Abstract
Detecting latitudinal range shifts of forest trees in response to recent climate change is difficult because of slow demographic rates and limited dispersal but may be facilitated by spatially compressed climatic zones along elevation gradients in montane environments. We resurveyed forest plots established in 1964 along elevation transects in the Green Mountains (Vermont) to examine whether a shift had occurred in the location of the northern hardwood–boreal forest ecotone (NBE) from 1964 to 2004. We found a 19% increase in dominance of northern hardwoods from 70% in 1964 to 89% in 2004 in the lower half of the NBE. This shift was driven by a decrease (up to 76%) in boreal and increase (up to 16%) in northern hardwood basal area within the lower portions of the ecotone. We used aerial photographs and satellite imagery to estimate a 91- to 119-m upslope shift in the upper limits of the NBE from 1962 to 2005. The upward shift is consistent with regional climatic change during the same period; interpolating climate data to the NBE showed a 1.1°C increase in annual temperature, which would predict a 208-m upslope movement of the ecotone, along with a 34% increase in precipitation. The rapid upward movement of the NBE indicates little inertia to climatically induced range shifts in montane forests; the upslope shift may have been accelerated by high turnover in canopy trees that provided opportunities for ingrowth of lower elevation species. Our results indicate that high-elevation forests may be jeopardized by climate change sooner than anticipated.
Keywords: climate change, range shift
Global climate is currently warming at an unprecedented rate with potentially profound and widespread effects on the distributions of ecological communities. Mean global temperature rose by 0.6°C over the past century, the rate of warming since 1976 has been greater than any other period during the last 1,000 years, and the decade 1990–1999 was the hottest in recorded history (1, 2). Recent climate change has been driven primarily by anthropogenic emissions of greenhouse gases (GHG), and warming is likely to continue at the same or an accelerated rate for the foreseeable future (3–6): global temperatures are predicted to rise by another 1.4–5.8°C by the year 2100 (7). Climate is an important determinant of species' ranges, and rising temperatures associated with GHG emissions are predicted to lead to species' migrations poleward or upward in elevation (8–10). Climate-linked range shifts have already been observed in some taxa (11, 12). Although forest composition and geographic distributions of canopy trees are expected to shift with global warming, it is not clear what level of inertia, or time lag, forests will display to climatic forcing nor how strong the relationship will be between warming and tree line rise (13–15). Historical reconstructions and models of forest response to climate change suggest that the natural pace of tree recruitment and canopy turnover result in century-scale responses of ecotones to climate change, which could make the identification of shifts in forest composition or range in response to recent climate change difficult (16–19). Montane environments provide an ideal environment for detecting shifts in forest distribution in response to climate change because of steep climatic gradients across elevation, which in many respects are analogous to latitudinal climatic gradients, but with distinct boundaries between forest types that facilitate detection of range shifts (Fig. 1) (20, 21).
Fig. 1.
The distinct zonation of northern hardwood and boreal forests with elevation on Mount Abraham in the Green Mountains of Vermont. [Reproduced with permission from ref. 39 (Copyright 2003, American Meteorological Society).]
We used historic vegetation plots and remotely sensed data to examine elevational shifts in the distribution of forests in the Green Mountains of Vermont in conjunction with regional climate change. In northeastern North America, montane forests exhibit distinct elevational zonation, with species' elevational ranges analogous to latitudinal range limits (22–24). The transition from northern hardwoods at lower elevations to boreal forest at upper elevations occurs across a narrow elevational zone [northern hardwood–boreal ecotone (NBE)], where there is a near-complete turnover from northern hardwoods [99.2% of the current basal area below 730 m above sea level (a.s.l.)] to boreal species (100% of the current basal area above 930 m a.s.l.) (Fig. 1). The location of the NBE is expected to be strongly related to local climate across the northeastern United States (24, 25). We examined changes in forest composition in vegetation plots at 11 elevations spaced at 61 m (200 ft) vertical increments from 550 to 1,160 m a.s.l. on the western slopes of three peaks in the Green Mountains of Vermont (Mount Abraham, Bolton Mountain, and Camels Hump), sampled originally in 1964 and then resampled in 2004 (26). We also used aerial photographs (1962 and 1995) and satellite imagery (2005) to estimate the location of the NBE at these time periods. We use these data to address the following questions: Has the location of the NBE shifted over the last half-century? Were these shifts consistent with regional climate change over this period?
Results and Discussion
Within the lower half of the NBE, the boreal species group declined while the northern hardwood group increased in abundance (Fig. 2). Boreal basal area declined by 76% (at 732 m) and 67% (at 792 m) from 1964 to 2004, representing a loss of 5.5 and 9.2 m2 ha−1, respectively. The decline of red spruce (Picea rubens) accounted for 65 and 54% of this loss, respectively, with montane paper birch (Betula papyrifera var. cordifolia) responsible for most of the remaining loss (30 and 56%) (Fig. 3). Balsam fir (Abies balsamea) accounted for 5% of the loss of boreal basal area at 732 m and increased in basal area at 792 m, partially offsetting the losses of red spruce and paper birch. The northern hardwood species group had an overall increase (3%) in basal area across these same elevations, which comprised a 4.8% decline (loss of 1.4 m2 ha−1) at 732 m and a 16% increase at 792 m (gain of 2.9 m2 ha−1) over the past 40 years. The modest increase of northern hardwoods does not reflect the high rate of species turnover occurring at these sites: A 78% (7.1 m2 ha−1) (Fig. 3) loss of American beech (Fagus grandifolia) basal area at 732 m is attributable to the invasion of the beech scale (beech bark disease) in the late 1960s, which has killed 80–90% of mature beech trees (27). The highest-elevation American beech (i.e., 792 m) increased in abundance probably because of the limited susceptibility of young trees at low densities to beech bark disease (27, 28). Declines of American beech in the lower portions of the ecotone were largely offset by large increases in sugar maple (Acer saccharum) basal area of 60% (6.8 m2 ha−1) and 115% (2.6 m2 ha−1) at 732 and 792 m.
