Abstract
Mitotic recombination between homologous chromosomes is a genetic technique for mosaic analysis in model organisms. The general application of this technique in the mouse depends on establishment of effective recombination systems for individual chromosomes and reliable and sensitive methods for detection of recombination events. Here, we established a Cre/LoxP-mediated recombination system in mice for mosaic analysis of full-length chromosome 17. Cre-mediated germ-line recombination between the homologous chromosomes was observed with ≈9% frequency in a progeny test. Mitotic recombination in somatic tissues was evaluated and scored in B and T lymphocytes with the aid of surface markers and fluorescent-activated cell sorting. We show that a lineage-specific Cre can induce mitotic recombination with a highly reproducible frequency of 0.5–1.0% in lymphoid progenitors. The recombination system established here allows for a simple and accurate detection and isolation of recombination events in live cells, making this system particularly attractive for mosaic analysis or mutagenesis studies in the immune system.
Keywords: CD2, Cre/Flp, germ lines, lymphocytes, germ cells
Mouse sequencing projects have revealed ≈30,000 genes in the genome (National Center for Biotechnology Information mouse genome resources). Thus far, >4,000 mouse strains carrying single gene mutations have been generated by gene targeting or other mutagenesis methods (1, 2). It is estimated that ≈30% of genes are essential in development and thus germ-line mutations in these genes will result in embryonic or neonatal lethal (3). Consequently, effects of these germ-line mutations on postnatal life and many disease models cannot be easily assessed with conventional methods. It is anticipated that the rest of the genome will be systematically mutated by either targeted or random mutagenesis approaches in the coming years (1, 4–6). Therefore, there is an increasing need to develop new and practical methods for comprehensive analysis of both viable and lethal mouse mutants.
Mitotic recombination-mediated mosaic analysis is a proven method for studying gene function in somatic tissues in model organisms such as Drosophila (7). Recombination between homologous chromosomes during mitosis provides an opportunity for segregation of heterologous alleles in somatic tissues. The mosaics resulting from mitotic recombination allows for functional analysis of homozygous clones in an otherwise heterozygous background. Mitotic recombination can be induced with a site-specific recombination system such as Cre/LoxP or Flp/FRT derived from bacteria P1 phage or yeast, respectively. The Flp/FRT-mediated mitotic recombination system has been developed and broadly used in Drosophila for the past 15 years (7, 8). More recently, the Cre/LoxP system has been successfully used to induce mitotic recombination in mouse ES cells and in mice (9, 10). The feasibility of this genetic tool for mosaic analysis in mice was further demonstrated in the loss of heterozygousity assay of tumor suppresser gene p27 with the Cre/LoxP system (11) and p53 with the Flp/FRT system (12).
Although these recent studies provide a proof of principles for mitotic recombination in mice, the general application of this technique in mice is still pending on the development of mitotic recombination systems to cover the full length of individual mouse chromosomes. Furthermore, the reported mitotic recombination frequency is generally low in somatic tissues and the methods available for detection, quantification, and isolation of the recombinant cells are still limited. Here, we sought to apply this important technique in the immune system by building a mitotic recombination system to cover the full length of chromosome 17.
Results
Construction of Recombination-Ready Alleles for Chromosome 17.
We chose chromosome 17 in this study for the potential application of mosaic analysis of the major histocompatibility locus in the immune system. We designed three gene-targeting constructs to insert both LoxP and FRT sequences at an intergenic region ≈5.7 million base pairs from the centromere end of chromosome 17 (Fig. 1a). Mitotic recombination at this location in heterozygous animals will produce cells that are homozygous for 94% of chromosome 17. Each targeting construct contains a unique marker located on the distal telomere side of the single LoxP and duplicated FRT sites for tracing recombination events between the two alleles. As illustrated in Fig. 1b, a site-specific recombination event involving either the LoxP or FRT site between the homologous chromosomes may result in multiple outcomes. Both the maternal and paternal chromosomes distal to the LoxP and FRT sequences will be retained in the same daughter cells when recombination occurs at the G0 phase, the G1 phase, or the G2 phase with Z segregation of sister chromatids. However, a mitotic recombination followed by X segregation (G2–X recombination) will generate two daughter cells carrying segregated alleles.
