Abstract
EndoS from Streptococcus pyogenes efficiently hydrolyzes the functionally important and conserved N-linked glycan of IgG in human blood. Repeated i.v. administration of EndoS in rabbits completely hydrolyzes the glycans of the whole IgG pool, despite the generation of anti-EndoS antibodies. EndoS administration had no apparent effects on the health of the animals. EndoS hydrolysis of the IgG glycan has profound effects on IgG effector functions, such as complement activation and Fc receptor binding, suggesting that the enzyme could be used as an immunomodulatory therapeutic agent against IgG-mediated diseases. We demonstrate here that EndoS indeed has a protective effect in a mouse model of lethal IgG-driven immune (or idiopathic) thrombocytopenic purpura. EndoS pretreatment of pathogenic antibodies inhibits the development of disease, and the enzyme also rescues mice from already established disease when severe thrombocytopenia and s.c. bleeding have developed. These results identify EndoS as a potential therapeutic agent against diseases where pathogenic IgG antibodies are important and further emphasize antibody glycans as possible targets in future therapies against antibody-mediated autoimmune conditions.
Keywords: autoimmunity, immunomodulation, Streptococcus pyogenes, endoglycosidase, glycosylation
The research field of pathogen-derived immunomodulatory molecules (IMs) is rapidly developing, and several IMs have been identified as potential immunotherapeutics that could be used to treat diseases connected with pathological innate and adaptive immune responses (1). EndoS, a secreted endoglycosidase from Streptococcus pyogenes, could represent an IM targeting the adaptive branch of the immune system via the conserved glycan in the effector part of human IgG (2, 3). EndoS specifically hydrolyzes the glycan on IgG between the two core GlcNAc residues (Fig. 1A). In contrast to many related endoglycosidases that require or are enhanced by denaturation of the glycoprotein, we have established that EndoS only hydrolyzes native IgG (no other substrate has been identified to date), suggesting that a protein–protein interaction between the enzyme and IgG provides this specificity (4). Treatment of human IgG with EndoS almost completely abolishes Fc-mediated binding to leukocytes, decreases complement activation through the classical pathway, and increases bacterial survival in blood (5). EndoS hydrolyzes all subclasses (1–4) of human IgG, both in purified form and in a plasma environment, leading to significantly decreased binding to purified and cell-bound Fc receptors (FcRs) (6).
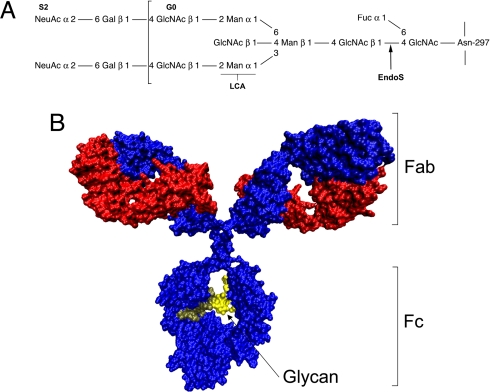
Fig. 1.
Structure and glycosylation of IgG. (A) Schematic representation of the fully substituted IgG heavy-chain glycan. S2 indicates the fully sialylated glycoform G0, and brackets indicate the extent of the G0 glycoform. LCA indicates the binding site for the LCA used in lectin experiments and EndoS the cleavage site for the enzyme. (B) Structural model of human IgG. IgG heavy chains are colored blue, and the light chains are colored red. Brackets indicate the antigen-binding Fab′ portion and the Fc effector portion of IgG. Arrow indicates the two conserved glycans (yellow) attached to Asn-297 of the heavy chains. The model was generated by using VMD 1.8.5 from a model deposited in the Protein Data Bank by M. Clark (Cambridge University, Cambridge, U.K.).
IgG is one of the most important molecules in adaptive immunity, and many of its structure–function relationships have been elucidated. The protein is a heterotetramer composed of two heavy and two light chains held together by disulfide bonds forming three protein domains separated by a flexible and protease-sensitive hinge region. The two identical Fab portions bind antigens, and the single Fc portion is responsible for effector functions, including binding and activation of complement factor C1q and FcRs on leukocytes (Fig. 1B) (7–9). Furthermore, the Fc portion contains a conserved complex biantennary glycan with a core fucose on each heavy chain attached to Asn-297 (Fig. 1A). Several studies indicate that these glycans are important for the structure and function of the Fc portion of the molecule (10, 11) and that altered IgG glycosylation is important in autoimmune diseases (12–14). Furthermore, a recent study showed that the antiinflammatory activity of IgG used in i.v. Ig (IVIG) therapy of inflammatory diseases depends on the fully sialylated S2 forms (see Fig. 1A) of IgG (15, 16).
Autoimmune disorders constitute a large group of diseases with various manifestations from mild to severe, but they all have in common that the immune system attacks and damages endogenous tissues and organs. Many autoimmune disorders have been thought to be largely dependent on the cellular branch of adaptive immunity mediated through T lymphocytes, but accumulating evidence suggests that B lymphocytes and autoantibodies are more important than previously appreciated for the development of disease (17). This also is true for acute and chronic organ-allograft rejection, where recent studies have shown that alloantibodies fixating complement on the allograft account for a substantial portion of graft-rejection episodes (18). Therapies, especially for the severe cases of both autoantibody-mediated autoimmune diseases and acute allograft rejection, focus on reducing antibody titers and production through plasmapheresis, immunosuppressants, antibodies directed toward B cells, and IVIG.