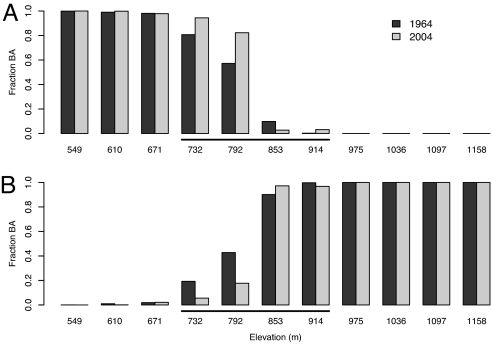
Fig. 2.
Relative composition of forest stands (as fraction of basal area) by northern hardwood (A) and boreal (B) tree species by elevation in 1964 and 2004. These data were collected along elevational transects on three mountains: Bolton Mountain, Camels Hump, and Mount Abraham. The elevational range of the ecotone is indicated by the underscoring. The boreal fraction of forest stands at the two lower elevations of the ecotone decreased from 19% to 6% at 732 m and from 43% to 18% at 792 m, and the deciduous fraction increased from 81% to 94% at 732 m and from 57% to 82% at 792 m.
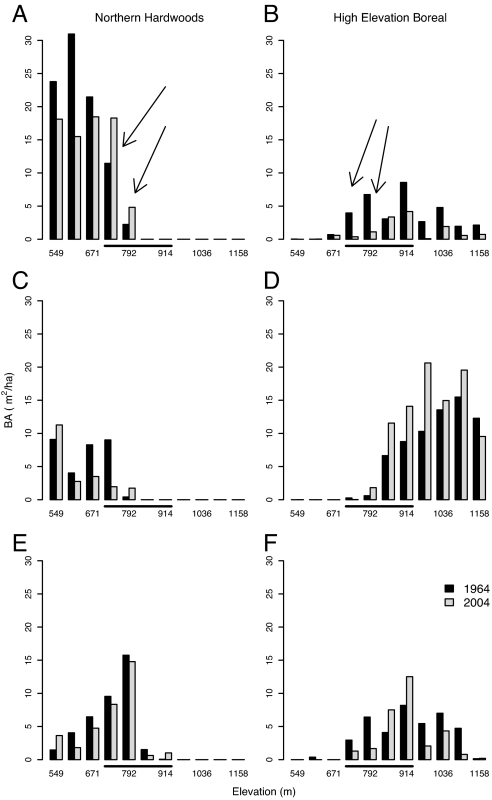
Fig. 3.
Basal area of dominant tree species of northern hardwood (A, C, and E) and boreal (B, D, and F) forests by elevation for 1964 and 2004. Sugar maple (A), red spruce (B), American beech (C), balsam fir (D), yellow birch (E), and paper birch (F). The elevational range of the ecotone is indicated by the underscoring. The shift in the ecotone has been driven both by increases in northern hardwood species at their upper elevation limit (e.g., A, arrows) and decreases in boreal species at their lower limits (e.g., B, arrows).
In the upper half of the NBE, the basal area of the boreal group increased by 62% (at 853 m) and 21% (at 914 m), corresponding to an increase in basal area of 8.6 and 5.3 m2 ha−1. The greater basal area of the boreal group was driven by increases of balsam fir (57% and 55% of increased basal area at 853 and 914 m) and paper birch (40% and 45% of increased basal area at 853 and 914 m) (Fig. 3) after large declines of red spruce in the 1960s and 1970s associated with acid deposition (29, 30). There was an overall increase of 4.8% in northern hardwood basal area in the upper half of the NBE, which resulted from a basal area increase of 0.08 m2 ha−1, but which also included a 15-fold increase in northern hardwood basal area at 914 m. Plots of size structure are consistent with our interpretation of demographic processes: increases in basal area correspond to increases in density of smaller size classes, indicative of recruitment and growth, whereas decreases in basal area correspond to loss of all size classes, with the exception of the root-sucker sprouting response of American beech to the beech bark disease [supporting information (SI) Figs. 6 and 7]. Northern hardwood basal area declined at elevations below the ecotone, primarily driven by decreases in American beech and sugar maple that reflect the effects of beech bark disease and a regional decline in sugar maple (27, 31, 32). At elevations above the ecotone, declines in boreal basal area were driven by large declines in red spruce (71% decline) and paper birch (57% decline), but were partially offset by increased basal area of balsam fir (Fig. 3), which was likely able to capture canopy space opened by mortality of red spruce and paper birch. The decline of the red spruce is likely related to increased winter injury from acidic deposition, which is more severe at higher elevations (33, 34). Paper birch may also be adversely affected by increasing frequency of freeze–thaw events associated with climate change (35). The differential mortality responses of the boreal species emphasize the importance of species' individualistic responses to environmental perturbations and climate change.
