) for 1 min, frozen in liquid nitrogen, and stored at −80°C for thylakoid-associated protein analysis (see below).
Pulse Labeling of Proteins.
Equal numbers of cells (10 μg of Chl in 1 ml) were illuminated with 200 μmol photons m−2 s−1 for 0, 12, or 24 h at 25°C or 0, 6, 12, or 24 h at 34°C before adding [35S]methionine/cysteine (10 μCi ml−1) for 90 or 30 min depending on whether the samples were incubated at 25°C or 34°C, respectively. The cells were collected and stored for protein analysis (see below).
SDS Gel Analysis of Total Cellular Proteins.
After the different heat treatments, the cells were collected by centrifugation (16,000 × g) for 1 min, suspended in 50 mM ice-cold Bis-Tris (pH 6.8, 75 μl) containing protease inhibitor mixture (Sigma–Aldrich), and sonicated for 20 s with a W-385 sonicator microtip probe (Heat Systems, 1-s cycle time, 60% duty cycle, 3.5 output control setting; Ultrasonics). The cellular protein was solubilized by diluting 4-fold with LDS sample buffer (Invitrogen) containing NuPAGE-reducing agent (Invitrogen) and incubating at 70°C for 5 min. Cell debris was removed by centrifugation (16,000 × g) for 5 min, and equal volumes of the supernatant (corresponding to 0.05 μg of Chl in the 25°C treatment) were separated by SDS/PAGE (NuPAGE Novex 4–12% Bis-Tris gel; Invitrogen) in Mes-SDS running buffer according to manufacturer specifications (Invitrogen). The separated protein was either visualized by Coomassie blue staining (GelCode blue stain reagent; Pierce) or blotted onto PVDF membrane (see below).
Separation of Thylakoid Membrane Proteins.
Stored cells (see above) were suspended in 1 ml of extraction buffer [50 mM Hepes (pH 7.6), 0.1 M sorbitol, 10 mM NaCl, and 5 mM MgCl2] and lysed by passing through a French pressure cell (SLM Instruments) at 140 MPa. Thylakoid membranes were collected by centrifugation at 16,000 × g for 3 min at 2°C, and the thylakoid-associated proteins were solubilized with LDS sample buffer, reducing agent, and 70°C treatment and then separated by SDS/PAGE as described above.
Protein Blotting and [35S] Detection.
After SDS/PAGE, proteins were blotted onto immobilon PVDF (Millipore) membrane by using a Hoeffer semidry blot apparatus according to supplier specifications. The transferred protein was visualized on the PVDF membrane by Coomassie blue staining before removing with 95% (vol/vol) methanol washes, and the membrane was used for immunoblotting (see below) or exposed to a Storage phosphor screen (Molecular Dynamics). The [35S]-labeled proteins were detected with a PhosphorImager (Molecular Dynamics) and [35S] incorporation was quantified by using Scion Image software (Scion Corporation). To estimate changes in the level of labeled proteins, a dilution series of initial sample was used for calibration.
Immunoblot Analyses.
Antibodies specific to the D1 protein (AgriSera AB) and acpPC from Amphidinium carterae (10) were used to probe the PVDF protein blots as described (44). Immunoreactive peptides were visualized by probing with alkaline-phosphatase-conjugated secondary antibodies and developing with the AP conjugate substrate kit (BioRad). The level of visualized proteins was quantified by using Scion image software. To estimate changes in the level of visualized proteins, a dilution series of initial sample was used for calibration.
Quantitative RT-PCR.
Total cellular RNA was isolated and purified with an RNeasy Plant Mini-Kit (Qiagen). Then 100 μg of RNA was digested with Turbo DNA-free DNase I (Ambion) according to the manufacturer's instructions. Quantitative RT-PCR was performed with acpPC primers (5′- GACTCGGTGCTGTGGAGG-3′ and 5′-CAAGGGGGTCACCGAAGTT-3′) in OTcH-1 and (5′-CAAGGCTATCGAAAAGGCTC-3′ and 5′-CAAGGGGGTCACCGAAGTT-3′) in CS-73 and with ub52 primers (5′-GGGAAGCAACTGGAAGATGG-3′ and 5′-AAAAGTCGCAAGCTGGGTTC-3′) in both OTcH-1 and CS-73 with Real-Time One-Step RNA PCR Kit Ver. 2.0 (Takara) according to the manufacturer's instructions. The following thermal profile was used for PCRs: 42°C for 15 min, 95°C for 2 min, 40 cycles of 95°C for 20 s, and 62°C for 30 s.