Immune (or idiopathic) thrombocytopenic purpura (ITP) is a relatively common autoimmune disorder, with an incidence of 5.5–6.6 per 100,000 people (19). ITP is caused by autoantibodies directed toward antigens on platelets (20), and platelets coated with IgG autoantibodies are cleared by FcR expressing tissue macrophages predominantly in the liver and spleen, causing the characteristic low platelet count and mucocutaneous bleeding (21). Current treatment of ITP focuses on reducing the production of pathogenic IgG and on reduction of platelet clearance by IVIG, splenectomy, corticosteroids, nonspecific immunosuppressants, monoclonal antibodies against B and T cells, and plasmapheresis (21, 22).
Because of the EndoS-specificity for IgG, its effects on IgG effector functions, and the importance of IgG in autoimmune disorders and allograft rejection, we hypothesized that EndoS could be used in vivo as a potential therapeutic agent against antibody-mediated diseases. This hypothesis was further stimulated by our recent finding that pretreatment of arthritogenic autoantibodies with EndoS abrogates the development of disease in a mouse model of collagen-induced arthritis (23). The IgG-specific protease IdeS also has been shown to be efficient in a similar model system (24) and in the mouse model of ITP used in this study (25). For EndoS to work as a useful agent against pathological IgG in vivo, it needs to fulfill several criteria. For instance, it cannot have any major adverse effects on animals; it must be active in a complex environment, such as human blood or the circulation of a live animal at reasonable concentrations; and, finally, it should work as a treatment in an animal model of an antibody-mediated disease.
Here we show that purified EndoS at low concentrations efficiently hydrolyzes the glycan on IgG in human blood, that EndoS can be injected i.v. in rabbits without adverse effects, and that EndoS at low concentrations hydrolyzes the glycans of the entire IgG pool in rabbits despite repeated administration and generation of antibodies toward EndoS. Furthermore, in a mouse model of lethal IgG-mediated thrombocytopenia, EndoS treatment inhibits the development of disease, but also rescues mice from already established disease with severe thrombocytopenia and s.c. bleeding. These results identify EndoS as a potential therapeutic agent in diseases where IgG antibodies play an important pathogenic role.
Results
EndoS Efficiently Hydrolyzes the IgG Glycans in Human Blood.
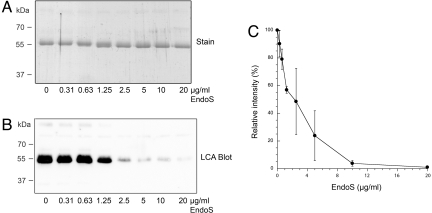
To function as a therapeutic agent against pathological IgG, EndoS needs to be active at low concentrations in whole human blood. To investigate this enzymatic activity, increasing final concentrations (0, 0.31, 0.63, 1.25, 2.5, 5, 10, and 20 μg/ml) of EndoS were incubated in heparinized human blood from healthy volunteers, followed by purification of IgG on immobilized protein G. There was no difference in binding efficiency to protein G between fully glycosylated IgG and EndoS-treated IgG (data not shown), which is in concordance with our previous findings for the IgG-binding protein H from S. pyogenes and protein A from Staphylococcus aureus (2). SDS/PAGE analysis of the samples showed that increasing concentrations of EndoS gradually shift the IgG heavy chain to an ≈3-kDa smaller apparent molecular mass and that almost no full-size heavy chain could be seen above a concentration of 2.5–5 μg/ml EndoS (Fig. 2A). The Lens culinaris agglutinin (LCA) lectin blot analysis of the same samples showed that increasing concentration of EndoS gradually gives a lower carbohydrate signal and that there is virtually no signal above EndoS concentrations of 2.5–5 μg/ml (Fig. 2B). We have previously shown that the lack of lectin signals corresponds well with complete IgG glycan hydrolysis as analyzed by mass spectroscopy (3). Furthermore, peak density analysis shows a dose–response curve, which flattens out to background levels around an EndoS concentration of 5 μg/ml (Fig. 2C). These results demonstrate that ≈5 μg/ml EndoS within 1 h completely hydrolyzes the IgG pool in human blood. Thus, EndoS shows a remarkably efficient hydrolysis of the functionally important IgG glycan in the complex environment of human blood.
Fig. 2.
EndoS hydrolyzes IgG in whole human blood. (A) SDS/PAGE analysis of purified IgG from human blood incubated with increasing concentrations of EndoS. (B) LCA lectin blot analysis of purified IgG from whole human blood incubated with increasing concentrations of EndoS. (C) Densitometric analysis of the lectin blot on IgG purified from human blood incubated with increasing concentrations of EndoS. Values are presented as the percentage of signal from control sample incubated with buffer alone. Means and standard errors were calculated from three independent experiments using blood from three different donors.
EndoS Hydrolyzes the IgG Glycans in Vivo in Rabbits.