We analyzed elevational changes in (log-transformed) basal area separately for each species group (northern hardwood and boreal) over the period 1964 to 2004 by using an information theoretic approach to distinguish between five alternative models of response (36): 1, A null model indicating no effect of elevation or year on basal area; 2, a model with a “year” term that allows for a change in basal area across the two sample periods but no effect of elevation; 3, a model with an “elevation” term that allows for an elevational effect on basal area, e.g., declining abundance as a species group's elevational range limit is approached, but no change in this effect across time; 4, a “year + elevation” model that allows for an effect of both time and elevation on basal area, but without any interaction between the two; 5, last, a “year × elevation” model that is similar to model 4 but also allows for an interaction between “year” and “elevation.” This model accommodates an upslope movement of species groups: a positive interaction term indicates a reduction in the rate of basal area decline with increasing elevation, which would be consistent with an upward shift in the distribution. This final model with the year × elevation interaction was selected for both the northern hardwood and boreal groups, indicating a directional shift in the distribution of each group with respect to elevation, although there was also some support for model 3 (elevation) and model 4 (elevation + year) for the northern hardwood and boreal groups, respectively (Table 1). Estimates of the interaction term for the selected models were positive, indicating an upslope shift in the distributions of both the northern hardwood and boreal groups (Table 2).
Table 1.
Competing models of forest change over the period 1964–2004
| Model | K | Log-like | AICc | ΔAICc |
|---|---|---|---|---|
| Northern hardwood forest | ||||
| Null | 2 | −373.5 | 751.1 | 52.5 |
| Year | 3 | −373.1 | 752.4 | 53.8 |
| Elevation | 3 | −346.7 | 699.6 | 1.0 |
| Elevation + year | 4 | −346.2 | 700.5 | 2.0 |
| Elevation × year | 5 | −344.1 | 698.6 | 0.0 |
| Boreal forest | ||||
| Null | 2 | −339.8 | 683.6 | 89.9 |
| Year | 3 | −339.0 | 684.3 | 90.5 |
| Elevation | 3 | −295.0 | 596.2 | 2.5 |
| Elevation + year | 4 | −293.1 | 594.5 | 0.8 |
| Elevation × year | 5 | −291.6 | 593.7 | 0.0 |
Number of parameters (K), log-likelihood, and model selection results for models fitting log-transformed basal area distributions of northern hardwood and boreal groups.
Table 2.
Parameter estimates of the elevation × year models of log-transformed northern hardwood and boreal basal area
| Parameter | Estimate | SE |
|---|---|---|
| Northern hardwood forest | ||
| Intercept | 8.54 | 0.81 |
| Elevation | −0.0082 | 0.0012 |
| Year | −2.50 | 1.17 |
| Elevation:year | 0.0034 | 0.0017 |
| Boreal forest | ||
| Intercept | −5.31 | 1.09 |
| Elevation | 0.0086 | 0.0012 |
| Year | −3.16 | 1.58 |
| Elevation:year | 0.0030 | 0.0018 |
The positive sign of the elevation:year interaction term for both the boreal and northern hardwood groups indicates that there was an upward shift in basal area from 1964 to 2004 for both forest groups, consistent with an upslope change in their distribution. The sign of the elevation term indicates that basal area decreases with elevation for the northern hardwood component and increases for the boreal component. The negative year effects indicate that basal area declined for both groups from 1964 to 2004, consistent with the effects of insect pathogens and acid rain on these forests (27, 29).
