Abstract
Caspase-1 cleaves the inactive IL-1β and IL-18 precursors into active inflammatory cytokines. In Salmonella-infected macrophages, caspase-1 also mediates a pathway of proinflammatory programmed cell death termed “pyroptosis.” We demonstrate active caspase-1 diffusely distributed in the cytoplasm and localized in discrete foci within macrophages responding to either Salmonella infection or intoxication by Bacillus anthracis lethal toxin (LT). Both stimuli triggered caspase-1-dependent lysis in macrophages and dendritic cells. Activation of caspase-1 by LT required binding, uptake, and endosome acidification to mediate translocation of lethal factor (LF) into the host cell cytosol. Catalytically active LF cleaved cytosolic substrates and activated caspase-1 by a mechanism involving proteasome activity and potassium efflux. LT activation of caspase-1 is known to require the inflammasome adapter Nalp1. In contrast, Salmonella infection activated caspase-1 through an independent pathway requiring the inflammasome adapter Ipaf. These distinct mechanisms of caspase-1 activation converged on a common pathway of caspase-1-dependent cell death featuring DNA cleavage, cytokine activation, and, ultimately, cell lysis resulting from the formation of membrane pores between 1.1 and 2.4 nm in diameter and pathological ion fluxes that can be blocked by glycine. These findings demonstrate that distinct activation pathways elicit the conserved cell death effector mechanism of caspase-1-mediated pyroptosis and support the notion that this pathway of proinflammatory programmed cell death is broadly relevant to cell death and inflammation invoked by diverse stimuli.
Keywords: single-cell analysis, apoptosis, inflammasome, inflammation, programmed cell death
The caspase-1 protease causes cell death and cleaves the precursors of IL-1β and IL-18, producing mature inflammatory cytokines. Caspase-1 has emerged as a critical determinant of both pathological inflammation and resistance to infectious diseases. Notably, caspase-1-deficient mice are protected from endotoxic shock (1, 2), ischemic injury (3, 4), and inflammatory bowel disease (5), yet are more susceptible to infection, including salmonellosis (6, 7). Salmonella enterica serovar Typhimurium is a pathogen that invades host macrophages and stimulates caspase-1-dependent cell death (8). Salmonella-infected macrophages produce activated IL-1β and IL-18 and undergo rapid lysis with the release of inflammatory intracellular contents, and thus the term “pyroptosis” is used to describe this form of proinflammatory cell death (9).
Activation of caspase-1 occurs via induced proximity in inflammasomes or pyroptosomes, which are protein complexes analogous to the apoptosis-inducing apoptosome (10, 11). Inflammasomes contain NOD-like receptor (NLR) family proteins, which are cytosolic pattern-recognition receptors stimulated by infectious agents and endogenous danger signals. Salmonella-induced activation of caspase-1 requires the host NLR protein Ipaf, as well as the bacterial type III secretion system (T3SS) and flagellin (10). The NLR protein Nalp3 activates caspase-1 in response to extracellular ATP binding to cell surface P2X7 receptors (12), and Nalp1 is required for the activation of caspase-1 and macrophage death in response to anthrax lethal toxin (LT), a critical virulence factor of Bacillus anthracis (13). LT entry into host cells has been elegantly characterized, yet the mechanism(s) of cytotoxicity are incompletely defined. Further, it is unclear how inflammasomes are regulated or how different stimuli activate caspase-1. Events downstream of caspase-1 activation, other than IL-1β and IL-18 activation, have only recently been described for Salmonella infection (14). Here we demonstrate that LT intoxication and Salmonella infection activate caspase-1 via two distinct pathways, which converge to cause host cell death using an apparently conserved program of caspase-1-mediated pyroptosis. Together with the observations that caspase-1 is involved in a wide variety of pathological conditions, our data support the idea that the proinflammatory programmed cell death pathway of pyroptosis is of broad biological relevance to cell death and inflammation triggered by diverse stimuli.
Results
LT and Salmonella Stimulate Caspase-1-Dependent Death of Macrophages and Dendritic Cells (DCs).
We examined the activation of caspase-1 by staining macrophages with a fluorescent peptide (FAM-YVAD-FMK) that binds specifically and irreversibly to active caspase-1. Whereas mock-infected macrophages have no detectable active caspase-1 (Fig. 1A), those infected with Salmonella contain active caspase-1 in large bright foci and diffusely throughout the cell [Fig. 1B and supporting information (SI) Fig. 9]. The localization of active caspase-1 in LT-treated macrophages was strikingly similar, with discrete brightly staining foci and diffuse active caspase-1 (Fig. 1C). LT has been suggested to stimulate macrophage apoptosis (15). However, a highly sensitive assay did not detect activity of the central apoptotic effector, caspase-3, in LT-treated macrophages (SI Fig. 10 and SI Materials and Methods).
Fig. 1.