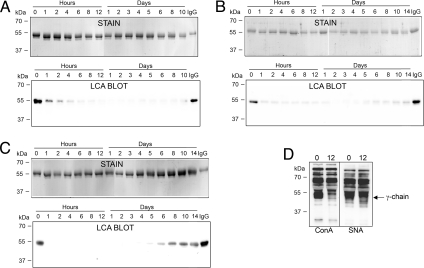
To further substantiate the potential use of EndoS as a therapeutic agent, we investigated the IgG glycan-hydrolyzing activity of EndoS in the circulation of live animals. Three rabbits were injected three times (at days 0, 35, and 130) i.v. with 1 mg each of EndoS, corresponding to an approximate EndoS:IgG ratio of 1:2,000, given that EndoS distributes in blood only. Serum samples were withdrawn at 0, 1, 2, 4, 6, 8, and 12 h and after 1, 2, 3, 4, 5, 6, 8, 10, and 14 days (only after injection two and three) after each injection. Serum IgG was analyzed for glycosylation status by using SDS/PAGE and lectin blot analyses as described for human blood in the previous section. Before the first injection, the apparent molecular mass of the heavy chains of IgG was comparable to fully glycosylated intact rabbit IgG (Fig. 3A, stain, hour 0, and IgG). In contrast, 1 h after EndoS injection, there already was a partial shift of the IgG heavy chains toward an ≈3-kDa smaller protein band. Four hours after EndoS injection, the IgG heavy chains were completely shifted to the lower apparent molecular mass form, and this was sustained until the last sample from day 10 after injection (Fig. 3A, stain, hour 4 to day 10). Lectin blot analysis revealed that the IgG heavy chain carbohydrate signal was nearly abolished 6–8 h after EndoS injection, and this was sustained until day 10, where there was only a slight increase in lectin signal (Fig. 3A, LCA blot). The IgG glycan hydrolysis patterns were nearly identical after injections 2 and 3, compared with the animals that had not previously been exposed to EndoS, with the exception that IgG reacting with the lectin started to appear ≈6 days earlier after the third injection (Fig. 3 B and C).
Fig. 3.
EndoS hydrolyzes IgG in vivo in rabbits. (A) SDS/PAGE (stain) and lectin blot analysis (LCA blot) of purified IgG from serum samples withdrawn from a representative rabbit at indicated time points after the first i.v. injection of 500 μg of EndoS (0 days). (B) SDS/PAGE (stain) and lectin blot analysis (LCA blot) of purified IgG from serum samples withdrawn from the rabbit at indicated time points after a second administration (35 days) of EndoS. (C) SDS/PAGE (stain) and lectin blot analysis (LCA blot) of purified IgG from serum samples withdrawn from the rabbit at indicated time points after a third administration (135 days) of EndoS. (D) Lectin blot analysis of total serum taken before (0) and 12 h (12) after the first injection of EndoS. ConA, Concavalin A; SNA, Sambucus nigra lectin. Arrow to the right indicates the position of the γ-chain of IgG.
Our previous studies have indicated that EndoS only interacts with native glycosylated IgG and not with closely related glycoproteins, such as IgA or IgM (4, 6, 26). To investigate whether EndoS had any activity on other rabbit serum glycoproteins in vivo, we analyzed total serum from a rabbit before and 12 h after the first injection of EndoS by using two different lectins, Concavalin A (ConA) and Sambucus nigra lectin (SNA). This revealed that the reactivity patterns using both the broad-specificity ConA lectin and the sialic acid-specific SNA lectin were nearly identical before and after EndoS injection, except for reduced reactivities at the position of the γ-chain of IgG in the sample taken after injection (Fig. 3D, ConA, SNA, and γ-chain).
None of the three rabbits showed any signs of disease after any of the EndoS injections as judged by normal weight gain and normal group behavior. Furthermore, the levels of protein G-purified total serum IgG were not significantly altered during the course of the experiment (data not shown). Taken together, these results show that low concentrations of EndoS efficiently hydrolyze the heavy-chain glycan on the whole-rabbit IgG pool in vivo without altering the IgG concentration or having any detectable activity on other serum glycoproteins. Furthermore, previous i.v. exposure to EndoS does not significantly affect the in vivo enzymatic activity of EndoS.
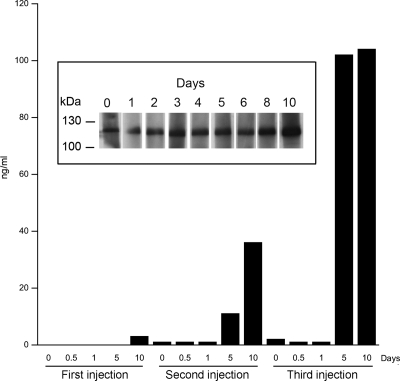
Because EndoS had full activity when injected repeatedly into rabbits, we wanted to determine the immune response against the enzyme. This immune response is of particular interest because most humans have been infected with S. pyogenes and carry antibodies against EndoS (27). Serum samples from rabbits were analyzed for reactivity against purified EndoS. Already before the first injection, there were antibodies reacting with EndoS in Western blot (Fig. 4Inset, day 0), suggesting that rabbits also are exposed to EndoS or a related molecule, giving rise to anti-EndoS IgG antibodies. There was only a slight increase in reactivity toward EndoS 10 days after injection (Fig. 4 Inset). Western blots were not practical to use for an analysis of samples obtained after the second and third injections because of very high signal levels (data not shown). Therefore, samples before and after all three injections also were analyzed by ELISA. This revealed that, just before the second injection of EndoS, the reactivity against EndoS was comparable or slightly higher than before the first injection, and the reactivity did not increase until 5 days after injection as determined by ELISA (Fig. 4, first and second injections). From days 5–10 after the second injection, the reactivity against EndoS gradually increased (Fig. 4, second injection, days 5–10). Before the third injection of EndoS, the reactivity against EndoS was slightly higher than before the second injection, and the reactivity did not increase during the first day after injection (Fig. 4, third injection, days 0–1). From days 5–10 after the third injection, the reactivity against EndoS increased dramatically (Fig. 4, third injection, days 5–10). These results indicate that there are antibodies directed toward EndoS in unexposed animals and that EndoS elicits an immune response in rabbits upon repeated i.v. exposure. However, these antibodies do not interfere with the activity of EndoS in the circulation during three consecutive administrations. Furthermore, repeated administration does not affect the ≈12-h circulation time (defined as the ability to detect EndoS) of the enzyme as analyzed by immunoprecipitation and Western blot analysis of EndoS from rabbit serum samples (data not shown).
