Abstract
We recently described the direct effects of thyroid-stimulating hormone (TSH) on bone and suggested that the bone loss in hyperthyroidism, hitherto attributed solely to elevated thyroid hormone levels, could at least in part arise from accompanying decrements in serum TSH. Recent studies on both mice and human subjects provide compelling evidence that thyroid hormones and TSH have the opposite effects on the skeleton. Here, we show that TSH, when injected intermittently into rodents, even at intervals of 2 weeks, displays a powerful antiresorptive action in vivo. By virtue of this action, together with the possible anabolic effects shown earlier, TSH both prevents bone loss and restores the lost bone after ovariectomy. Importantly, the osteoclast inhibitory action of TSH persists ex vivo even after therapy is stopped for 4 weeks. This profound and lasting antiresorptive action of TSH is mimicked in cells that genetically overexpress the constitutively active ligand-independent TSH receptor (TSHR). In contrast, loss of function of a mutant TSHR (Pro → Leu at 556) in congenital hypothyroid mice activates osteoclast differentiation, confirming once again our premise that TSHRs have a critical role in regulating bone remodeling.
Keywords: osteoclast, osteoporosis, pituitary, osteoblast, bisphosphonate
Clinical data available since von Recklinghausen's first description of thyrotoxic bone disease, the strong correlation between fracture risk and serum thyroid-stimulating hormone (TSH), recent evidence that TSH receptor (TSHR) polymorphisms are associated with low bone mass, and evidence that bone loss occurs in patients with subclinical hyperthyroidism with normal and low TSH levels all support a role for low TSH in the pathogenesis of hyperthyroid osteoporosis, hitherto attributed solely to high circulating levels of thyroid hormones (1–4).
Evidence supporting this premise comes in parallel from genetically manipulated mice lacking receptors for either TSH or thyroid hormones (5, 6). We demonstrated that TSHR deficiency induces a high-turnover osteoporosis, with elevated bone formation and resorption even in TSHR haploinsufficient mice that have normal thyroid function. We found that in ex vivo cell cultures continually exposed to TSH, both osteoblastic bone formation and osteoclastic bone resorption were suppressed. Although these studies point to a primary function for TSH in skeletal homeostasis, data have reestablished a role for the two thyroid hormone receptor (TR) isoforms, TRα and TRβ (6). The deficiency of TRα induces osteosclerosis in adult mice, whereas hyperthyroid TRβ-null mice display osteopenia despite high circulating TSH levels (6). Thus, evidence from human and mouse studies underscore the paradigm that both thyroid hormone excess and low TSH levels contribute to hyperthyroid bone loss (7).
Hyperthyroidism can arise from adenomatous or inflammatory diseases of the thyroid or from overzealous replacement with thyroid hormones. Hyperthyroidism is ≈10-fold more common in women, with an incidence of 1 in 1,000 women per year. Also, >10% of postmenopausal women in the United States receive thyroid hormone replacement therapy, and ≤20% of these women are overreplaced, inducing subclinical hyperthyroidism (8). One in two women over the age of 50 also has postmenopausal osteoporosis, with a fracture incidence that far outweighs the combined incidence of breast cancer, heart disease, and stroke (9). Thus, there is considerable overlap in the postmenopausal population suffering from hyperthyroidism and osteoporosis. Because intermittent TSH has been shown to have antiresorptive actions in vivo (10), it is possible that postmenopausal women on thyroid hormone therapy with suppressed TSH levels may benefit from recombinant human TSH (rhTSH) therapy. A robust skeletal response to intermittent rhTSH is not unexpected particularly because parathyroid hormone (PTH), although proresorptive at high circulating levels, is profoundly anabolic when administered intermittently (11).
In this study, we have examined the effect of TSH administered in varying regimens between three times a week (thrice per week) and once every 1 or 2 weeks (once per week, once per 2 weeks) to ovariectomized rats or mice. Administered to rats at the three stated regimens, TSH caused a reversal of ovariectomy-induced bone loss, as well as increased bone strength in a dose-dependent manner. In mice, TSH prevented the fall in bone mass 6 and 8 weeks postovariectomy. That the in vivo effects were mainly due to sustained antiresorptive actions of TSH was testified by significant and persistent decrements in osteoclast differentiation ex vivo 4 weeks after cessation of TSH. Consistent with these and our previous in vitro data (5, 12), the transgenic overexpression of native TSHR or constitutively active TSHR (caTSHR) in osteoclasts under a tartrate-resistant acid phosphatase (TRAP) promoter substantially inhibited osteoclastogenesis. In line with this antiresorptive action, the presence of a signaling-deficient TSHR in hypothyroid (hyt) mice was associated with stimulated osteoclastogenesis. Overall, the data suggest that TSH injected intermittently as far apart as every 2 weeks protects against bone loss by inhibiting osteoclastic resorption.