Lethal toxin and Salmonella stimulate caspase-1-dependent lysis of macrophages and DCs. (A–C) Macrophages were treated with PBS (A), S. typhimurium (S.T) (B), or LT (C), and active caspase-1 was identified by FAM-YVAD staining (green). Macrophages counterstained with TOPRO-3 (blue) were visualized by confocal fluorescence microscopy. (B and C) Discrete foci of active caspase-1 are indicated by filled arrowheads. (B) Bacteria stained by TOPRO-3 are indicated by open arrowheads. (D and E) Macrophages (MΦ) or DCs were treated with LT or S.T in the presence of the specific caspase-1 inhibitor YVAD or the negative control inhibitor zFA. LDH released by dying cells was quantified; means ± SD are shown. *, P < 0.05 versus medium.
LT and Salmonella are both cytotoxic for macrophages (8, 16), and we confirmed cell lysis by measuring the release of cytosolic lactate dehydrogenase (LDH). LT-intoxicated and Salmonella-infected macrophages underwent caspase-1-dependent lysis that was prevented by the specific caspase-1 inhibitor YVAD (17), but not the negative control inhibitor zFA (Fig. 1D and SI Fig. 11). Caspase-1-independent release of LDH by cells treated with H2O2 was not affected by YVAD or zFA (Fig. 1D). DCs also are targets of LT (18) and Salmonella (19), and lysis of LT-intoxicated and Salmonella-infected DCs was blocked specifically by YVAD (Fig. 1E), indicating caspase-1-dependent death in both cell types.
LT consists of two proteins, protective antigen (PA) and lethal factor (LF). PA binds cell surface receptors, triggering receptor-mediated endocytosis of the toxin complex (20). Endosome acidification causes a conformational change in PA, allowing LF translocation into the host cell cytosol, where it acts as a metalloprotease cleaving several mitogen-activated protein kinase kinases (MEKs), including MEK3 (21). Ammonium chloride (NH4Cl) prevents the acidification of intracellular compartments, traps LF within membrane-bound endosomes (16), and prevents caspase-1 activation in response to LT (Fig. 2A). Caspase-1 specifically cleaves IL-18, and, in macrophages treated with LT, the production of mature IL-18 was inhibited by NH4Cl (Fig. 2B). LT containing catalytically inactive E687C mutant LF did not activate caspase-1 (Fig. 2 A and B). Therefore, although extracellular ATP binding the cell surface P2X7 receptor activates caspase-1 (12), PA interactions with cellular receptors alone are not sufficient to trigger caspase-1 activation. LF translocation from the endosome into the host cell cytosol and LF catalytic activity are both required for LT-induced caspase-1 activation. Similarly, Salmonella entry into macrophages and delivery of stimulatory ligand(s) to the cytosol via the bacterial T3SS are required for cell death and caspase-1 activation (10). In contrast, although acidification of the Salmonella-containing phagosome is necessary for the expression of bacterial virulence genes (22), Salmonella-infected macrophages activated caspase-1 and secreted mature IL-18 in the presence of NH4Cl (Fig. 2 A and B). Thus, we sought to investigate the mechanism of LT-induced caspase-1 activation and cell death, with the aim of testing the possibility that different stimulatory pathways, used by Salmonella and LT, trigger caspase-1 activation and evoke a conserved program causing cell death.
Fig. 2.
LT requires acidification of intracellular compartments and catalytic activity to stimulate caspase-1. Macrophages were treated with PBS, LT, LT containing catalytically inactive E687C mutant LF, or S. typhimurium (S.T) in the presence or absence of 10 mM NH4Cl to inhibit the acidification of intracellular compartments. (A) Macrophages containing active caspase-1 were identified by FAM-YVAD staining; means ± SD are shown. *, P < 0.05 versus LT. (B) Mature IL-18 was detected in supernatants by Western blot.
Caspase-1 Activation by LT Is Ca2+-Dependent.