Fig. 4.
Rabbit antibody response to EndoS. Serum samples were withdrawn from the rabbit at indicated time points after the first (0 days), second (35 days), and third (135 days) injections of EndoS. Serum samples after the first (0 days), second (35 days), and third (135 days) injections were used as primary antisera in an ELISA experiment with immobilized EndoS. Increase in concentration (ng/ml) of anti-EndoS IgG compared with concentration before first injection is presented. One representative experiment is shown. (Inset) The serum samples after the first injection were used as primary antisera in a Western blot on separate membrane strips with SDS/PAGE-separated purified EndoS.
EndoS Rescues Mice from Lethal Antibody-Mediated Thrombocytopenia.
Having established that EndoS efficiently hydrolyzes the IgG glycan in vivo and that animals tolerated administration of the enzyme, we subjected our hypothesis to the ultimate test: Could EndoS be used to treat a serious IgG-mediated disease? The disease model chosen was a mouse model of ITP. In this model, polyclonal rabbit IgG directed against mouse platelets (αPLT-IgG) is injected i.p., leading to severe thrombocytopenia, bleedings, and ultimately death at higher doses of IgG (28, 29).
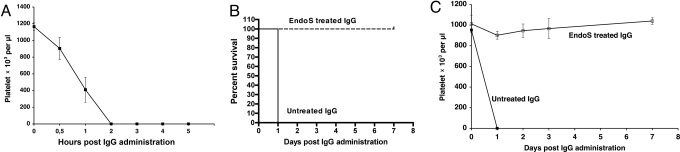
In a pilot experiment, three female BALB/c mice were injected with 1.2 mg of αPLT-IgG, and platelet counts were followed over time by using flow cytometry and microscopy. This revealed that all three mice rapidly developed thrombocytopenia, and death occurred within 24 h after αPLT-Ig administration (Fig. 5A).
Fig. 5.
EndoS pretreatment of pathogenic IgG antibodies inhibits antibody-mediated thrombocytopenia in mice. (A) Female BALB/c mice (n = 3) received i.p. injections of rabbit anti-mouse platelet IgG (αPLT-IgG). Blood samples were taken at regular intervals, and platelet counts were determined by using flow cytometry. (B) Survival plots of BALB/c mice injected with αPLT-IgG that were pretreated with GST-EndoS (n = 4) or GST (n = 4). (C) Platelet counts over time as determined by flow cytometry on blood samples from mice that had received αPLT-IgG pretreated with GST-EndoS.
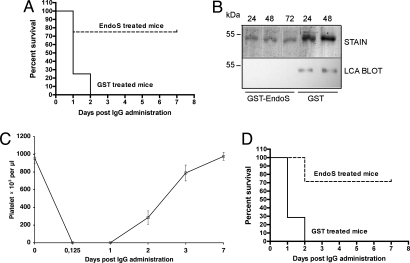
Next, we tested whether pretreatment of αPLT-IgG with GST-EndoS, or GST as a control, before administration to mice had any effects on the development of disease and survival rate. All animals (n = 4) injected with GST-EndoS-treated αPLT-IgG survived without developing any signs of disease, whereas all animals (n = 4) injected with GST-treated αPLT-Ig developed severe s.c. bleeding and died within 24 h (Fig. 5B). This represents a statistically significant difference between the two groups of animals (P = 0.0082). Furthermore, daily platelet count analysis by flow cytometry revealed that GST-EndoS-treated αPLT-IgG had no significant effect on mouse platelet count, whereas GST-treated αPLT-IgG caused a rapid drop in platelet counts (Fig. 5C). These experiments demonstrated that EndoS treatment of αPLT-IgG ex vivo abrogated the pathogenicity of the IgG antibodies, results that, in combination with the in vivo activity of EndoS, stimulated us to investigate whether EndoS could be administered to mice after initiation of disease to prevent the development of lethal thrombocytopenia. Mice (n = 8 per group) were injected with 1.2 mg of αPLT-IgG, followed by i.p. injection of 100 μg of GST-EndoS or GST 3 h after the administration of αPLT-Ig. All animals (eight of eight) that were treated with GST died within 2 days, whereas only two of eight animals treated with GST-EndoS died (Fig. 6A). This represents a statistically significant difference in survival rate between the groups (P = 0.003). SDS/PAGE and lectin blot analysis of total IgG from GST-EndoS or GST treated mice showed that the heavy-chain glycan was completely hydrolyzed at 24, 48, and 72 h after αPLT-IgG treatment in GST-EndoS-treated animals, whereas the IgG in GST-treated animals was fully glycoslated until death occurred at 24 h (Fig. 6B). Furthermore, the platelet count as analyzed by flow cytometry showed that administration of αPLT-IgG induces a rapid fall in platelet count, but in GST-EndoS-treated mice the platelet count began to rise steadily and reached normal values after 2–3 days (Fig. 6C).