Results
Intermittently Administered Rat TSH Restores Ovariectomy-Induced Bone Loss and Improves Bone Strength in Rats.
One of our groups has shown recently that native rat TSH administered thrice per week to Sprague–Dawley rats 7 months postovariectomy caused a significant dose-dependent increase in areal bone mineral density (BMD), cortical thickness, and trabecular bone volume, number, and thickness (10). Providing evidence for an effect of intermittent TSH administration on the skeleton in vivo, this study also confirmed our in vitro studies showing direct effects of TSH on osteoclastogenesis (5, 10). Furthermore, because rat TSH did not increase serum thyroid hormone levels (10), this further confirmed that the effect of TSH on the skeleton was independent of the thyroid axis.
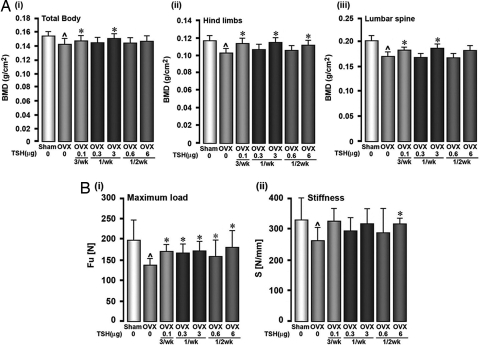
The present study was designed to explore whether the intervals between two TSH doses could be extended even further. Rats were used because cancellous bone remodeling is high postovariectomy, and lower concentrations of an osteoclast inhibitor can be used to demonstrate effects. We compared the thrice per week rat TSH regimen to once per week and once per 2 weeks. The dose for any given extended interval regimen was the same as the sum of the doses given individually over that period. For example, if 0.1 μg were given thrice per week, the cumulative dose for the once per 2 weeks regimen was 0.6 μg. The BMD measurements performed at 8 weeks after commencement of therapy showed significant increases in BMD at the total body, lumbar spine, and hind limbs, compared with ovariectomized controls. Although this increase was significant at 0.1 μg thrice per week and 3 μg once per week at all sites, the 6 μg once per 2 weeks dose increased BMD significantly only at the hind limbs (Fig. 1A).
Fig. 1.
TSH restores ovariectomy-induced bone loss. (A and B) BMD (A) and bone strength (B) declines over 28 weeks due to ovariectomy of Sprague–Dawley rats (4–6 months old) are restored with purified rat TSH injected at the stated doses three times a week (3/wk), once a week (1/wk), and once every two weeks (1/2 wk). Bone strength parameters include maximum load and stiffness (units as stated). Data are shown as mean ± SEM (n = 12 rats per group). P values calculated by ANOVA Dunett test comparing treatment versus no treatment in ovariectomized rats (*, P < 0.05) or ovariectomy versus sham control (⋀, P < 0.05).
In parallel experiments, bones dissected from the rats at 16 weeks were subject to a three-point bending test to examine for changes in biomechanical properties, namely maximum load and stiffness, as well as to microcomputed tomography (μCT) to evaluate changes in trabecular and cortical parameters. Maximal load, an excellent surrogate of bone quality, was decreased expectedly after ovariectomy, but these decrements were restored with all treatment regimens (Fig. 1Bi). Stiffness remained unchanged with all treatments (Fig. 1Bii). Interestingly, bone strength was restored at lower doses (e.g., 0.3 μg once per week or 0.6 μg once per 2 weeks) that did not fully restore the decrements in BMD. This finding is not unexpected because enhancements in bone strength and reductions in fracture risk can occur in the absence of large BMD changes (13).
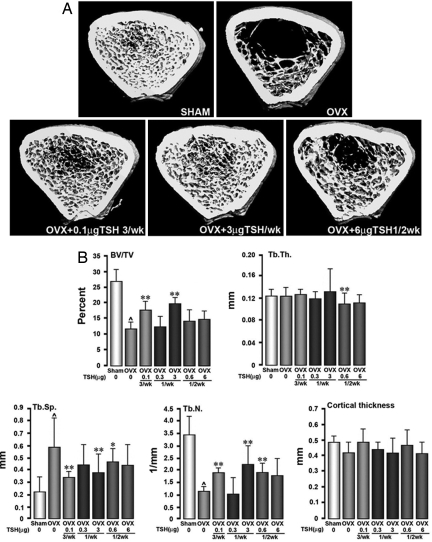
Representative μCT images showing both cortical and trabecular bone in the tibial diaphysis of rats are shown in Fig. 2A. Fig. 2B shows that ovariectomy profoundly reduced bone volume (BV/TV) and trabecular number (Tb.N) and consequently increased trabecular spacing (Tb.Sp), but without affecting trabecular thickness (Tb.Th). Rat TSH at 0.1 μg thrice per week, 3 μg once per week, and 0.6 μg once per 2 weeks significantly elevated Tb.N and reduced Tb.Sp, compared with ovariectomized controls. Surprisingly, observed increases and decreases in Tb.N and Tb.Sp, respectively, with 6 μg once per 2 weeks did not achieve statistical significance. Importantly, cortical bone remained unaffected with all of the treatment regimens. Overall, therefore, when injected intermittently, rat TSH restores ovariectomy-induced bone loss and architectural deterioration, hence improving bone strength.