Ca2+ is involved in LT-induced cytotoxicity because both Ca2+-free culture medium and Ca2+ channel blockers protect macrophages from LT (23), suggesting a role for Ca2+ in caspase-1 activation. Ca2+-free medium and the Ca2+ channel blocker, verapamil, both prevented caspase-1 activation after LT treatment, but not Salmonella infection (Fig. 3A), indicating that Ca2+ is not always involved in caspase-1 activation, but is necessary for the pathway induced by LT. To address the mechanism of this Ca2+ requirement, we examined cleavage of LF substrate, MEK3. Catalytically active LF failed to cleave MEK3 in macrophages treated with NH4Cl (Fig. 3B Upper). However, MEK3 cleavage was restored in macrophages treated with LT and NH4Cl after cellular disruption with detergent (Fig. 3B Lower), consistent with NH4Cl allowing LT uptake, but trapping LF in a detergent-soluble membrane-bound compartment (16). Similarly, verapamil blocked MEK3 proteolysis in LT-treated macrophages (Fig. 3B Upper) unless the cells were treated with detergent to disrupt membrane-bound compartments (Fig. 3B Lower). Therefore, like NH4Cl, verapamil rescues cells from LT-induced caspase-1 activation by interfering with LF translocation to the cytosol perhaps by disrupting the trafficking of intracellular compartments (24). In contrast, Ca2+-free medium completely prevented MEK3 proteolysis in LT-treated macrophages, even in detergent lysates (Fig. 3B). LF added directly to these lysates cleaved MEK3 (Fig. 3B), indicating that extracellular Ca2+ is necessary for the processes leading to significant LT uptake by primary bone marrow-derived macrophages (BMDMs). Interestingly, extracellular Ca2+ is not required for LT uptake by the J774A.1 macrophage-like cell line (23). Therefore, caspase-1 activation by LT requires extracellular Ca2+ to permit LT uptake by BMDM and translocation of catalytically active LF into the cytosol, which is blocked by both NH4Cl and the Ca2+ channel blocker, verapamil. In contrast, Salmonella infection activates caspase-1 independently of extracellular Ca2+ and Ca2+ channels sensitive to verapamil.
Fig. 3.
Calcium is required for caspase-1 activation by LT: target cell interaction and LF translocation into the cytosol. Macrophages were treated with LT or S. typhimurium (S.T) in Ca2+-free medium with 1 mM EDTA or in medium containing 150 μM verapamil, a Ca2+ channel blocker. (A) Macrophages containing active caspase-1 were identified by FAM-YVAD staining; means ± SD are shown. *, P < 0.05 versus medium. (B) Cleaved LF substrate MEK3 (arrow) in LT-intoxicated macrophages was detected by Western blot. Where indicated, macrophages treated with detergent released membrane-enclosed LF and cleaved MEK3 was detected by Western blot in the lysates.
Proteasome Activity and Potassium Efflux Are Required for Caspase-1 Activation by LT Subsequent to LF-Mediated Proteolytic Events.
LT-induced cytotoxicity requires proteasome-mediated protein degradation (25). In addition, proteasome-dependent degradation of the Raf-1 kinase occurs in Salmonella-infected macrophages and has been suggested to be involved in the death of these cells (26). The proteasome inhibitors MG-132 and lactacystin prevented caspase-1 activation (Fig. 4A) and secretion of mature IL-18 (Fig. 4B) in response to LT intoxication, but not Salmonella infection. Neither inhibitor altered proteolysis of the LF target, MEK3 (Fig. 4C), which is consistent with results observed for MEK1 (25). These findings indicate that a proteasome-dependent process mediates caspase-1 activation after LT uptake and LF proteolysis of cytosolic proteins, whereas Salmonella infection activates caspase-1 independently of proteasome activity. A well described function of the proteasome is NF-κB activation, and NF-κB potentiates caspase-1 activation by ATP-mediated P2X7 receptor stimulation (27). The inhibitor Bay 11-7085, which blocks NF-κB activation (27), did not prevent lysis of LT-treated cells (data not shown), indicating that LT-stimulated caspase-1 activation and cell lysis require other proteasome-dependent processes.
Fig. 4.
LT activation of caspase-1 requires proteasome activity subsequent to LF-mediated proteolytic events. (A) Macrophages treated with LT or S. typhimurium (S.T) in the presence of proteasome inhibitors (1 μM MG-132 or 5 μM lactacystin) were examined for active caspase-1 by FAM-YVAD staining; means ± SD are shown. *, P < 0.05 versus medium. (B) Mature IL-18 was detected in supernatants by Western blot. (C) Cleaved MEK3 (arrow) in LT intoxicated macrophages was detected as in Fig. 3.
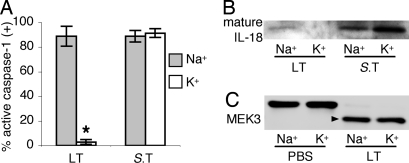
P2X7 receptor ligation stimulates caspase-1 by causing the efflux of intracellular potassium, and caspase-1 activation by this stimulus is prevented in cell culture medium with sodium replaced by potassium to eliminate the gradient for potassium efflux (28, 29). To test the hypothesis that potassium efflux also is required for caspase-1 activation induced by LT intoxication and Salmonella infection, macrophages were treated in medium with sodium replaced by potassium. Elimination of the gradient for potassium efflux selectively prevented caspase-1 activation (Fig. 5A) and secretion of mature IL-18 (Fig. 5B) in response to LT intoxication, but not Salmonella infection. This finding indicates that potassium efflux is not universally required for inflammasome formation or caspase-1 activation, but is differentially involved in caspase-1 stimulation by LT intoxication. Macrophages treated with LT in medium with sodium replaced by potassium contained cleaved MEK3 (Fig. 5C), indicating that the uptake and proteolytic function of LF were not affected. Rather, potassium efflux is required for caspase-1 activation downstream of LF substrate proteolysis.