Fig. 6.
EndoS rescues mice from lethal IgG-mediated thrombocytopenia. (A) Survival plots of BALB/c mice injected with αPLT-IgG, followed by GST-EndoS (n = 8) or GST (n = 8) treatment 3 h after αPLT-IgG administration. (B) SDS/PAGE analysis (stain) and LCA lectin blot analysis (LCA blot) of IgG purified from GST-EndoS or GST-treated mice 24, 48, or 72 (only GST-EndoS) h after injection of αPLT-Ig. (C) Blood samples were taken at regular intervals, and platelet counts were determined by using flow cytometry in mice that received GST-EndoS treatment. (D) Survival plots of BALB/c mice injected with αPLT-Ig, followed by GST-EndoS (n = 7) or GST (n = 7) treatment at the onset of clear signs of intraabdominal bleeding (5–7 h after αPLT-Ig administration).
To challenge our hypothesis further, we attempted to mimic the clinical situation of ITP patients. When these patients seek medical attention, the platelet count is often very low, and s.c. and other bleeding complications are already manifest. Therefore, we induced disease in mice (n = 14) with αPLT-IgG, but did not initiate treatment with GST-EndoS or GST until animals exhibited clearly visible cutaneous hematomas 5–7 h after αPLT-IgG injection. In these experiments, five of seven mice treated with GST-EndoS survived and recovered, whereas all mice (seven of seven) treated with GST died within 2 days, again representing a statistically significant difference in the survival rate between the two groups (P = 0.0015) (Fig. 6D). Combined, our results demonstrate that the pathogenic properties of αPLT-IgG in mice depends on the glycosylation state of the antibodies and that EndoS both ex vivo and in vivo drastically reduces the pathogenicity of anti-platelet IgG antibodies.
Discussion
We have demonstrated that purified EndoS from S. pyogenes at low concentrations efficiently hydrolyzes the conserved N-linked glycan on IgG heavy chains in whole human blood. Assuming an IgG plasma concentration of 10 mg/ml, EndoS completely hydrolyzes the glycans of the IgG pool in 1 h at an EndoS:IgG ratio of 1:2,000. Of particular interest is the finding that EndoS has no detectable effects on other plasma proteins, and we have been able to show previously that, despite its activity on IgG, EndoS has no activity on the closely related molecules, IgA and IgM (2). The specificity of EndoS for the N-linked glycans of a specific glycoprotein (IgG) makes it stand out from the other known enzymes in family 18 of glycosyl hydrolases. Most of these enzymes, including EndoF1–3 from Elizabethkingia meningoseptica (formerly Flavobacterium meningosepticum), are specific for certain glycan structures that can be attached to any protein or peptide and are enhanced by or require denaturation of the protein backbone (30). This is in complete contrast to EndoS, which exclusively hydrolyzes native IgG or IgG Fc, suggesting that the substrate specificity depends on protein–protein interactions between the enzyme and IgG Fc (2, 4). Furthermore, we have recently shown that EndoS does not interact with IgG lacking most of its heavy-chain glycans, suggesting a complex interaction between enzyme and substrate involving both protein–protein and protein–glycan interactions (6, 26).
Our in vivo experiments in rabbits show that EndoS efficiently hydrolyzes the heavy-chain glycans on the whole IgG pool without any noticeable adverse effects or signs of disease, and the glycan hydrolysis does not significantly alter the total plasma concentration of IgG. The glycosylation state has been shown to influence the proteolytic resistance of IgG (31). Therefore, our results suggest that the core N-acetylglucosamine (with or without a core fucose) provides comparable proteolytic resistance as intact IgG. Furthermore, the largely unchanged total IgG concentration might indicate that the interaction of IgG hydrolyzed by EndoS with the protective FcRn (32) is not altered in the same way as for the other FcRs (6).
Because EndoS hydrolysis of IgG does not affect the antigen-binding Fab portion, IgG functions such as neutralization of enzymes, and toxins are probably not affected. Furthermore, repeated administration and the development of anti-EndoS antibodies does not significantly reduce the circulation time and has only minor effects on the activity of the enzyme. A possible explanation for this is that other EndoS molecules rapidly hydrolyze antibodies directed toward EndoS, and EndoS–antigen complexes can therefore not be efficiently removed by the reticuloendothelial system. However, it should be noted that after the third injection, where there were high antibody titers against EndoS, IgG reactivity with lectin reappeared earlier than after the two prior injections. This finding is most likely due to a partial antibody-mediated removal of EndoS.