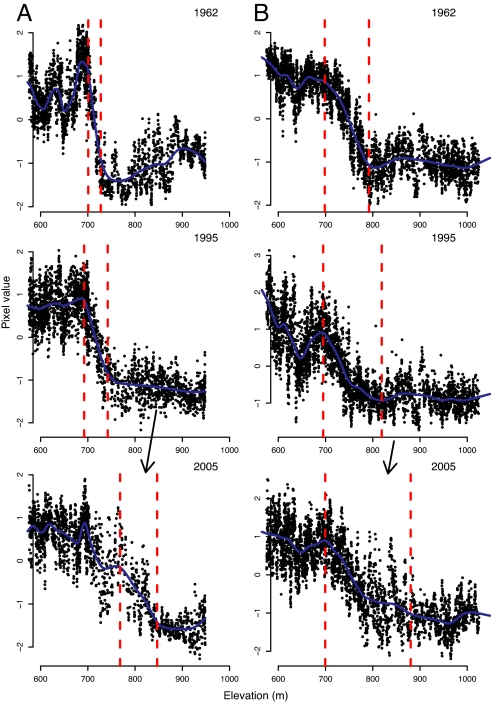
We used remotely sensed data to confirm these ecotonal shifts and to estimate the amount of upslope movement in the northern hardwood to boreal ecotone. We identified the location of the NBE in 1962 (aerial photographs), 1995 (aerial photographs), and 2005 (satellite images). We estimated the upper and lower limits of the ecotones by fitting a two change-point model to pixel values along narrow transects upslope through the NBE on Camels Hump and Mount Abraham (37). Pixel values that are representative of northern hardwoods have higher values than boreal forest, making it possible to distinguish these two groups (Fig. 4). We excluded Bolton Mountain because the extensive presence of snow in the aerial photographs made distinguishing forest types problematic. The first change-point represents the lower limit of the ecotone, whereas the second change-point represents the upper limit. We estimated that the upper limit of the ecotone moved upslope 119 m on Camels Hump and 92 m on Mount Abraham from 1962 to 2005, with most of this upward movement occurring between 1995 and 2005 (104 and 69 m, respectively) (Fig. 4 and Table 3). The lower limit of the ecotone moved upslope an estimated 67 m for Camels Hump, but did not move upslope for Mount Abraham. The pattern of change for Mount Abraham was similar to Camels Hump (i.e., ingrowth of northern hardwoods as indicated by higher pixel numbers), despite the lack of upward shift detected in the lower ecotonal limit (Fig. 4). We expect that continued incremental ingrowth of northern hardwoods in the ecotone on Mount Abraham would result in a rapid shift of the estimated change-point to higher elevations. The differential upslope movement of the upper and lower ecotonal limits has resulted in an increased breadth of the transitions zone (Table 3), which may be indicative of a rapid vegetative response that has not yet reached an equilibrium with climate.
Fig. 4.
Upslope shift of the ecotone between northern hardwood and boreal forest. The location of the ecotone is shown in 1962, 1995, and 2005 for Camels Hump (A) and Mount Abraham (B). The black points represent the normalized pixel values (z scores) from aerial photographs or satellite images for pixels along narrow transects that cross from northern hardwood to boreal forest. Higher pixel values are indicative of northern hardwood forests. Identical transects were examined in all 3 years for each mountain. The blue line represents the smoothed model fit to these data, and the red dashed lines represent the estimated change-point locations, which delineate the ecotone. The arrows in the 2005 graphs indicate elevations where northern hardwood species have moved into former areas of boreal forest.
Table 3.
Estimated locations of northern hardwood to boreal forest ecotone using a change-point model
| 1962 | 1995 | 2005 | |
|---|---|---|---|
| Camels Hump | |||
| Lower limit | 700 (1.1) | 692 (1.0) | 768 (2.4) |
| Upper limit | 727 (1.2) | 742 (5.3) | 846 (4.0) |
| Mount Abraham | |||
| Lower limit | 699 (3.2) | 694 (7.1) | 696 (11.6) |
| Upper limit | 792 (1.2) | 815 (15.1) | 884 (10.9) |
The mean and standard deviation (in parentheses) of lower and upper elevational limits (in meters a.s.l.) of ecotone were estimated from remotely sensed data for Camels Hump and Mount Abraham. We excluded Bolton Mountain from this analysis because high amounts of snow in early photographs made determination of ecotone location difficult.
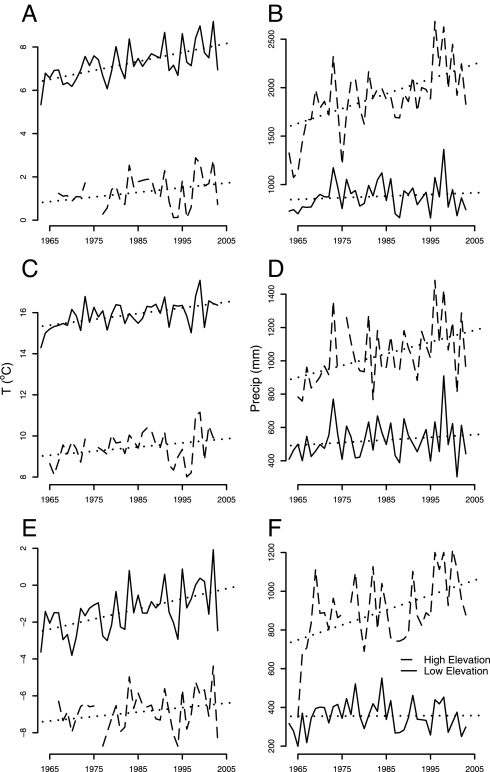
The upward shift in composition and location of the ecotone is consistent with regional trends in climate observed at two nearby climate stations over this 40-year period: a station at Burlington International Airport (100.6 m a.s.l., “low elevation”) and a station at the summit of Mount Mansfield (1,204 m a.s.l., “high elevation”). An annual warming trend of 0.042 and 0.022°C was observed between 1963 and 2003 at the low- and high-elevation stations, respectively (Fig. 5). This resulted in an increase in average annual temperature at the low-elevation station (+1.66°C) that was nearly twice that of the high-elevation station (+0.86°C). Warming trends varied with season at the low-elevation station: “winter” season (i.e., October to March) temperature increases were approximately twice as great as that during the “summer” (i.e., April to September): +1.1°C (summer) vs. +2.2°C (winter) over 40 years. Seasonal differences were much less pronounced at the high-elevation station: +0.80°C (summer) vs. + 0.96°C (winter). Changes in precipitation were also dependent on elevation (Fig. 5). Total annual precipitation increased by 67 mm (8% increase) and 612 mm (38% increase) at the low- and high-elevation stations, respectively, over 40 years. At the low-elevation station, 94% of this increase occurred in summer precipitation, whereas seasonal differences were less pronounced at the high-elevation station: 46% of the increase occurred in summer and 54% in winter. The observed increases in precipitation are consistent with regional predictions of increased precipitation in the northeastern United States with global warming in the Intergovernmental Panel on Climate Change (IPCC) Fourth Assessment Report (38). The large increases in high-elevation precipitation may result from orographic features; this source of uncertainty in regional climate predictions is emphasized in the latest IPCC assessment because of the poor representation of landscape features in climate models (38). We interpolated these climatic changes to the center of the ecotone (830 m): the result was a projected increase of 1.13°C in average yearly temperature, 1.38°C in winter, and 0.91°C in summer, in 2003 compared with 1963. We estimated that annual precipitation in the ecotone increased by 427 mm, with this increase nearly equally divided between winter and summer.