Fig. 5.
Potassium efflux is required for caspase-1 activation by LT, but is dispensable for pyroptosis induced by Salmonella. Macrophages were treated with LT or S. typhimurium (S.T) in standard medium containing high Na+ or modified medium containing Na+ replaced by K+. (A) Macrophages containing active caspase-1 were identified by FAM-YVAD staining; means ± SD are shown. *, P < 0.05 versus Na+ medium. (B) Mature IL-18 was detected in supernatants by Western blot. (C) Cleaved MEK3 (arrow) in LT-intoxicated macrophages was detected as in Fig. 3.
Pyroptosis Stimulated by LT and Salmonella Features a Common Mechanism of Lysis Mediated by Pore Formation.
We previously described caspase-1-dependent pores in Salmonella-infected macrophages that contribute to the lysis of these cells (14). Plasma membrane pores dissipate cellular ionic gradients, but retain large cytoplasmic constituents, leading to net increased osmotic pressure, water influx, cell swelling, and osmotic lysis (30). Osmotic lysis is prevented by osmoprotectants with molecular diameters greater than the functional diameter of the pores (31), and, importantly, osmoprotection is not observed when irregular membrane damage occurs (32). Osmoprotectants with molecular diameters of ≥2.4 nm (33) rescued both LT-intoxicated and Salmonella-infected macrophages from lysis, whereas molecules <1.1 nm had no effect; none prevented lysis of H2O2-treated controls (Fig. 6A). Osmoprotective PEG 1450 and PEG 2000 did not inhibit caspase-1 activation, demonstrating that their effect is specific for lysis (Fig. 6B). Thus, caspase-1-dependent lysis stimulated by both LT intoxication and Salmonella infection is mediated by a common mechanism involving the formation of plasma membrane pores between 1.1 and 2.4 nm in diameter.
Fig. 6.
Lethal toxin and Salmonella stimulate a common mechanism of lysis mediated by pore formation. (A–C) Macrophages were treated with LT, S. typhimurium (S.T), or 5 mM H2O2 in the presence of osmoprotectants of varying sizes (A and B) or glycine (B and C), which nonspecifically inhibits ion fluxes. (A and C) LDH released by dying cells was quantified; means ± SD are shown. *, P < 0.05 versus medium. (B) Macrophages containing active caspase-1 were identified by FAM-YVAD staining; means ± SD are shown.
Osmotic lysis due to membrane pores results from cellular loss of ionic equilibrium (31), where pathological ion fluxes can be nonspecifically inhibited by the cytoprotective agent glycine (34, 35). Glycine inhibits swelling and lysis of Salmonella-infected macrophages, without preventing formation of plasma membrane pores (14), and glycine also prevented lysis of LT-treated macrophages (Fig. 6C). Caspase-1 activation in response to both LT and Salmonella was unaffected by glycine (Fig. 6B). Together these data indicate that a common pathway of caspase-1-dependent lysis during pyroptosis is mediated by membrane pores between 1.1 and 2.4 nm in diameter and pathological ion fluxes that can be blocked by glycine.
Caspase-1-Dependent DNA Cleavage Is a Common Feature of Pyroptosis Stimulated by LT and Salmonella.
Degradation of chromosomal DNA is a well recognized feature of apoptosis, but also occurs during pyroptosis of Salmonella-infected macrophages (35). Using the TUNEL reaction, we found damaged DNA in LT-treated macrophages (Fig. 7), which was completely prevented by YVAD. Thus, caspase-1-dependent DNA cleavage is an additional common consequence of pyroptosis in response to both LT intoxication and Salmonella infection.
Fig. 7.
Caspase-1-dependent DNA fragmentation occurs in LT-treated macrophages and during Salmonella infection. Macrophages were treated with PBS, LT, or S. typhimurium (S.T) in the presence of the specific caspase-1 inhibitor YVAD or the negative control inhibitor zFA. Macrophages containing damaged DNA were identified by TUNEL staining; means ± SD are shown. *, P < 0.05 versus medium.