Fig. 2.
Loss of trabecular bone structure over 28 weeks due to ovariectomy of Sprague–Dawley rats (4–6 months), measured by μCT, is restored with purified rat TSH injected at stated doses of three times a week (3/wk), once a week (1/wk), and once every 2 weeks (1/2 wk). (A) Representative micrographs. (B) Measured parameters include BV/TV, Tb.Th, Tb.N, Tb.Sp, and cortical thickness. Data are shown as mean ± SD (n = 12 rats per group). P values calculated by ANOVA Dunett's test comparing treatment versus no treatment in ovariectomized rats (*, P < 0.05; **, P < 0.01) or ovariectomy versus sham control (⋀, P < 0.01).
Intermittently Administered hTSH Prevents Ovariectomy-Induced Bone Loss in Mice.
We next used ovariectomized mice to (i) determine whether, in addition to restoring bone mass, rhTSH could prevent ovariectomy-induced bone loss; (ii) examine the time dependence of the rhTSH effects; (iii) study whether the effects of rhTSH persisted, despite a treatment-free interval; and (iv) evaluate the properties of rhTSH-exposed osteoclasts in ex vivo cultures.
Groups of sham-operated and ovariectomized mice (8–10 mice per group) were injected thrice per week with vehicle, one of two doses of rhTSH (0.07 or 0.7 μg), or one dose of PTH (2.4 μg). Unlike the restoration protocol in rats, in the mouse, injections were initiated immediately after ovariectomy or sham operation and continued for 8 weeks. Pellets containing 0.56 μg β-estradiol also were implanted in groups of ovariectomized and sham-operated mice. The injections were stopped at 8 weeks, and the mice were given a further 4-week treatment-free period before final BMD measurements, killing, and bone marrow isolation for ex vivo cultures. BMD measurements at the total body, lumbar spine (L2–L4), and femur were made at times 0, 6, 8, and 12 weeks.
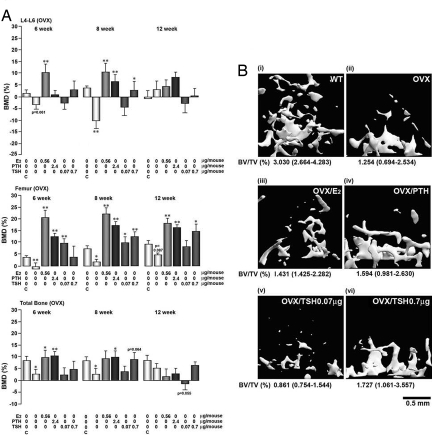
Fig. 3A shows a significant BMD decline at the lumbar spine, femur, and total body at both 6 and 8 weeks when ovariectomized mice were compared with respective sham-operated controls. Further comparisons were made at each time point between treatment and zero-dose controls. With estrogen and PTH (positive controls), the ovariectomy-induced BMD loss at all sites was prevented at 6 and 8 weeks, with the exception of the lumbar spine response to PTH at 6 weeks. With the lowest rhTSH dose (0.07 μg thrice per week), there was a prevention of ovariectomy-induced bone loss at the femur at 6 and 8 weeks; no significant differences with respect to zero dose were noted at other sites. At 0.7 μg rhTSH thrice per week, however, broadly similar responses to PTH and estrogen were observed. Thus, the BMD decline was reversed at 8 weeks at the lumbar spine and femur, with a marginally significant effect (P = 0.064) at the total body. No changes in serum T4 levels were noted with any treatment at 8 weeks, compared with basal levels (Table 1).
Fig. 3.
TSH prevents ovariectomy-induced bone loss. (A and B) BMD declines (A) and trabecular loss (B) due to ovariectomy (OVX) of mice (12 weeks old) are restored with rhTSH, (thyrogen, doses as stated) injected three times a week, rhPTH injected three times a week, or the s.c. implantation of estrogen pellets (E2). Treatments with rhTSH or rhPTH ended at 8 weeks, after which the mice were given a 4-week treatment-free period with the exception of E2. Bones were harvested for μCT and bone marrow cultures at 12 weeks after BMD measurements (see Fig. 4A). (B) μCT images of trabecular bone at the distal femoral metaphysis, together with estimates of BV/TV. Data are shown as mean ± SEM (n = 8–10 mice per group). The P values are calculated by unpaired Student's t test comparing treatment versus no treatment in ovariectomized mice (*, P < 0.05; **, P < 0.01) or ovariectomy versus sham control (⋀, P < 0.01). Median value and ranges are given for BV/TV.