Fig. 5.
Temperature and precipitation at Burlington International Airport (“low elevation”; solid curves) and the summit of Mount Mansfield (“high elevation”; dashed curves) for the period from 1963 to 2003. (A) Monthly mean temperature smoothed with a 12-month running mean. (B) Total annual precipitation. (C) Growing season (April–September) mean temperature. (D) Total growing season (April–September) precipitation. (E) Winter (October–March) mean temperature. (F) Total winter (October–March) precipitation. Precipitation is reported in rainfall equivalent. The dotted lines represent least-squares fitted lines.
We estimated the expected upslope shift in the ecotone based on the temperature lapse rate and the observed temperature rise at the low- and high-elevation stations. We note that that the annual lapse rate (the decrease in temperature per 100-m increase in elevation) increased from 0.49 to 0.58°C from 1963 to 2003 because of the differential warming at the low- and high-elevation stations. The seasonal lapse rates changed from 0.57 to 0.60°C per 100 m in summer and from 0.42 to 0.56°C per 100 m in winter from 1963 to 2003, respectively. We used the more conservative 2003 lapse rates to predict the upslope movement in the ecotone based on observed temperature change interpolated to the ecotonal elevation. The result was an estimated upslope movement of 208 m based on annual temperature change and lapse rates, 164 m upslope based on summer change and lapse rates, and 266 m upslope based on winter change and lapse rates. These upslope shifts are also consistent with rising regional cloud ceilings: The height of cloud ceilings in the northeastern United States has increased at the rate of 6.3 m per year for an expected 250-m rise over our 40-year period (39). This projected rise is significant because cloud ceiling height is thought to be closely associated with the location of the transition between northern hardwood and boreal forests (26, 39). These projected shifts in climatic zones were greater than observed upslope shifts in the NBE: the largest upslope shift estimated from remote sensing data were 119 and 67 m for the upper and lower limits of the ecotone. This suggests that there remains a large disequilibrium between vegetation and climate and that the northern hardwood zone should continue moving up into the boreal zone even in the absence of additional regional warming.
We propose that the upslope movement of the ecotone between northern hardwood and boreal species is the result of climatic change that has promoted the growth and recruitment of northern hardwoods at higher elevations. We suggest that the rapid pace of upslope movement of the NBE resulted from a change in climatic conditions that has favored northern hardwoods that were already present in the lower half of the ecotone at the expense of boreal species. The upward shift of the NBE was accelerated by declines in boreal species, most notably red spruce, which created new recruitment opportunities for northern hardwoods. We expect that the rapid movement of the NBE may be a transient response dependent on individuals of hardwood species already present in boreal stands. In this case, subsequent rates of upslope migration will likely be slower as northern hardwood species expand into areas lacking preestablished individuals. It is unclear whether mortality of red spruce reflects the more general decline associated with acid deposition observed in the 1960s and 1970s (29, 30), is a more direct response to warming (40), or is perhaps an interaction between acid deposition and climate change.
The upward movement of the ecotone is not the result of spatial patterns of canopy disturbance, although the rate of upslope shift was likely accelerated by canopy turnover. The concurrent declines of American beech and sugar maple at lower elevations and red spruce at higher elevations, would ensure a supply of canopy gaps across the ecotone, allowing for downslope movement of boreal species if climatic conditions favored this shift. Species sorting within canopy gaps along the ecotone is sensitive to climate and the movement of northern hardwood species upslope is consistent with more temperate climatic conditions. This accelerated gap-phase mechanism of species turnover allowed for relatively short-term climate change (≈40 years) to lead to detectable changes in forest composition, contrary to models of ecotonal shift (14, 41). In summary, we propose that the rapid upslope shift in the ecotone is the result of climate-driven shifts in the competitive balance between northern hardwood and boreal tree species in the ecotone in conjunction with increased canopy turnover at elevational range limits. This accelerated mode of ecotonal shift is not likely to be unique to northeastern North America: In many mountainous areas, increased severity of mortality agents such as insect outbreaks, pathogens, and air pollution are affecting forest health (42–44). Climate-induced shifts in the wake of increased canopy mortality may allow for more rapid species shifts than without such disturbances, and could threaten high-elevation montane forests sooner than expected.