Discussion
Our findings describe distinct mechanisms by which anthrax LT intoxication and Salmonella infection activate host cell caspase-1 to cause cell death by converging on the common pathway of caspase-1-mediated pyroptosis. Although exogenous ATP and anthrax LT bind specific macrophage surface receptors and activate caspase-1, activation by LT requires delivery of its metalloproteinase LF subunit into the cytoplasm. Ca2+-dependent uptake of the LT complex precedes LF cytoplasmic translocation, which requires endosome acidification and is inhibited by the Ca2+ channel blocker, verapamil. LF proteolytic activity is necessary, but not sufficient. In addition to cleaving MEKs and possibly other substrate proteins, caspase-1 activation requires proteasome activity and potassium efflux (Fig. 8) and the inflammasome protein Nalp1 (13). Salmonella activates caspase-1 by an independent pathway (Fig. 8) that requires the inflammasome protein Ipaf (10). Caspase-1 activated in response to LT intoxication and Salmonella infection exhibits the same pattern of localization diffusely throughout the cell and in discrete, brightly staining foci. These foci may represent active caspase-1 localized within Ipaf- and Nalp1-containing inflammasomes, respectively. Similar structures containing oligomers of the adaptor protein ASC have recently been visualized and termed “pyroptosomes” (11). Caspase-1 activated by both stimuli mediates a common pathway of caspase-1-dependent cell death, or pyroptosis, featuring DNA cleavage, cytokine activation, and lysis mediated by the formation of membrane pores between 1.1 and 2.4 nm in diameter. Osmotic lysis during pyroptosis is blocked by osmoprotectants and glycine. This process is not limited to macrophages; both LT intoxication and Salmonella infection stimulate caspase-1-dependent lysis of DCs.
Fig. 8.
Lethal toxin and Salmonella use distinct mechanisms to elicit the common pathway of caspase-1-dependent pyroptosis. (Upper) The LT complex consisting of PA and LF is taken up by macrophages in a Ca2+-dependent manner. Endosome acidification, which is blocked by NH4Cl, triggers a conformational change in PA, allowing translocation of LF into the cytosol. The Ca2+ channel blocker verapamil also inhibits LF translocation. In the cytosol, LF proteolytically cleaves MEK and other substrates, after which caspase-1 activation requires proteasome activity, potassium efflux, and the inflammasome protein Nalp1. Salmonella infection stimulates caspase-1 by an independent pathway requiring ligand(s) delivered by the bacterial type III secretion system (T3SS) and the inflammasome protein Ipaf. (Lower) Caspase-1 activated by both stimuli mediates a common pathway of pyroptosis: cell death featuring DNA fragmentation, secretion of activated inflammatory cytokines, and lytic release of inflammatory intracellular contents mediated by the formation of membrane pores between 1.1 and 2.4 nm in diameter. Osmotic lysis during pyroptosis is blocked by osmoprotectants and the cytoprotective agent glycine.
Although Ca2+ and potassium fluxes are necessary for caspase-1 activation in response to some stimuli (11, 36, 37), the ability of Salmonella infection to activate caspase-1 independently of these ion fluxes demonstrates that they are not absolutely required for inflammasome formation as previously hypothesized. However, LT activation of caspase-1 through Nalp1 requires potassium efflux (Fig. 5), and the Nalp3 inflammasome (12) and the ASC pyroptosome (11) also respond to potassium efflux-inducing agents, suggesting that potassium efflux may be a common signal for both Nalp-containing inflammasomes and the pyroptosome. The route of potassium efflux during LT intoxication is unclear. Although potassium efflux can occur through the P2X7 receptor ion channel, a role for this channel in LT intoxication was previously excluded (38).
Our results suggest that it is unlikely that Nalp1 is directly activated by recognition of LF as a ligand because catalytically inactive E687C mutant LF shares a virtually identical structure with active LF (39), but catalytically inactive LF does not activate caspase-1. Additionally, the inhibition of potassium efflux or proteasome activity prevents caspase-1 activation, but not MEK proteolysis, demonstrating that the presence of cytosolic LF and MEK cleavage are not sufficient to stimulate the inflammasome. LF may cleave additional, as yet unknown, targets, leading to the degradation of Nalp1 inhibitors or the production of activating factors that trigger inflammasome function.
Apoptosis was initially described simply based on common morphological features of dying cells observed in different physiological contexts (40). It is now well recognized that apoptosis represents a conserved pathway of cell death stimulated by diverse molecular mechanisms. The extrinsic, intrinsic, and ER stress pathways each includes a plethora of specific signaling mechanisms that all converge on the common outcome of apoptosis (41). Our findings demonstrate that pyroptosis also is a conserved program of cell death occurring in response to distinct signals. In contrast to the noninflammatory outcome of apoptosis, pyroptosis is characterized by activation of the inflammatory cytokines, IL-1β and IL-18, and lysis with release of inflammatory intracellular contents.
In vivo, caspase-1 activation in response to innate immune recognition of microbe-associated patterns helps provide host resistance to infectious pathogens, including Salmonella (6, 7), Shigella (42), Legionella (43, 44), Francisella (45), Listeria (46), and Yersinia (47). Caspase-1 also provides host-protective functions during B. anthracis infection; human DCs and alvelovar macrophages produce IL-1β in response to spore infection (48, 49). IL-1β production in BALB/c mice correlates with early control of bacterial dissemination (50). Correspondingly, LT-deficient mutants exhibit greater initial dissemination after murine inoculation with spores or vegetative bacilli (51). In addition to its protective role in innate immunity, caspase-1 plays a role in the pathogenesis of conditions featuring inflammation and cell death, including neurodegenerative diseases (52), inflammatory bowel disease (5), endotoxic shock (1, 2), myocardial infarction (3, 53), cerebral ischemia (54), and renal injury (4, 55). Therefore, the conserved proinflammatory programmed cell death pathway of pyroptosis is likely to have broad biological significance.