Table 1.
Effect of recombinant human TSH administered to mice three times a week on serum thyroxine levels
| Serum T4 (μg/dl) | 1 week | P | n | 8 week | P | n |
|---|---|---|---|---|---|---|
| OVX-buffer | 1.87 ± 0.134 | 0.06 | 8 | 1.46 ± 0.199 | 0.19 | 6 |
| OVX-E2 | 1.52 ± 0.118 | 0.45 | 9 | 1.60 ± 0.09 | 0.25 | 9 |
| OVX-PTH | 1.64 ± 0.142 | 0.13 | 8 | 1.71 ± 0.131 | 0.18 | 7 |
| OVX-TSH (0.07 μg) | 2.03 ± 0.166 | 0.23 | 8 | 1.95 ± 0.22 | 0.07 | 7 |
| OVX-TSH (0.7 μg) | 1.59 ± 0.169 | 0.09 | 8 | 1.48 ± 0.137 | 0.38 | 6 |
Serum thyroxine (T4) levels measured at 1 and 8 weeks in sham-operated or ovariectomized (OVX) mice that received recombinant human thyroid-stimulating hormone (TSH, doses as stated) thrice per week, recombinant human parathyroid hormone (PTH, 2.4 μg) thrice per week, or estrogen pellets (E2). Statistics shown as mean ± SEM, Student's ttest compared mean serum T4 of each treatment to OVX-buffer; Pvalues and number (n) as stated. There also was no difference between sham-operated control (serum T4 = 1.50 ± 0.092 mg/dl) and OVX (above).
We next examined the effect of the 4-week treatment-free interval on the persistence of the BMD responses to the various treatment protocols. No significant differences between the treatment and zero-dose groups were noted at the lumbar spine and total body at 12 weeks. Importantly, however, the responses to estrogen, PTH, and the 0.7 μg rhTSH dose were sustained even after the treatment-free interval. Fig. 3B presents representative μCT images of trabecular bone at 12 weeks: The profound reduction in BV/TV with ovariectomy was reversed with PTH, estrogen, and 0.7 μg rhTSH thrice per week.
Both Intermittent and Continuous TSHR Activation Induce Osteoclastic Inhibition.
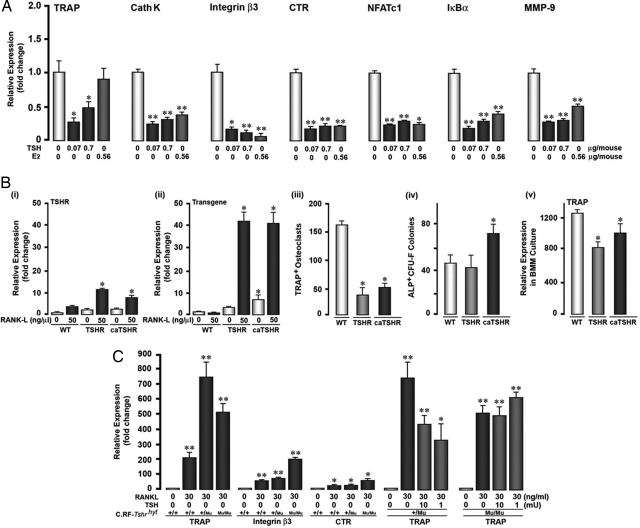
We have shown previously that sustained TSH application in vitro inhibits osteoclast formation and the expression of genes critical to osteoclastogenesis (5, 12). Additionally, we have shown that retroviral infection of cloned RAW-C3 cells with the native or caTSHR attenuates osteoclast differentiation (12). Here we examine whether rhTSH, given thrice per week for 8 weeks, followed by a treatment-free period of 4 weeks, can persistently inhibit osteoclast differentiation. Thus, we studied the expression of gene markers, namely TRAP, cathepsin K, integrin β3, metalloproteinase-9, NFATc1, and Iκ-Bα, in bone marrow-derived osteoclast precursors by real-time PCR after a 5-day culture with the osteoclastogenic cytokines RANK-L and M-CSF.
Cells isolated from wild-type mice treated previously with 0.07 or 0.7 μg rhTSH showed strong and significant attenuations in the expression of all seven genes despite a 4-week treatment-free period (Fig. 4A). Sustained estrogen treatment, used as a positive control, likewise suppressed all genes but TRAP. Prior intermittent PTH treatment expectedly failed to suppress gene expression (data not shown).