Methods
Vegetation plots were initially set up in 1964 at elevation intervals of 61 m (200 ft) along transects on the western slope of three of the tallest peaks in the Green Mountains: Mount Abraham (44°07′ N, 72°56′ W, 1,221 m), Bolton Mountain (44°27′ N, 72°50′ W, 1,121 m), and Camels Hump (44°19′ N, 72°53′ W, 1,244 m). The study plots ranged in elevation from 549 to 1,158 m on Mount Abraham and Camels Hump, and from 549 to 1,097 m on Bolton Mountain, because the summit is lower than 1,158 m. Northern hardwood forest occurred in the lower elevation plots below ≈730 m and was dominated by sugar maple (Acer saccharum Marsh.), American beech (Fagus grandifolia Ehrh.), and yellow birch (Betula alleghaniensis Britton). Boreal forest dominated the higher elevation plots above 930 m and was primarily composed of red spruce (Picea rubens Sarg.), balsam fir (Abies balsamea Mill.), and montane paper birch [Betula papyrifera var. cordifolia (Regel) Fern]. The NBE occurs between ≈730 and 930 m (26). Our study areas experienced minimal selective logging (less than ≈700 m) (22, 26, 45), which probably had little effect on species composition (46); all forest stands were in a late-successional stage in 1964 (26, 45). These slopes were initially selected because they share similar exposure, are not affected by secondary ridges, and did not show any recent disturbance (22, 26). At each elevation, 2–10 plots, each 30.5 × 3.05 m (100 × 10 ft), were placed parallel to each other and perpendicular to the contour with 15.2 m between the long axes (22). The species and diameter at 1.37 m above ground [diameter at breast height (dbh)] of all live trees >2 cm dbh were recorded. In the summer of 2004, we relocated the original forest stands and resampled the vegetation at the same elevations by using the same methodology. We relocated the original forest stands through use of original, detailed field notes, maps, and, for Camels Hump, the inclusion of participants from the 1964 study. The study plots were relocated with respect to elevation by using both a handheld global positioning system (GPS) and a barometric altimeter (±3 m). Thus, we are confident that we sampled the target elevations within the same forest stands, if not the original plots. Finally, any errors in relocating the vegetation plots was random with respect to elevation and would not be expected to bias our results toward an upslope shift.
We used remotely sensed data to examine the elevational shift in the ecotone detected in the field plots. Distinguishing montane forest types is simplified by phenological differences between the two primary forest types: Northern hardwood forest is deciduous, whereas high-elevation conifer forest is predominately evergreen (Fig. 1). Black-and-white historical aerial photographs that were taken in early spring, before leaf-out, were available, allowing the separation of these two forest types. We digitized aerial photographs taken in 1962 and 1995, and obtained Quickbird (Satellite Imaging Corporation, Houston, TX) satellite images from 2005 that encompassed all three of our study sites to estimate the change in ecotone position. We examined the study areas in both the aerial photographs and satellite imagery, and orthorectified the images when needed to correct for distortion or shifts from terrain features by using georeferenced control points (>15 per photograph). The result was an orthophoto mosaic of ≈0.5-m resolution for aerial photographs and 0.6 (panchromatic) for the satellite imagery. We used the ratio of red to infrared bands to classify the Quickbird images because they were taken in the fall before leaf-off: this resulted in an image resolution of 3 m, but allowed for easy separation of northern hardwood and boreal components. We then extracted the values of pixels along narrow transects (≈6 m wide) that crossed the NBE on each of our study sites and averaged the pixel values across the width of the transect. The same transects were analyzed across multiple years for each mountain. We excluded Bolton Mountain because the extensive presence of snow in the aerial photographs made distinguishing the forest groups problematic. The pixel values were normalized (centered with unit variance) and the resulting z-scores were analyzed as described below.
We analyzed elevational changes in basal area distributions by using an information-theoretic approach (36). In this analysis, we focused on the six dominant species that comprised 97% of the basal area sampled in 1964 and 2004 combined: sugar maple (36%), American beech (11%), and yellow birch (16%), which are the three dominant species in the northern hardwood forest (“deciduous” species); and red spruce (7%), balsam fir (17%), and montane paper birch (9%), which are the three dominant species at upper elevations (“boreal” species). Inclusion of the other minor components of these forests did not alter our results. We pooled species into deciduous and boreal groups to analyze changes in the NBE (26). Basal area distributions of northern hardwood and boreal groups were log-transformed and then fit by using Gaussian linear models. Combinations of plot and year from which a species group was absent were excluded from that particular model. Model selection was performed by using Akaike's information criterion with a small-sample correction (AICc). This model selection criterion balances model complexity and model fit. The best-fit model has a ΔAICc of 0, and models with a ΔAICc ≤ 2 have some support (36). Data analysis was performed by using the R statistical software package (R Foundation for Statistical Computing, www.r-project.org).