Materials and Methods
Cell Culture.
From BALB/c femur exudates, BMDMs were cultured for 7 days in DMEM and 30% L cell-conditioned medium (14); DCs were cultured with 20 ng/ml GM-CSF (56). Cells were treated with 100 μM YVAD or zFA, 10 mM NH4Cl, 150 μM verapamil, 1 μM MG-132, 5 μM lactacystin, 5 mM glycine, or 60 mM osmoprotectants for 1 h before induction of cell death. Ca2+-free medium contained 1 mM EDTA. K+-substituted medium contained 110.34 mM KCl, 5.8 mM NaCl, 44.1 mM KHCO3, and 0.906 mM KH2PO4; control medium contained 110.34 mM NaCl, 5.8 mM KCl, 44.1 mM NaHCO3, and 0.906 mM NaH2PO4; both contained 1.8 mM CaCl2, 0.248 μM Fe(NO3)3, 0.813 mM MgSO4, 0.4 mM glycine, 25 mM d-glucose, 5 mM Hepes, 0.2 mg·ml−1 l-glutamine, 0.05 mM β-mercaptoethanol, 25× MEM vitamins, 12.5× BME amino acids, and 5% FCS. Cells were treated with 1 μg·ml−1 PA and 1 μg·ml−1 LF or catalytically inactive E687C mutant LF (List Biological) for 2 h unless otherwise indicated. Late-log cultures of S. typhimurium SL1344 grown in L-broth with 0.3 M NaCl were used to infect cells (10:1) for 90 min (35). Release of cytoplasmic LDH was determined from triplicate samples by using the Cytotox 96 kit and calculated as 100 × (experimental LDH − spontaneous LDH)/(maximum LDH − spontaneous LDH).
Fluorescence Microscopy.
Macrophages stained for active caspase-1 (FAM-YVAD-FMK; Immunochemistry Technologies) on glass coverslips were fixed and counterstained with TO-PRO-3. TUNEL staining of damaged DNA was performed as described previously (35), and cells were counterstained with TO-PRO-3. At least 295 cells were examined for each experimental condition by using a Leica SL confocal microscope; percent positive is relative to the total cell population. Images were reduced in size with Adobe Photoshop.
Western Analysis.
To analyze MEK3 cleavage in intact cells treated with LT for 1 h, protein extracts were prepared in SDS sample buffer with 1% Triton X-100. For detergent-treated cells, where MEK3 cleavage is examined in cellular lysates after releasing any active LF trapped in membranous compartments, macrophages treated with LT for 1 h were incubated on ice for 5 min in 1% Triton X-100; adding SDS sample buffer stopped proteolytic activity. For IL-18 immunoblotting, serum-free macrophage supernatant was concentrated by diafiltration. Proteins were separated by SDS/PAGE, transferred to nitrocellulose membranes, and interrogated with anti-MEK-3 antibody or anti-IL-18 antibody (Santa Cruz Biotechnology).
Statistics.
Data were analyzed by unpaired two-tailed Student's t test.
Supplementary Material
Acknowledgments.
We thank Matthew Johnson for technical assistance; Carleen Collins, Ferric Fang, and the Cookson laboratory for critical manuscript review; and the W. M. Keck Center for confocal microscopy support. This work was supported by National Institutes of Health Grants AI47242 and P50 HG02360, Poncin and Achievement Rewards for College Scientist Fellowships (to S.L.F.), and National Institute of General Medical Sciences Public Health Service National Research Service Award Grant T32 GM07270 (to T.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707370105/DC1.