Fig. 4.
Gain and loss of function of TSHR results in opposite effects on osteoclast differentiation in ex vivo cultures. (A) Effect of TSH (doses as stated) injected three times a week for 8 weeks or the s.c. implantation of estrogen pellets (E2) for 12 weeks on the expression of the osteoclast differentiation markers TRAP, cathepsin K (CathK), integrin β3, calcitonin receptors (CTR), NFATc1, IκBα, and metalloproteinase-9 (MMP-9) after a TSH-free period of 4 weeks. (B) Endogenous murine TSHR expression (i), transgenic human TSHR expression (ii), TRAP-positive (TRAP+) cell formation (iii), alkaline-phosphatase-positive CFU-f (ALP+ CFU-f) numbers (iv), and TRAP mRNA expression (v) in bone marrow-derived hematopoetic stem cells (i, ii, iii, and v) or stromal cells (iv) obtained from mice transgenically expressing either hTSHR or caTSHR. (C) TRAP, integrin β3, and CTR mRNA expression in hematopoetic stem cells isolated from wild-type (+/+), heterozygotic (+/mu), or homozygotic (mu/mu) hyt mice that were exposed to RANK-L and/or TSH (as shown). Data are shown as mean ± SEM. The P values were calculated by unpaired Student's t test comparing treatment versus zero-dose control or wild-type cells as appropriate (*, P < 0.05; **, P < 0.01).
Gain- and loss-of-function experiments using genetic strategies further demonstrated that TSH effects on the osteoclast were specific and profound. We tested the hypothesis that genetically induced gain of function of the TSHR will suppress osteoclast differentiation, whereas loss of function will enhance it. Bone marrow cells were isolated from transgenic mice that overexpress the human TSHR or caTSHR in osteoclasts under a TRAP promoter. We confirmed that RANK-L induced the expression of endogenous mouse TSHR, as well as of both transgenes (Fig. 4 Bi and Bii). The culture of transgenic osteoclast precursors with RANK-L and M-CSF revealed a significant dampening of osteoclastogenesis compared with wild-type cells, whereas CFU-F (early osteoblast) formation was stimulated only with caTSHR (Fig. 4 Biii and Biv). The antiosteoclastogenic effect was paralleled by reduced TRAP mRNA expression, which also was consistent with that seen with intermittent rhTSH injection (cf. Fig. 4A). The antiresorptive actions of intermittent and continuous TSHR activation were therefore, in essence, similar.
For complementary loss-of-function studies, osteoclast gene expression was similarly studied in precursors isolated from the hyt mouse, which bears an inactivating mutation in the TSHR gene, rendering the receptor signaling-deficient and, hence, functionless. Fig. 4C shows that the osteoclastic expression of TRAP, integrin β3, and calcitonin receptor was dramatically up-regulated in both haploinsufficient and homozygous hyt mice. That this enhancement arose specifically from TSHR inactivation was verified by showing that rhTSH reversed this stimulation in heterozygotic hyt osteoclasts, but not in homozygotic cells.
Discussion
Here, we provide direct evidence that TSH given thrice per week or over intervals as widely spaced as 2 weeks can restore the lost bone that follows ovariectomy, apart from, importantly, restoring bone architecture and bone strength. We also show that, at least in the mouse model, thrice per week injections can mimic the actions of intermittent PTH or constant estrogen in preventing bone loss. Furthermore, we find that TSH exerts its skeletal effect primarily by inhibiting osteoclastic bone resorption, as is evident from the persistent inactivation of osteoclastogenesis observed ex vivo even after a 4-week treatment-free period. Moreover, the antiresorptive action of rhTSH injection in mice is mimicked by TSHR or caTSHR overexpression specifically in osteoclasts. In contrast, TSHR inactivation in the hyt mouse stimulates osteoclast differentiation, providing even stronger genetic evidence for a key role for TSHRs in adult skeletal remodeling.
There is debate about whether low TSH levels or high thyroid hormone levels in hyperthyroid states contribute to the bone loss in hyperthyroidism (6, 14). We are of the opinion that the two hormones have a reciprocal role (7, 15) Our demonstration of increased osteoclast formation in a TSHR signaling-deficient hyt mouse once again offers clear genetic evidence for a role of the TSHR in osteoclast regulation. The fact that rhTSH added to hyt osteoclast cultures fails to reduce enhanced differentiation but suppresses haploinsufficient and wild-type cells provides further proof for the direct, receptor-dependent inhibition of osteoclastogenesis by TSH. Basset et al. (14) confirm that hyt mice have osteoporosis, which would be fully consistent with an increase in bone resorption not quantitated by this group through traditional histomorphometry or resorption marker measurements.