In the mouse model of ITP, EndoS had dramatic positive effects on the platelet count and survival, both when pathogenic antibodies were pretreated with the enzyme and when EndoS was administered early or late during the course of disease. In this model, in vivo hydrolysis of IgG glycans has been used as an experimental treatment of an autoimmune disease. The mechanisms underlying the positive effects of EndoS are, from a theoretical viewpoint, quite clear because we have previously shown that EndoS hydrolysis of IgG inhibits IgG of all subclasses from binding to FcRs and also reduces complement activation (5, 6). Not only does EndoS inhibit IgG from binding FcRs, but it also can release already FcR-bound IgG by hydrolysis of the heavy-chain glycan. It also should be noted that there seems to be one IgG–FcR interaction that is not affected like the others: EndoS-hydrolyzed IgG does, under certain circumstances, bind better to human FcRIIb than nonhydrolyzed IgG (6). In the context of antiinflammatory activity, this finding might be of relevance because IgG interactions with FcRIIb have been shown to be important for the antiinflammatory activity of IVIG that is used to treat autoimmune conditions (33, 34). Therefore, one could speculate that EndoS under certain circumstances may have a dual antiinflammatory activity by directly inhibiting the binding of pathogenic IgG to activating FcRs and shifting toward the inhibitory action mediated through FcRIIb.
The properties of EndoS make it an attractive alternative to current therapies of conditions involving pathogenic antibodies, especially in the light of several recent studies establishing the IgG glycan as a key to IgG effector modulation. This includes our own findings that EndoS hydrolysis of this glycan nearly abolishes complement activation through the classical pathway and reduces binding to FcRs on leukocytes (5, 6). Based on our current and previous observations, we suggest that EndoS could be used to treat conditions where IgG antibodies play a pathogenic role, including autoimmune diseases as exemplified here by ITP and acute antibody-mediated organ allograft rejections.
Materials and Methods
EndoS Activity in Human Blood.
Recombinant untagged and GST-tagged EndoS were expressed and purified to ≈95% homogeneity as described (4). Untagged recombinant enzyme is designated EndoS throughout the article and the tagged form GST-EndoS. Increasing final concentrations (0, 0.31, 0.63, 1.25, 2.5, 5, 10, and 20 μg/ml) of EndoS was incubated in 500 μl of heparinized human blood from three different healthy volunteers with rotation end over end for 1 h at 37°C. Samples were centrifuged at 720 × g for 10 min at 4°C, followed by purification of total IgG in plasma by using protein G Sepharose according to the manufacturer's instructions (GE Healthcare Biosciences). Purified IgG was separated on SDS/10% PAGE, stained with Coomassie blue, or electroblotted onto PVDF (Immobilon-P; Millipore). Glycosylated IgG was detected by using 5 μg/ml biotinylated LCA lectin and 1 μg/ml streptavidin-horseradish peroxidase (Vector Laboratories) and SuperSignal West Pico peroxidase substrate (Pierce). Membranes were analyzed by using a Chemidoc XRS imaging system and Quantity One image analysis software (BioRad).
EndoS Activity in Rabbits.
Swedish lop rabbits with a body weight of ≈3 kg were injected i.v. at days 0, 35, and 130 with 1 mg of EndoS, corresponding to an approximate EndoS:IgG ratio of 1:2,000 given that EndoS distributes in blood only. Animals were housed in an animal facility and were checked for signs of disease hourly for the first 12 h and daily for the duration of the study. After EndoS injections, serum samples were withdrawn at 0, 1, 2, 4, 6, 8, and 12 h, and 1, 2, 3, 4, 5, 6, 8, and 10 days. After injections 2 and 3, samples also were taken after 14 days. Serum IgG was analyzed for glycosylation status by using SDS/PAGE and lectin blot analysis as described above for human blood. Total serum protein glycosylation at 0 and 12 h after the first EndoS injection was analyzed by separation of 0.1 μl of serum on SDS/10% PAGE, blotting to PVDF, and detection by using 1 μg/ml biotinylated ConA or SNA (both from Vector Laboratories), 1 μg/ml streptavidin-horseradish peroxidase, and substrate as above. The Laboratory Animal Ethics Committee of Malmö/Lund has approved the animal experiments.
Detection of EndoS Antibodies in Rabbits.
Purified untagged EndoS was separated on SDS/10% PAGE and electroblotted onto PVDF that was cut into 1.5-mm strips. Strips were incubated with 1:500 dilutions of serum samples from the first injection, followed by incubation with peroxidase-labeled goat anti-rabbit antibodies (Pierce). Strips were developed by using chemiluminescence as described above for lectin blots. For ELISA experiments, 2 μg of EndoS was used to coat microtiter plates (Nunc), followed by blocking with 20 mg/ml BSA in PBS. Sera from animals before EndoS injections and 0.5, 1, 5, and 10 days after injections were used as primary antiserum in serial dilutions of 1:100 to 1:200,000. Peroxidase-labeled goat anti-rabbit antibodies (Pierce) were used as secondary antibodies and ABTS (Roche) as a peroxidase substrate. A standard curve for rabbit IgG was generated by coating microtiter plates as above with serial dilutions of polyclonal rabbit IgG (Sigma–Aldrich) and peroxidase-labeled goat anti-rabbit antibodies as secondary antibodies. Plates were analyzed at 405 nm in a Victor3 multilabel reader (PerkinElmer).