Supplementary Material
Acknowledgments.
We thank Dr. Roger G. Hiller (Macquarie University, North Ryde, NSW, Australia) for supplying antibody against acpPC and Dr. David C. Haywood (Australian National University) for providing an acpPC cDNA sequence. This work was supported by the Japan Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad (to S.T.), the Australian Research Council to the Centre of Excellence in Plant Energy Biology (to M.B.), and the Discovery Project DP0450564 (to S.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708554105/DC1.
References
- 1.Niyogi KK. Photoprotection revisited: Genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:333–359. doi: 10.1146/annurev.arplant.50.1.333. [DOI] [PubMed] [Google Scholar]
- 2.Kleppel GS, Dodge RE, Reese CJ. Changes in pigmentation associated with the bleaching of stony corals. Limnol Oceanogr. 1989;34:1331–1335. [Google Scholar]
- 3.Porter JW, Fitt WK, Spero HJ, Rogers CS, White MW. Bleaching in reef corals: Physiological and stable isotopic responses. Proc Natl Acad Sci USA. 1989;86:9342–9346. doi: 10.1073/pnas.86.23.9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warner ME, Fitt WK, Schmidt GW. The effects of elevated temperature on the photosynthetic efficiency of zooxanthellae in hospite from four different species of reef coral: A novel approach. Plant Cell Environ. 1996;19:291–299. [Google Scholar]
- 5.Takahashi S, Nakamura T, Sakamizu M, van Woesik R, Yamasaki H. Repair machinery of symbiotic photosynthesis as the primary target of heat stress for reef-building corals. Plant Cell Physiol. 2004;45:251–255. doi: 10.1093/pcp/pch028. [DOI] [PubMed] [Google Scholar]
- 6.Venn AA, Wilson MA, Trapido-Rosenthal HG, Keely BJ, Douglas AE. The impact of coral bleaching on the pigment profile of the symbiotic alga, Symbiodinium. Plant Cell Environ. 2006;29:2133–2142. doi: 10.1111/j.1365-3040.2006.001587.x. [DOI] [PubMed] [Google Scholar]
- 7.Hoeghguldberg O. Climate change, coral bleaching and the future of the world's coral reefs. Mar Freshwater Res. 1999;50:839–866. [Google Scholar]
- 8.Glynn PW. Coral reef bleaching: Facts, hypotheses and implications. Glob Change Biol. 1996;2:495–509. [Google Scholar]
- 9.Prézelin BB. In: The Biology of Dinoflagellates. Taylor M, editor. Oxford: Blackwell; 1987. pp. 174–223. [Google Scholar]
- 10.Hiller RG, Wrench PM, Gooley AP, Shoebridge G, Breton J. The major intrinsic light-harvesting protein of Amphidinium: Characterization and relation to other light-harvesting proteins. Photochem Photobiol. 1993;57:125–131. doi: 10.1111/j.1751-1097.1993.tb02267.x. [DOI] [PubMed] [Google Scholar]
- 11.Iglesias-Prieto R, Trench RK. Acclimation and adaptation to irradiance in symbiotic dinoflagellates. II. Response of chlorophyll-protein complexes to different photon-flux densities. Marine Biol. 1997;130:23–33. [Google Scholar]
- 12.Aro EM, Virgin I, Anderson B. Photoinhibition of photosystem II: Inactivation, protein damage and turnover. Biochim Biophys Acta. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- 13.Warner ME, Fitt WK, Schmidt GW. Damage to photosystem II in symbiotic dinoflagellates: A determinant of coral bleaching. Proc Natl Acad Sci USA. 1999;96:8007–8012. doi: 10.1073/pnas.96.14.8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krieger-Liszkay A. Singlet oxygen production in photosynthesis. J Exp Bot. 2005;56:337–346. doi: 10.1093/jxb/erh237. [DOI] [PubMed] [Google Scholar]