Interestingly, the same group finds high bone mass in TRα-null mice despite normal TSH levels (6), which again would be fully expected from the undisputed proresorptive action of thyroid hormones, documented through organ culture studies since the 1970s (16, 17). Likewise, Basset and colleagues (6, 14) report that thyrotoxic TRβ-null and hypothyroid Pax8−/− mice are osteoporotic despite elevated TSH levels, a finding that is again not unexpected from potential osteoblast-inhibitory effects of TSH at high plasma concentration (5). A detailed static and dynamic histomorphometric analysis, rather than the study of mineralization by nontraditional back-scattered scanning EM (6, 14), should allow for clarity on the relative effects of TSH on bone formation and resorption in favorite genetic models of hypo- and hyperthyroidism.
No matter to what extent low TSH levels might play a role in human hyperthyroidism, evidence for the skeletal effects of TSH is indeed becoming compelling. There is abundant evidence for an inhibitory effect of TSH on osteoclast formation and function in vitro (5, 10, 12). TSHR haploinsufficient mice have osteopenia and enhanced osteoclastogenesis in the face of normal thyroid function (5). Patients with subclinical hyperthyroidism or those oversuppressed with thyroxine have osteoporosis (18). Furthermore, serum TSH levels correlate with fracture risk (2), TSHR polymorphisms correlate with bone mass (3), and even a single TSH injection causes a precipitous drop in resorption markers in the absence of changes in thyroid hormone levels (19).
With that said, can TSH be used as a drug for osteoprotection in humans? In other words, is there a dosing regimen that could, via its antiresorptive actions shown here and possible anabolic actions shown previously (10), prevent bone loss or restore the lost bone? Is such a regimen safe vis-à-vis causing iatrogenic hyperthyroidism, particularly in older individuals? Here we show that TSH injected in regimens ranging from thrice per week through once per week to once per 2 weeks can restore the lost bone after ovariectomy in rats. Biomechanical testing revealed impressive gains in bone strength, interestingly at lower doses than those that enhanced BMD. Restoration was evident in the trabecular bone, whereas the cortical bone was not affected in rats postovariectomy. Our densitometric and μCT studies on cortical bone in the mouse using TSH thrice per week are fully consistent with the rat data. However, in the mouse, profound effects of TSH also were noted on the femur, which has a significant cortical bone component.
A single dose of TSH may have a long-lasting effect by causing a persistent inhibition of osteoclastogenesis evident even after a 4-week treatment-free period. Furthermore, although not directly evaluated in this study, dynamic histomorphometry in the rat provides good evidence for an increase in bone formation (10). An anabolic action of intermittent TSH, like that of intermittent PTH (11), which is otherwise proresorptive, would not be unexpected considering the presence of TSHRs on mature osteoblasts (5, 10). If so, TSH could be an antiosteoporotic drug with a dual action: a powerful antiresorptive response, shown here, coupled with an anabolic action, shown previously (10), with a net profound effect on preventing bone loss and restoring lost bone.
Therefore, what might be the clinical niche for TSH in the future considering that rhTSH is already in for the management of thyroid cancer patients? Nonthyroid cancer patients supplemented with thyroxine for hypothyroidism could indeed be one group. If low TSH levels underlie, at least in part, the osteoporosis and high fracture risk, per Bauer et al. (2), then injecting rhTSH to prevent bone loss would make clinical sense. The issue is whether intermittent TSH can cause iatrogenic thyrotoxicosis, and for this, the body of evidence to date is negative (10, 19). In our previous study (10), as well as this one, we did not detect increments in serum thyroid hormone levels. Likewise, Maziotti et al. (19) found no such increments in patients receiving a single dose of rhTSH that suppressed bone resorption. We expect that future efficacy and safety studies with standard rhTSH preparations should pave the way for the use of TSH to avert specific forms of osteoporosis.
Methods
Skeletal Phenotyping.
Areal BMD measurements were performed by using a small animal bone densitometer (Piximus; Lunar) (4). Whole-body BMD (the cranium excluded) and region-specific measurements of the whole body, spine (L4–L6), and femur were made. The instrument was calibrated each time before use by employing a phantom per the manufacturer's recommendation.
The mice or rats were killed per our Institutional Animal Care and Use Committee-approved protocol, and the femora was isolated and cleaned. Volumetric measurements by μCT (Desktop_CT 40; Scanco Medical AG) used methods previously reported (10). The scans were performed at a 13-μm (rat) or a 20-μm (mouse) resolution. All morphometric parameters were determined by using direct 3D reconstruction. Cortical thickness (Cort.Th) was determined in a mid-diaphyseal segment. Trabecular bone parameters measured in the distal metaphysic included BV/TV, Tb.Th, Tb.N, Tb.Sp, and trabecular connectivity density (Conn.D) (20).