Mouse Model of IgG-Mediated Thrombocytopenia.
Rabbit antiserum against mouse platelets was purchased from Inter-Cell Technologies. The IgG fraction was isolated from this serum by using protein G Sepharose. Protein purity was confirmed by SDS/PAGE analysis, and protein concentration was determined by using advanced protein assay reagent (Cytoskeleton). For experiments using pretreated IgG, purified rabbit anti-mouse platelet IgG was incubated with GST-EndoS or GST, purified as described (4), at an enzyme to substrate ratio of 1:500 at 37°C for 24 h, followed by removal of GST-EndoS and GST on a glutathione-Sepharose (GE Healthcare). IgG glycan hydrolysis was confirmed by SDS/PAGE and lectin blotting using LCA as described above. Female BALB/c mice (≈20 g in body weight) were housed under standard conditions of light and temperature and were fed standard laboratory chow and water ad libitum. Then 1.2 mg of anti-mouse platelet IgG (untreated, EndoS-treated, or GST-treated) in 0.25 ml of PBS was administered to the animals by i.p. injection. Animals were monitored for mucocutaneous bleeds, physical activity, and isolation from the group, and the survival time was recorded.
Blood Sampling from Mice and Platelet Analysis.
Immediately before the injection of rabbit anti-mouse platelet IgG and at regular intervals during the course of the experiment, blood samples were collected from mice. From the prewarmed tail vein, 5 μl of whole blood was collected into tubes containing 45 μl of 0.1 M sodium citrate/citric acid in PBS (pH 6.5). The platelet population in these blood samples was identified by flow cytometry. Samples were labeled with hamster anti-mouse CD-61 PE (BD Biosciences) for 10 min at room temperature; then 10 μl of SPHERO Rainbow Calibration Particles (BD Biosciences) were added to each tube to enable counting. The RBC populations were lysed by using Utilyse (Dako), and the samples were analyzed on a FacsCalibur flow cytometer (BD Biosciences) in the logarithmic mode. The platelet number in the blood samples after lysis of RBCs was confirmed by manual counting in a Neubauer chamber.
Acknowledgments.
We thank Ulla Johannesson for excellent technical assistance. This work was supported by Swedish Research Council Grants 2005-4791 (to M.C.) and 2005-7480 (to L.B.), the Swedish Government Funds for Clinical Research (L.B.), the Kock Foundation (M.C. and L.B.), the Jeansson Foundation (M.C.), the Zoégas Foundation (M.C.), the Bergvall Foundation (M.C.), the Österlund Foundation (M.C., O.S., and L.B.), the Groschinsky Foundation (M.C.), the Crafoord Foundation (M.C.), the Royal Physiografic Society (M.C and O.S.), the Hansa Medical AB (to L.B.), and a Swedish Research Council Assistant Professorship (to M.C.).
Footnotes
Conflict of interest statement: Hansa Medical, which funded this study in part, has filed a patent application on the in vivo use of EndoS. M.C., O.S., and L.B. are listed as inventors, and the application is pending.
This article is a PNAS Direct Submission.
See Commentary on page 4081.
References
- 1.Fallon PG, Alcami A. Pathogen-derived immunomodulatory molecules: Future immunotherapeutics? Trends Immunol. 2006;27:470–476. doi: 10.1016/j.it.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Collin M, Olsén A, Endo S. A novel secreted protein from Streptococcus pyogenes with endoglycosidase activity on human IgG. EMBO J. 2001;20:3046–3055. doi: 10.1093/emboj/20.12.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collin M, Fischetti VA. A novel secreted endoglycosidase from Enterococcus faecalis with activity on human immunoglobulin G and ribonuclease B. J Biol Chem. 2004;279:22558–22570. doi: 10.1074/jbc.M402156200. [DOI] [PubMed] [Google Scholar]
- 4.Collin M, Olsén A. Effect of SpeB and EndoS from Streptococcus pyogenes on human immunoglobulins. Infect Immun. 2001;69:7187–7189. doi: 10.1128/IAI.69.11.7187-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collin M, et al. EndoS and SpeB from Streptococcus pyogenes inhibit immunoglobulin-mediated opsonophagocytosis. Infect Immun. 2002;70:6646–6651. doi: 10.1128/IAI.70.12.6646-6651.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allhorn M, Olin A, Nimmerjahn F, Collin M. Human IgG/FcγR interactions are modulated by streptococcal IgG glycan hydrolysis. PLoS One. 2008;3:e1413. doi: 10.1371/journal.pone.0001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics. J Mol Graphics. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 8.Clarke VA, Platt N, Butters TD. Cloning and expression of the β-N-acetylglucosaminidase gene from Streptococcus pneumoniae. Generation of truncated enzymes with modified aglycon specificity. J Biol Chem. 1995;270:8805–8814. doi: 10.1074/jbc.270.15.8805. [DOI] [PubMed] [Google Scholar]
- 9.Burton DR. Immunoglobulin G: Functional sites. Mol Immunol. 1985;22:161–206. doi: 10.1016/0161-5890(85)90151-8. [DOI] [PubMed] [Google Scholar]