Changes in climate were examined by using daily weather data from two local stations chosen for their proximity to our study sites, for the length and completeness of their records, and because they span the elevation gradient of the Green Mountains: (i) Historical climate network (HCN) (available from http://www.ncdc.noaa.gov/) data from Burlington International Airport, located in the Champlain Valley just to the west of the Green Mountains (100.6 m elevation, 44°28′ N, 73°09′ W); HCN datasets are intended for the study of decade- and century-scale climate trends and have been corrected for changes in station location, time of observation bias, and urban heat effects (47, 48); (ii) National Climatic Data Center data from the summit of Mount Mansfield (1,204.0 m elevation, 44°32′ N, 72°49′ W), located just north of Bolton Mountain. Daily mean temperatures were calculated as the mean of daily maximum and minimum temperature. The daily means were used to calculate monthly means from which annual and seasonal trends were inferred. Precipitation in rainfall equivalent was first summed on a monthly basis from which annual and seasonal trends were calculated. Two seasons were considered: (i) “summer” (April to September) and (ii) “winter” (previous October to March of the current year). Trends in seasonal and annual summaries from both the low- and high-elevation stations from 1963 to 2003 (the 40-year period beginning and ending with the growing season before those during which sampling occurred) were fit by using linear Gaussian models.
We estimated the lower and upper limits of the NBE by using a two change-point model of pixel values (37). The change-point model is a piecewise linear regression with the transition points between adjacent linear regions unknown. We constrained the slope within the first and third regions to be 0 (i.e., horizontal lines), whereas the slope of the second region was unconstrained. We allowed for a single variance across regions. Because adjacent pixel values were likely to be autocorrelated, we accounted for spatial structure by using a Gaussian Markov Random Field (37, 49). We fit these models by using a Bayesian methodology, allowing us to quantify both the uncertainty in the change-point locations and the parameters defining the species abundance by means of probability distributions (50). We estimated the upper and lower ecotone locations in 1962, 1995, and 2005 by using the remotely sensed data described above, and then examined the shift in the ecotonal boundaries across time. A detailed description of this modeling approach is given in ref. 37. Bayesian analyses require a prior distribution over all unknown parameters: Our general strategy was to employ diffuse or noninformative prior distributions, so that final inferences depended solely or almost solely on the data. We fit our models by using Markov Chain Monte Carlo methods (50), programmed in winBUGS (www.mrc-bsu.cam.ac.uk/bugs/). We used a 5,000 burn in, and then generated an additional 200,000 samples that were thinned to 10,000 samples, and assessed convergence of our sampler visually.
Supplementary Material
Acknowledgments.
We thank three anonymous reviewers for their helpful comments. This work was supported by the University of Vermont Agricultural Experiment Station federal formula funds, Project VT-H01113, received from the Cooperative State Research, Education, and Extension Service, U.S. Department of Agriculture.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708921105/DC1.
References
- 1.Mann ME, Bradley RS, Hughes MK. Nature. 1998;392:779–787. [Google Scholar]
- 2.Easterling DR, Evans JL, Groisman PY, Karl TR, Kunkel KE, Ambenje P. Bull Am Meteorol Soc. 2000;81:417–425. [Google Scholar]
- 3.Stott PA, Tett SFB, Jones GS, Allen MR, Mitchell JFB, Jenkins GJ. Science. 2000;290:2133–2137. doi: 10.1126/science.290.5499.2133. [DOI] [PubMed] [Google Scholar]
- 4.Karl TR, Trenberth KE. Science. 2003;302:1719–1723. doi: 10.1126/science.1090228. [DOI] [PubMed] [Google Scholar]
- 5.Hansen J, Nazarenko L, Ruedy R, Sato M, Willis J, Del Genio A, Koch D, Lacis A, Lo K, Menon S, et al. Science. 2005;308:1431–1435. doi: 10.1126/science.1110252. [DOI] [PubMed] [Google Scholar]
- 6.Meehl GA, Washington WM, Collins WD, Arblaster JM, Hu AX, Buja LE, Strand WG, Teng HY. Science. 2005;307:1769–1772. doi: 10.1126/science.1106663. [DOI] [PubMed] [Google Scholar]
- 7.Wigley TML. Science. 2005;307:1766–1769. doi: 10.1126/science.1103934. [DOI] [PubMed] [Google Scholar]
- 8.Kullman L. J Ecol. 2002;90:68–77. [Google Scholar]
- 9.Penuelas J, Boada M. Glob Chang Biol. 2003;9:131–140. [Google Scholar]