- 15.Stat M, Carter D, Hoegh-Guldberg O. The evolutionary history of Symbiodinium and scleractinian hosts: Symbiosis, diversity, and the effect of climate change. Perspect Plant Ecol Evol Syst. 2006;8:23–43. [Google Scholar]
- 16.Rowan R, Powers DA. A molecular genetic classification of zooxanthellae and the evolution of animal–algal symbioses. Science. 1991;251:1348–1351. doi: 10.1126/science.251.4999.1348. [DOI] [PubMed] [Google Scholar]
- 17.Ishikura M, et al. Isolation of new Symbiodinium strains from Tridacnid giant clam (Tridacna crocea) and sea slug (Pteraeolidia ianthina) using culture medium containing giant clam tissue homogenate. Mar Biotechnol. 2004;6:378–385. doi: 10.1007/s10126-004-1800-7. [DOI] [PubMed] [Google Scholar]
- 18.ten Lohuis MR, Miller DJ. Light-regulated transcription of genes encoding peridinin chlorophyll a proteins and the major intrinsic light-harvesting complex proteins in the dinoflagellate Amphidinium carterae Hulburt (Dinophycae): Changes in cytosine methylation accompany photoadaptation. Plant Physiol. 1998;117:189–196. doi: 10.1104/pp.117.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lesser MP, Stochaj WR, Tapley DW, Shick JM. Bleaching in coral reef anthozoans: Effects of irradiance, ultraviolet radiation, and temperature on the activities of protective enzymes against active oxygen. Coral Reefs. 1990;8:225–232. [Google Scholar]
- 20.Mattoo AK, Hoffman-Falk H, Marder JB, Edelman M. Regulation of protein metabolism: Coupling of photosynthetic electron transport to in vivo degradation of the rapidly metabolized 32-kilodalton protein of the chloroplast membranes. Proc Natl Acad Sci USA. 1984;81:1380–1384. doi: 10.1073/pnas.81.5.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tyystjärvi E, Aro EM. The rate constant of photoinhibition, measured in lincomycin-treated leaves, is directly proportional to light intensity. Proc Natl Acad Sci USA. 1996;93:2213–2218. doi: 10.1073/pnas.93.5.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakamoto W. Protein degradation machineries in plastids. Annu Rev Plant Biol. 2006;57:599–621. doi: 10.1146/annurev.arplant.57.032905.105401. [DOI] [PubMed] [Google Scholar]
- 23.García-Lorenzo M, Żelisko A, Jackowski G, Funk C. Degradation of the main photosystem II light-harvesting complex. Photochem Photobiol Sci. 2005;4:1065–1071. doi: 10.1039/b506625e. [DOI] [PubMed] [Google Scholar]
- 24.Żelisko A, García-Lorenzo M, Jackowski G, Jansson S, Funk C. AtFtsH6 is involved in the degradation of the light-harvesting complex II during high-light acclimation and senescence. Proc Natl Acad Sci USA. 2005;102:13699–13704. doi: 10.1073/pnas.0503472102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toller WW, Rowan R, Knowlton N. Zooxanthellae of the Montastraea annularis species complex: Patterns of distribution of four taxa of Symbiodinium on different reefs and across depths. Biol Bull. 2001;201:348–359. doi: 10.2307/1543613. [DOI] [PubMed] [Google Scholar]
- 26.Rowan R, Knowlton N. Intraspecific diversity and ecological zonation in coral algal symbiosis. Proc Natl Acad Sci USA. 1995;92:2850–2853. doi: 10.1073/pnas.92.7.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowan R, Knowlton N, Baker A, Jara J. Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature. 1997;388:265–269. doi: 10.1038/40843. [DOI] [PubMed] [Google Scholar]
- 28.Hiller RG, Wrench PM, Sharples FP. The light-harvesting chlorophyll a-c-binding protein of dinoflagellates: A putative polyprotein. FEBS Lett. 1995;363:175–178. doi: 10.1016/0014-5793(95)00297-m. [DOI] [PubMed] [Google Scholar]