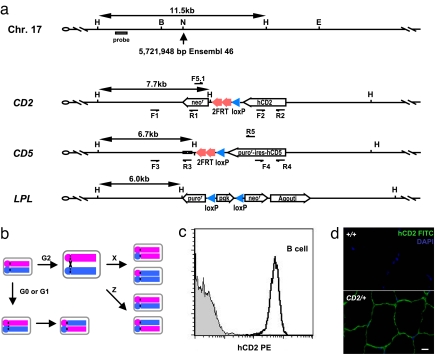
Fig. 1.
Modifications of chromosome 17 for mitotic recombination. (a) Schematic diagram of the wild-type, CD2, CD5, and LPL alleles around the insertion site of chromosome 17. The precise location of insertion site is based on Ensembl release 46. The hCD2 and hCD5-ires-puror markers are transcribed by the β-actin promoter, and the agouti marker is transcribed by the K14 promoter. F1/R1, F2/R2, F3/R3, F4/R4, and F5.1/R5 are primer pairs for evaluation of recombination across the LoxP or FRT sites. Restriction sites are as follows: B, BglII; E, EcoR V; H, HindIII; N, NheI. (b) Conceptual overview of the mitotic recombination system. Although recombination may occur in the G0, G1, and G2 phases of the cell cycle, only G2 recombination followed by X segregation will produce daughter cells carrying the segregated alleles. (c) Expression of hCD2 in lymph node B cells. Gray area is from a wild-type control mouse, and the dark line indicates data from a CD2/+ mouse. (d) Detection of hCD2 expression in the skeletal muscle of a CD2/+ mouse. (Scale bar: 10 μm.)
Mouse strains carrying the LoxP-FRT-CD2 (referred to as CD2 here after), LoxP-FRT-CD5 (referred to as CD5 here after), or LoxP-FRT-LPL (referred to as LPL here after) alleles were successfully generated after gene targeting in ES cells [supporting information (SI) Appendix]. Mice homozygous or transheterozygous for these alleles were phenotypically indistinguishable from their wild-type littermate controls during 2 years of breeding history of these strains, indicating that the introduction of these selection markers at the chosen site did not cause inadvertent effects on development. The expression efficiency of the surface markers for the CD2 and CD5 alleles was evaluated. The CD2 allele displayed a high and uniform expression level of the human CD2 (hCD2) marker in mature lymphocytes (Fig. 1c and SI Appendix) and skeletal muscle (Fig. 1d). The human CD5 marker from the CD5 allele showed varying levels of expression in different lineages of lymphocytes (SI Appendix). The high and uniform expression of the hCD2 marker made the CD2 allele particularly useful in tracing the recombination events in lymphocytes (see below).
Cre-Mediated Germ-Line Recombination on Chromosome 17.
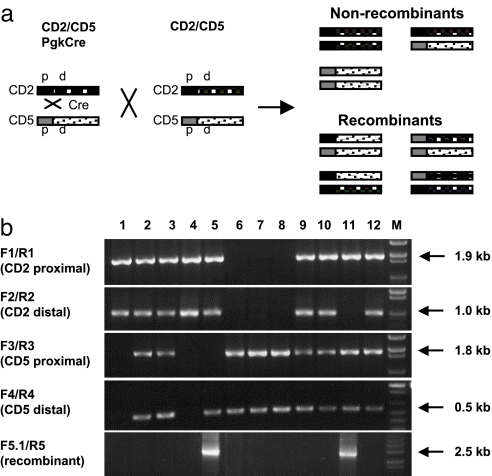
We first evaluated the Cre mediated recombination between the CD2 and CD5 alleles in the germ line. Mice transheterozygous for the CD2 and CD5 allele and carrying a Pgk-Cre transgene (CD2/CD5;Pgk-Cre) were produced and crossed with Cre-negative CD2/CD5 transheterozygous mice. The Pgk-Cre transgene drives Cre expression broadly in somatic and germ-line cells (unpublished data). Interchromosomal recombination occurring in the germ line of CD2/CD5;Pgk-Cre mice will result in offspring carrying a recombinant chromosome. PCR analysis of both CD2 and CD5 alleles was performed on the progeny obtained from the cross (Fig. 2 and SI Appendix). Each allele was tested with proximal- and distal-specific primer pairs. In the absence of germ-line recombination, we would expect to see a cosegregation of the distal and proximal markers for each allele (Fig. 2a). A recombination between the proximal and distal markers would result in the segregation of the proximal and distal sequences from the same allele. Indeed, progeny with segregated patterns were identified (mice 5 and 11 in Fig. 2b and SI Appendix). These mice were further analyzed by PCR with one primer derived from the CD2 allele and the other from the CD5 allele. A PCR product of the predicted size and sequence was obtained from mice 5 and 11, confirming the presence of the recombinant chromosome in these mice (Fig. 2b). Overall, we identified 8 of 87 (9.2%) progeny in this cross carrying a recombinant chromosome.