- 10.Nose M, Wigzell H. Biological significance of carbohydrate chains on monoclonal antibodies. Proc Natl Acad Sci USA. 1983;80:6632–6636. doi: 10.1073/pnas.80.21.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution. Biochemistry. 1981;20:2361–2370. [PubMed] [Google Scholar]
- 12.Dube R, et al. Agalactosyl IgG in inflammatory bowel disease: Correlation with C-reactive protein. Gut. 1990;31:431–434. doi: 10.1136/gut.31.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rademacher TW, Williams P, Dwek RA. Agalactosyl glycoforms of IgG autoantibodies are pathogenic. Proc Natl Acad Sci USA. 1994;91:6123–6127. doi: 10.1073/pnas.91.13.6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahman MAA, Isenberg DA. Glycosylation of IgG in rheumatic disease. In: Isenberg DA, Rademacher TW, editors. Abnormalities of IgG Glycosylation and Immunological Disorders. Chichester, UK: Wiley; 1996. pp. 101–118. [Google Scholar]
- 15.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 16.Burton DR, Dwek RA. Immunology sugar determines antibody activity. Science. 2006;313:627–628. doi: 10.1126/science.1131712. [DOI] [PubMed] [Google Scholar]
- 17.Lim PL, Zouali M. Pathogenic autoantibodies: Emerging insights into tissue injury. Immunol Lett. 2006;103:17–26. doi: 10.1016/j.imlet.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 18.Colvin RB, Smith RN. Antibody-mediated organ-allograft rejection. Nat Rev Immunol. 2005;5:807–817. doi: 10.1038/nri1702. [DOI] [PubMed] [Google Scholar]
- 19.McMillan R. Therapy for adults with refractory chronic immune thrombocytopenic purpura. Ann Intern Med. 1997;126:307–314. doi: 10.7326/0003-4819-126-4-199702150-00007. [DOI] [PubMed] [Google Scholar]
- 20.Woods VL, et al. Autoantibodies against platelet glycoprotein Ib in patients with chronic immune thrombocytopenic purpura. Blood. 1984;64:156–160. [PubMed] [Google Scholar]
- 21.Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346:995–1008. doi: 10.1056/NEJMra010501. [DOI] [PubMed] [Google Scholar]
- 22.British Committee for Standards in Haematology Task Force. Guidelines for the investigation and management of idiopathic thrombocytopenic purpura in adults, children and in pregnancy. Br J Haematol. 2003;120:574–596. doi: 10.1046/j.1365-2141.2003.04131.x. [DOI] [PubMed] [Google Scholar]
- 23.Nandakumar KS, et al. Endoglycosidase treatment abrogates IgG arthritogenicity: Importance of IgG glycosylation in arthritis. Eur J Immunol. 2007;37:2973–2982. doi: 10.1002/eji.200737581. [DOI] [PubMed] [Google Scholar]
- 24.Nandakumar KS, Johansson BP, Björck L, Holmdahl R. Blocking of experimental arthritis by cleavage of IgG antibodies in vivo. Arthritis Rheum. 2007;56:3253–3260. doi: 10.1002/art.22930. [DOI] [PubMed] [Google Scholar]
- 25.Johansson BP, Shannon O, Björck L. IdeS: A bacterial proteolytic enzyme with therapeutic potential. PLoS One. 2008;3:e1692. doi: 10.1371/journal.pone.0001692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allhorn M, Olsén A, Collin M. EndoS from Streptococcus pyogenes is hydrolyzed by the cysteine proteinase SpeB and requires glutamic acid 235 and tryptophans for IgG glycan-hydrolyzing activity. BMC Microbiol. 2008;8:3. doi: 10.1186/1471-2180-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Åkesson P, et al. Low antibody levels against cell wall-attached proteins of Streptococcus pyogenes predispose for severe invasive disease. J Infect Dis. 2004;189:797–804. doi: 10.1086/381982. [DOI] [PubMed] [Google Scholar]
- 28.Fujimi S, et al. Platelet depletion in mice increases mortality after thermal injury. Blood. 2006;107:4399–4406. doi: 10.1182/blood-2005-09-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dominguez V, Govezensky T, Gevorkian G, Larralde C. Low platelet counts alone do not cause bleeding in an experimental immune thrombocytopenic purpura in mice. Haematologica. 2003;88:679–687. [PubMed] [Google Scholar]
- 30.Tarentino AL, Plummer THJ. Enzymatic deglycosylation of asparagine-linked glycans: Purification, properties, and specificity of oligosaccharide-cleaving enzymes from Flavobacterium meningosepticum. Methods Enzymol. 1994;230:44–57. doi: 10.1016/0076-6879(94)30006-2. [DOI] [PubMed] [Google Scholar]
- 31.Raju TS, Scallon BJ. Glycosylation in the Fc domain of IgG increases resistance to proteolytic cleavage by papain. Biochem Biophys Res Commun. 2006;341:797–803. doi: 10.1016/j.bbrc.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 32.Roopenian DC, Akilesh S. FcRn: The neonatal Fc receptor comes of age. Nat Rev Microbiol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 33.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–486. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 34.Nimmerjahn F, Ravetch JV. The antiinflammatory activity of IgG: The intravenous IgG paradox. J Exp Med. 2007;204:11–15. doi: 10.1084/jem.20061788. [DOI] [PMC free article] [PubMed] [Google Scholar]