- 29.Sharples FP, Wrench PM, Ou KL, Hiller RG. Two distinct forms of the peridinin-chlorophyll a-protein from Amphidinium carterae. Biochim Biophys Acta. 1996;1276:117–123. doi: 10.1016/0005-2728(96)00066-7. [DOI] [PubMed] [Google Scholar]
- 30.Norris BJ, Miller DJ. Nucleotide sequence of a cDNA clone encoding the precursor of the peridinin-chlorophyll a-binding protein from the dinoflagellate Symbiodinium sp. Plant Mol Biol. 1994;24:673–677. doi: 10.1007/BF00023563. [DOI] [PubMed] [Google Scholar]
- 31.Chen YB, Durnford DG, Koblizek M, Falkowski PG. Plastid regulation of Lhcb1 transcription in the chlorophyte alga Dunaliella tertiolecta. Plant Physiol. 2004;136:3737–3750. doi: 10.1104/pp.104.038919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones RJ, Hoegh-Guldberg O, Larkum AWD, Schreiber U. Temperature-induced bleaching of corals begins with impairment of the CO2 fixation mechanism in zooxanthellae. Plant Cell Environ. 1998;21:1219–1230. [Google Scholar]
- 33.Leggat W, Whitney S, Yellowlees D. Is coral bleaching due to the instability of the zooxanthellae dark reactions? Symbiosis. 2004;37:137–153. [Google Scholar]
- 34.Tchernov D, et al. Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc Natl Acad Sci USA. 2004;101:13531–13535. doi: 10.1073/pnas.0402907101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lesser MP. Oxidative stress in marine environments: Biochemistry and physiological ecology. Annu Rev Physiol. 2006;68:253–278. doi: 10.1146/annurev.physiol.68.040104.110001. [DOI] [PubMed] [Google Scholar]
- 36.Asada K. The water–water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- 37.Nishiyama Y, et al. Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J. 2001;20:5587–5594. doi: 10.1093/emboj/20.20.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kojima K, et al. Oxidation of elongation factor G inhibits the synthesis of the D1 protein of photosystem II. Mol Microbiol. 2007;65:936–947. doi: 10.1111/j.1365-2958.2007.05836.x. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi S, Murata N. Interruption of the Calvin cycle inhibits the repair of photosystem II from photodamage. Biochim Biophys Acta. 2005;1708:352–361. doi: 10.1016/j.bbabio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi S, Murata N. Glycerate-3-phosphate, produced by CO2 fixation in the Calvin cycle, is critical for the synthesis of the D1 protein of photosystem II. Biochim Biophys Acta. 2006;1757:198–205. doi: 10.1016/j.bbabio.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Aarti D, Tanaka H, Ito H, Tanaka A. High light inhibits chlorophyll biosynthesis at the level of 5-aminolevulinate synthesis during de-etiolation in cucumber (Cucumis sativus) cotyledons. Photochem Photobiol. 2007;83:171–176. doi: 10.1562/2006-03-06-RA-835. [DOI] [PubMed] [Google Scholar]
- 42.Mansour MP, Volkman JK, Jackson AE, Blackburn SI. The fatty acid and sterol composition of five marine dinoflagellates. J Phycol. 1999;35:710–720. [Google Scholar]
- 43.Jeffrey SW, Humphrey GF. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz. 1975;167:191–194. [Google Scholar]
- 44.Takahashi S, Bauwe H, Badger M. Impairment of the photorespiratory pathway accelerates photoinhibition of photosystem II by suppression of repair process and not acceleration of damage process in Arabidopsis thaliana. Plant Physiol. 2007;144:487–494. doi: 10.1104/pp.107.097253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.