- 10.Krajick K. Science. 2004;303:1600–1602. doi: 10.1126/science.303.5664.1600. [DOI] [PubMed] [Google Scholar]
- 11.Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin J-M, Hoegh-Guldberg O, Bairlein F. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- 12.Parmesan C, Yohe G. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 13.Peters RL, Darling JDS. Bioscience. 1985;35:707–717. [Google Scholar]
- 14.Neilson RP. Ecol Appl. 1993;3:385–395. doi: 10.2307/1941907. [DOI] [PubMed] [Google Scholar]
- 15.Daniels LD, Veblen TT. Ecology. 2004;85:1284–1296. [Google Scholar]
- 16.Weinstein DA. In: Landscape Boundaries: Consequences for Biotic Diversity and Ecological Flows. Hansen AJ, di Castri F, editors. New York: Springer; 1992. pp. 379–393. [Google Scholar]
- 17.Starfield AM, Chapin F. S., III Ecol Appl. 1996;6:842–864. [Google Scholar]
- 18.Loehle C. Can J For Res. 2000;30:1632–1645. [Google Scholar]
- 19.Loehle C. Environ Manage. 2003;32:106–115. doi: 10.1007/s00267-003-0017-2. [DOI] [PubMed] [Google Scholar]
- 20.Kupfer JA, Cairns DM. Prog Phys Geogr. 1996;20:253–272. [Google Scholar]
- 21.Diaz HF, Grosjean M, Graumlich L. Clim Change. 2003;59:1–4. [Google Scholar]
- 22.Siccama TG. Department of Botany. Burlington, Vermont: University of Vermont; 1968. p. 396. [Google Scholar]
- 23.Siccama TG, Bliss M, Vogelmann HW. Bulletin of the Torrey Botanical Club. 1982;109:162–168. [Google Scholar]
- 24.Cogbill CV, White PS. Vegetatio. 1991;94:153–175. [Google Scholar]
- 25.Arris LL, Eagleson PS. Water Resour Res. 1994;30:1–9. [Google Scholar]
- 26.Siccama TG. Ecol Monogr. 1974;44:325–349. [Google Scholar]
- 27.Lovett GM, Canham CD, Arthur MA, Weathers KC, Fitzhugh RD. BioScience. 2006;56:395–405. [Google Scholar]
- 28.Griffin JM, Lovett GM, Arthur MA, Weathers KC. Can J For Res. 2003;33:1754–1760. [Google Scholar]
- 29.Vogelmann HW, Badger GJ, Bliss M, Klein RM. Bulletin of the Torrey Botanical Club. 1985;112:274–287. [Google Scholar]
- 30.Vogelmann HW, Perkins TD, Badger GJ, Klein RM. Eur J Forest Pathol. 1988;18:240–249. [Google Scholar]
- 31.Horsley SB, Long RP, Bailey SW, Hallett RA, Wargo PM. North J Appl For. 2002;19:34–44. [Google Scholar]
- 32.Bailey SW, Horsley SB, Long RP, Hallett RA. Soil Sci Soc Am J. 2004;68:243–252. [Google Scholar]
- 33.Schaberg PG, DeHayes DH. In: Responses of Northern U.S. Forests to Environmental Change. Mickler R, Birdsey R, Hom J, editors. New York: Springer; 2000. pp. 181–227. [Google Scholar]
- 34.Lazarus BE, Schaberg PG, Hawley GJ, DeHayes DH. Can J For Res. 2006;36:142–152. [Google Scholar]
- 35.Auclair AND, Lill JT, Revenga C. Water Air Soil Pollut. 1996;91:163–186. [Google Scholar]
- 36.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretical Approach. 2nd Ed. New York: Springer; 2002. [Google Scholar]
- 37.Beckage B, Joseph L, Belisle P, Wolfson D, Platt W. New Phytol. 2007;174:456–467. doi: 10.1111/j.1469-8137.2007.01991.x. [DOI] [PubMed] [Google Scholar]
- 38.Intergovernmental Panel on Climate Change. Climate Change 2007: The Physical Science Basis. Cambridge, UK: Cambridge Univ Press; 2007. [Google Scholar]
- 39.Richardson AD, Denny EG, Siccama TG, Lee X. J Clim. 2003;16:2093–2098. [Google Scholar]
- 40.van Mantgem PJ, Stephenson NL. Ecol Lett. 2007;10:909–916. doi: 10.1111/j.1461-0248.2007.01080.x. [DOI] [PubMed] [Google Scholar]
- 41.Noble IR. Ecol Appl. 1993;3:396–403. doi: 10.2307/1941908. [DOI] [PubMed] [Google Scholar]
- 42.Neuvonen S, Niemela P, Virtanen T. Ecol Bull. 1999;47:63–67. [Google Scholar]
- 43.Rusek J. Ecol Appl. 1993;3:409–416. doi: 10.2307/1941910. [DOI] [PubMed] [Google Scholar]
- 44.Dale VH, Joyce LA, McNulty S, Neilson RP, Ayres MP, Flannigan MD, Hanson PJ, Irland LC, Lugo AE, Peterson CJ, et al. BioScience. 2001;51:723–734. [Google Scholar]
- 45.Whitney HE. PhD dissertation. Burlington, VT: Univ of Vermont; 1988. Disturbance and vegetation change on Camel's Hump, Vermont. [Google Scholar]
- 46.Martin CW, Bailey AS. For Ecol Manage. 1999;123:253–260. [Google Scholar]
- 47.Easterling DR, Karl TR, Lawrimore JH, Del Greco SA. United States Historical Climatology Network Daily Temperature, Precipitation, and Snow Data for 1871–1997. Oak Ridge, TN: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy; 1999. ORNL/CDIAC-118, NDP-070. [Google Scholar]
- 48.Keim BD, Wilson AM, Wake CP, Huntington TG. Geophys Res Lett. 2003;30:1404. [Google Scholar]
- 49.Besag J, Higdon D. J R Stat Soc B. 1999;61:691–717. [Google Scholar]
- 50.Gelman A, Carlin JB, Stern HS, Rubin DB. Bayesian Data Analysis. 2nd Ed. Boca Raton, FL: Chapman & Hall; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.