References
- 1.Li P, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 2.Wang W, et al. Endotoxemic acute renal failure is attenuated in caspase-1-deficient mice. Am J Physiol. 2005;288:F997–F1004. doi: 10.1152/ajprenal.00130.2004. [DOI] [PubMed] [Google Scholar]
- 3.Frantz S, et al. Targeted deletion of caspase-1 reduces early mortality and left ventricular dilatation following myocardial infarction. J Mol Cell Cardiol. 2003;35:685–694. doi: 10.1016/s0022-2828(03)00113-5. [DOI] [PubMed] [Google Scholar]
- 4.Melnikov VY, et al. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest. 2001;107:1145–1152. doi: 10.1172/JCI12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegmund B, Lehr HA, Fantuzzi G, Dinarello CA. IL-1 beta-converting enzyme (caspase-1) in intestinal inflammation. Proc Natl Acad Sci USA. 2001;98:13249–13254. doi: 10.1073/pnas.231473998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lara-Tejero M, et al. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J Exp Med. 2006;203:1407–1412. doi: 10.1084/jem.20060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raupach B, Peuschel SK, Monack DM, Zychlinsky A. Caspase-1-mediated activation of interleukin-1beta (IL-1beta) and IL-18 contributes to innate immune defenses against Salmonella enterica serovar Typhimurium infection. Infect Immun. 2006;74:4922–4926. doi: 10.1128/IAI.00417-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hersh D, et al. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: Intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 11.Fernandes-Alnemri T, et al. The pyroptosome: A supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 13.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 14.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 15.Popov SG, et al. Lethal toxin of Bacillus anthracis causes apoptosis of macrophages. Biochem Biophys Res Commun. 2002;293:349–355. doi: 10.1016/S0006-291X(02)00227-9. [DOI] [PubMed] [Google Scholar]
- 16.Friedlander AM. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J Biol Chem. 1986;261:7123–7126. [PubMed] [Google Scholar]
- 17.Garcia-Calvo M, et al. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem. 1998;273:32608–32613. doi: 10.1074/jbc.273.49.32608. [DOI] [PubMed] [Google Scholar]
- 18.Alileche A, Serfass ER, Muehlbauer SM, Porcelli SA, Brojatsch J. Anthrax lethal toxin-mediated killing of human and murine dendritic cells impairs the adaptive immune response. PLoS Pathog. 2005;1:e19. doi: 10.1371/journal.ppat.0010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Velden AW, Velasquez M, Starnbach MN. Salmonella rapidly kill dendritic cells via a caspase-1-dependent mechanism. J Immunol. 2003;171:6742–6749. doi: 10.4049/jimmunol.171.12.6742. [DOI] [PubMed] [Google Scholar]
- 20.Collier RJ, Young JA. Anthrax toxin. Annu Rev Cell Dev Biol. 2003;19:45–70. doi: 10.1146/annurev.cellbio.19.111301.140655. [DOI] [PubMed] [Google Scholar]
- 21.Pellizzari R, Guidi-Rontani C, Vitale G, Mock M, Montecucco C. Anthrax lethal factor cleaves MKK3 in macrophages and inhibits the LPS/IFNgamma-induced release of NO and TNFalpha. FEBS Lett. 1999;462:199–204. doi: 10.1016/s0014-5793(99)01502-1. [DOI] [PubMed] [Google Scholar]
- 22.Alpuche Aranda CM, Swanson JA, Loomis WP, Miller SI. Salmonella typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc Natl Acad Sci USA. 1992;89:10079–10083. doi: 10.1073/pnas.89.21.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatnagar R, Singh Y, Leppla SH, Friedlander AM. Calcium is required for the expression of anthrax lethal toxin activity in the macrophagelike cell line J774A. 1. Infect Immun. 1989;57:2107–2114. doi: 10.1128/iai.57.7.2107-2114.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hay JC. Calcium: A fundamental regulator of intracellular membrane fusion? EMBO Rep. 2007;8:236–240. doi: 10.1038/sj.embor.7400921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang G, Leppla SH. Proteasome activity is required for anthrax lethal toxin to kill macrophages. Infect Immun. 1999;67:3055–3060. doi: 10.1128/iai.67.6.3055-3060.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jesenberger V, et al. Protective role of Raf-1 in Salmonella-induced macrophage apoptosis. J Exp Med. 2001;193:353–364. doi: 10.1084/jem.193.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-kappaB-driven protein synthesis. J Immunol. 2005;175:7611–7622. doi: 10.4049/jimmunol.175.11.7611. [DOI] [PubMed] [Google Scholar]
- 28.Walev I, Reske K, Palmer M, Valeva A, Bhakdi S. Potassium-inhibited processing of IL-1 beta in human monocytes. EMBO J. 1995;14:1607–1614. doi: 10.1002/j.1460-2075.1995.tb07149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perregaux DG, Gabel CA. Human monocyte stimulus-coupled IL-1beta posttranslational processing: Modulation via monovalent cations. Am J Physiol. 1998;275:C1538–C1547. doi: 10.1152/ajpcell.1998.275.6.C1538. [DOI] [PubMed] [Google Scholar]
- 30.Clinkenbeard KD, Thiessen AE. Mechanism of action of Moraxella bovis hemolysin. Infect Immun. 1991;59:1148–1152. doi: 10.1128/iai.59.3.1148-1152.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lobo AL, Welch RA. Identification and assay of RTX family of cytolysins. Methods Enzymol. 1994;235:667–678. doi: 10.1016/0076-6879(94)35180-5. [DOI] [PubMed] [Google Scholar]