Bone strength was measured on rat bone by a material testing system (model 810; MTS System Corporation). The mid-shaft of the femur was subject to three-point bending to failure at a displacement rate of 0.1 mm/s (20) by using a 2.5-kN load cell (model 661, 14A-03). The maximal load and stiffness were calculated from the load-displacement curves (22).
Mouse Models.
Congenitally hypothyroid hyt mice were obtained from The Jackson Laboratory. These mice have an autosomal recessive mutation (Pro→Leu) in amino acid 556 of the fourth transmembrane domain of the TSHR (21), which affects TSH binding and downstream signaling. Thus, the mice are characterized by hypoplastic thyroid glands, have high circulating TSH, and show a growth and fertility response to thyroid hormone therapy. These TSHR signal-deficient mice were used to complement our previous studies using mice in which TSHRs were deleted (5). For gain-of-function studies, we generated transgenic mice by using a construct containing the gene encoding the human TSHR (hTSHR) or its constitutively active mutant, cahTSHR, TRAP promoter, and a growth hormone minigene. These mice display normal growth, development, and fertility.
TSH Preparations.
rhTSH was provided by Genzyme. Native rat TSH purified from rat pituitary gland was obtained from the National Hormone Peptide Program, National Institute of Diabetes and Digestive and Kidney, and A. F. Parlow (National Hormone and Peptide Program, University of California, Los Angeles Medical Center, Torrance, CA).
Ex Vivo Osteoclast and Osteoblast Cultures.
For osteoclastogenesis experiments, murine bone marrow cells were extracted from long bones, washed, and cultured in α-MEM with 10% FBS, 50 ng/ml M-CSF, and 1% penicillin/streptomycin for 48 h. Nonadherent hematopoetic stem cell precursors were purified with Ficoll-Paque Plus (Amersham Biotech). The interface cell layer was isolated for culture in α-MEM with 10% FBS, 50 ng/ml M-CSF, and 60 ng/ml RANK-L at 5 × 104 cells per well in 96-well plates (Costar). A TRAP kit (Sigma–Aldrich) was used to stain osteoclasts for counting (23).
To study osteoblast generation, bone marrow cells were cultured in the presence of 1 mM ascorbic acid-2-phosphate. At ≈3 days, multicellular fibroblastoid colonies (CFU-Fs) appeared that became alkaline phosphatase (AP)-positive over the following week. CFU-Fs were stained by using an AP kit per the manufacturer's recommendations (Sigma–Aldrich).
Real-Time PCR.
Total RNA was purified by using an RNeasy Mini kit (Qiagen) and converted into cDNA by using SuperScript II (Invitrogen). For PCR, we used platinum TaqDNA polymerase (Invitrogen) and specific primers. PCR was performed as before (23, 24).
Acknowledgments.
This work was supported by National Institutes of Health and Department of Veteran's Affairs (VA) Grants AG14917, DK70526, and AG23176 (to M.Z.) and AG12951 (to E.A.); a VA Merit Review (to M.Z. and B.S.M.); the Ministry of Science, Education, and Sports, Croatia (S.V.); the American Society for Bone and Mineral Research Outstanding Research Award (to L.S.); and the Endocrine Society Anthony Means' Basic Science Award (to J.I.).
Footnotes
Conflict of interest statement: R.S., J.M., and K.S. are employees of Genzyme Corporation.
References
- 1.Von Recklinghausen F. The fibrosis or deforming ostitis, osteomalacia and osteoplastic carcinosis in their respective relationships to each other (translated from German). In: Reimer G, editor. Festchrift Rudolpf Virchow. Berlin: Vollendung; 1891. [Google Scholar]
- 2.Bauer DC, Ettinger B, Nevitt MC, Stone KL. Risk for fracture in women with low serum levels of thyroid-stimulating hormone. Ann Intern Med. 2001;134:561–568. doi: 10.7326/0003-4819-134-7-200104030-00009. [DOI] [PubMed] [Google Scholar]
- 3.Albagha OME, Natrajan R, Reid DM, Ralston SH. The D727 polymorphism of the human thyroid stimulating hormone receptor is associated with bone mineral density and bone loss in women from the UK. J Bone Min Res. 2005;20(Supp 1):S341. [Google Scholar]
- 4.Biondi B, et al. Subclinical hyperthyroidism: Clinical features and treatment options. Eur J Endocrinol. 2005;152:1–9. doi: 10.1530/eje.1.01809. [DOI] [PubMed] [Google Scholar]
- 5.Abe E, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115:151–162. doi: 10.1016/s0092-8674(03)00771-2. [DOI] [PubMed] [Google Scholar]
- 6.Bassett JH, et al. Thyroid hormone excess rather than thyrotropin deficiency induces osteoporosis in hyperthyroidism. Mol Endocrinol. 2007;21:1095–1107. doi: 10.1210/me.2007-0033. [DOI] [PubMed] [Google Scholar]
- 7.Zaidi M, Sun L, Davies TF, Abe E. Low TSH triggers bone loss: Fact or fiction? Thyroid. 2006;16:1075–1076. doi: 10.1089/thy.2006.16.1075. [DOI] [PubMed] [Google Scholar]
- 8.Greenspan SL, Greenspan FS. The effect of thyroid hormone on skeletal integrity. Ann Intern Med. 1999;130:750–758. doi: 10.7326/0003-4819-130-9-199905040-00016. [DOI] [PubMed] [Google Scholar]
- 9.Stepnick LS. The frequency of bone disease. In: McGowan JA, Raisz LG, Noonan AS, Elderkin AL, editors. Bone health and osteoporosis: A Report of the Surgeon General. Washington, DC: Office of the U.S. Surgeon General; 2004. pp. 68–87. [Google Scholar]
- 10.Sampath TK, et al. Thyroid stimulating hormone restores bone volume, microarchitecture, and strength in aged ovariectomized rats. J Bone Miner Res. 2007;22:849–859. doi: 10.1359/jbmr.070302. [DOI] [PubMed] [Google Scholar]
- 11.Shoback D. Update in osteoporosis and metabolic bone disorders. J Clin Endocrinol Metab. 2007;92:747–753. doi: 10.1210/jc.2007-0042. [DOI] [PubMed] [Google Scholar]
- 12.Hase H, et al. TNF-alpha mediates the skeletal effects of thyroid-stimulating hormone. Proc Natl Acad Sci USA. 2006;103:12849–12854. doi: 10.1073/pnas.0600427103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummings SR. The paradox of small changes in bone density and reductions in risk of fracture with raloxifene. Ann NY Acad Sci. 2001;949:198–201. doi: 10.1111/j.1749-6632.2001.tb04021.x. [DOI] [PubMed] [Google Scholar]
- 14.Bassett JH, et al. A lack of thyroid hormones rather than excess TSH causes abnormal skeletal development in hypothyroidism. Mol Endocrinol. 2007;22:501–512. doi: 10.1210/me.2007-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun L, Davies TF, Blair HC, Abe E, Zaidi M. TSH and bone loss. Ann NY Acad Sci. 2006;1068:309–318. doi: 10.1196/annals.1346.033. [DOI] [PubMed] [Google Scholar]
- 16.Feinblatt JD, Tai LR, Leone RG. Secretion of a bone resorbing factor by chick thyroid glands in organ culture. Endocrinology. 1976;99:1363–1369. doi: 10.1210/endo-99-5-1363. [DOI] [PubMed] [Google Scholar]
- 17.Mundy GR, Shapiro JL, Bandelin JG, Canalis EM, Raisz LG. Direct stimulation of bone resorption by thyroid hormones. J Clin Invest. 1976;58:529–534. doi: 10.1172/JCI108497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burmeister LA, Flores A. Subclinical thyrotoxicosis and the heart. Thyroid. 2002;12:495–499. doi: 10.1089/105072502760143872. [DOI] [PubMed] [Google Scholar]
- 19.Mazziotti G, et al. Recombinant human TSH modulates in vivo C-telopeptides of type-1 collagen and bone alkaline phosphatase, but not osteoprotegerin production in postmenopausal women monitored for differentiated thyroid carcinoma. J Bone Miner Res. 2005;20:480–486. doi: 10.1359/JBMR.041126. [DOI] [PubMed] [Google Scholar]
- 20.Turner CH, Burr DB. Basic biomechanical measurements of bone: A tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 21.Gu WX, et al. The thyrotropin (TSH) receptor transmembrane domain mutation (Pro556-Leu) in the hypothyroid hyt/hyt mouse results in plasma membrane targeting but defective TSH binding. Endocrinology. 1995;136:3146–3153. doi: 10.1210/endo.136.7.7789342. [DOI] [PubMed] [Google Scholar]
- 22.Cesnjaj M, Stavljenic A, Vukicevic S. In vivo models in the study of osteopenias. Eur Clin Chem Clin Biochem. 1991;29:211–219. [PubMed] [Google Scholar]
- 23.Sun L, et al. FSH regulates bone mass. Cell. 2007;125:247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 24.Zhu LL, Zaidi S, Moonga BS, Troen BR, Sun L. RANK-L induces the expression of NFATc1, but not of NFkappaB subunits during osteoclast formation. Biochem Biophys Res Commun. 2005;326:131–135. doi: 10.1016/j.bbrc.2004.10.212. [DOI] [PubMed] [Google Scholar]