- 32.Viboud GI, Bliska JB. Measurement of pore formation by contact-dependent type III protein secretion systems. Methods Enzymol. 2002;358:345–350. doi: 10.1016/s0076-6879(02)58100-3. [DOI] [PubMed] [Google Scholar]
- 33.Scherrer R, Gerhardt P. Molecular sieving by the Bacillus megaterium cell wall and protoplast. J Bacteriol. 1971;107:718–735. doi: 10.1128/jb.107.3.718-735.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frank A, Rauen U, de Groot H. Protection by glycine against hypoxic injury of rat hepatocytes: Inhibition of ion fluxes through nonspecific leaks. J Hepatol. 2000;32:58–66. doi: 10.1016/s0168-8278(00)80190-7. [DOI] [PubMed] [Google Scholar]
- 35.Brennan MA, Cookson BT. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol. 2000;38:31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- 36.Andrei C, et al. Phospholipases C and A2 control lysosome-mediated IL-1 beta secretion: Implications for inflammatory processes. Proc Natl Acad Sci USA. 2004;101:9745–9750. doi: 10.1073/pnas.0308558101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dinarello CA. An IL-1 family member requires caspase-1 processing and signals through the ST2 receptor. Immunity. 2005;23:461–462. doi: 10.1016/j.immuni.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Moayeri M, Wickliffe KE, Wiggins JF, Leppla SH. Oxidized ATP protection against anthrax lethal toxin. Infect Immun. 2006;74:3707–3714. doi: 10.1128/IAI.00051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pannifer AD, et al. Crystal structure of the anthrax lethal factor. Nature. 2001;414:229–233. doi: 10.1038/n35101998. [DOI] [PubMed] [Google Scholar]
- 40.Kerr JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bredesen DE. Key note lecture: Toward a mechanistic taxonomy for cell death programs. Stroke. 2007;38:652–660. doi: 10.1161/01.STR.0000257802.82826.a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sansonetti PJ, et al. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity. 2000;12:581–590. doi: 10.1016/s1074-7613(00)80209-5. [DOI] [PubMed] [Google Scholar]
- 43.Zamboni DS, et al. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol. 2006;7:318–325. doi: 10.1038/ni1305. [DOI] [PubMed] [Google Scholar]
- 44.Molofsky AB, et al. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J Exp Med. 2006;203:1093–1104. doi: 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mariathasan S, Weiss DS, Dixit VM, Monack DM. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med. 2005;202:1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsuji NM, et al. Roles of caspase-1 in Listeria infection in mice. Int Immunol. 2004;16:335–343. doi: 10.1093/intimm/dxh041. [DOI] [PubMed] [Google Scholar]
- 47.Bergsbaken T, Cookson BT. Macrophage activation redirects Yersinia-infected host cell death from apoptosis to Caspase-1-dependent pyroptosis. PLoS Pathog. 2007;3:e161. doi: 10.1371/journal.ppat.0030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chakrabarty K, et al. Bacillus anthracis spores stimulate cytokine and chemokine innate immune responses in human alveolar macrophages through multiple mitogen-activated protein kinase pathways. Infect Immun. 2006;74:4430–4438. doi: 10.1128/IAI.00446-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pickering AK, et al. Cytokine response to infection with Bacillus anthracis spores. Infect Immun. 2004;72:6382–6389. doi: 10.1128/IAI.72.11.6382-6389.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Popov SG, et al. Systemic cytokine response in murine anthrax. Cell Microbiol. 2004;6:225–233. doi: 10.1046/j.1462-5822.2003.00358.x. [DOI] [PubMed] [Google Scholar]
- 51.Heninger S, et al. Toxin-deficient mutants of Bacillus anthracis are lethal in a murine model for pulmonary anthrax. Infect Immun. 2006;74:6067–6074. doi: 10.1128/IAI.00719-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ona VO, et al. Inhibition of caspase-1 slows disease progression in a mouse model of Huntington's disease. Nature. 1999;399:263–267. doi: 10.1038/20446. [DOI] [PubMed] [Google Scholar]
- 53.Pomerantz BJ, Reznikov LL, Harken AH, Dinarello CA. Inhibition of caspase 1 reduces human myocardial ischemic dysfunction via inhibition of IL-18 and IL-1beta. Proc Natl Acad Sci USA. 2001;98:2871–2876. doi: 10.1073/pnas.041611398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schielke GP, Yang GY, Shivers BD, Betz AL. Reduced ischemic brain injury in interleukin-1 beta converting enzyme-deficient mice. J Cereb Blood Flow Metab. 1998;18:180–185. doi: 10.1097/00004647-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 55.Faubel S, et al. Caspase-1-deficient mice are protected against cisplatin-induced apoptosis and acute tubular necrosis. Kidney Int. 2004;66:2202–2213. doi: 10.1111/j.1523-1755.2004.66010.x. [DOI] [PubMed] [Google Scholar]
- 56.Alaniz RC, Cummings LA, Bergman MA, Rassoulian-Barrett SL, Cookson BT. Salmonella typhimurium coordinately regulates FliC location and reduces dendritic cell activation and antigen presentation to CD4+ T cells. J Immunol. 2006;177:3983–3993. doi: 10.4049/jimmunol.177.6.3983. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.