Fig. 2.
Detection of germ-line recombination. (a) Diagram illustrating progeny genotypes resulting from the mating between CD2/CD5;Pgk-Cre and CD2/CD5 mice. The CD2 and CD5 alleles are highlighted by dark and light ink fill, respectively. The proximal sequence (p) and distal sequence (d) flanking the loxP sites are highlighted by solid and stippled patterns, respectively. (b) PCR analysis of germ-line recombination between the CD2 and CD5 alleles. Tail samples were from 12 progeny of a cross between CD2/CD5;Pgk-Cre and CD2/CD5 mice. Positions of PCR primers are shown in Fig. 1a. The size of each PCR product is indicated on the right. M, DNA size markers.
Induction of Mitotic Recombination in Lymphocytes with Pgk-Cre.
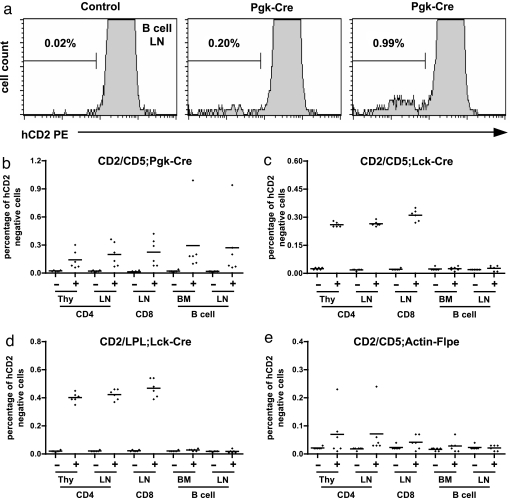
Mitotic recombination in somatic tissues was then evaluated in the lymphoid system with the aid of the hCD2 marker. Mitotic recombination between the CD2 and the other recombinant allele with a G2–X segregation should lead to a complete loss of hCD2 expression among half of the resulting daughter cells. We find that up to 1% of B lymphocytes have lost hCD2 expression in the presence of Pgk-Cre (Fig. 3 a and b), indicating the occurrence of mitotic recombination between the CD2 and CD5 alleles. However, the recombination frequency varies between individuals. A varying degree of mitotic recombination was also observed in CD4 single positive thymocytes and peripheral CD4 or CD8 T cells (Fig. 3b).
Fig. 3.
Detection of mitotic recombination in lymphocytes. (a) Representative FACS plots show the background signals in lymph node B cells of a control CD2/CD5 mouse and the appearance of hCD2 negative cells after mitotic recombination in two individual CD2/CD5;Pgk-Cre mice. (b) Summary of mitotic recombination result in CD4 T cells, CD8 T cells, and B cells from various lymphoid organs of CD2/CD5;Pgk-Cre mice and littermate controls. Thy, thymus; LN, lymph nodes; BM, bone marrow. +, Cre positive; −, Cre negative. Each dot represents data from one mouse. n = 6 for each genotype. (c) Summary of mitotic recombination in CD2/CD5;Lck-Cre and CD2/CD5 control mice. Labels, nomenclatures, and sample size are as in b. (d) Summary of mitotic recombination in CD2/LPL;Lck-Cre and CD2/LPL control mice. Labels, nomenclatures and sample size are as in b. (e) Summary of mitotic recombination test involving the Flp/FRT system. +, Flpe positive; −, Flpe negative. Other labels and sample size are as in b.
Induction of Mitotic Recombination with a T Cell-Specific Cre.
Lymphoid progenitors normally go through multiple rounds of expansion before becoming mature lymphocytes. Therefore, lymphoid progenitors are ideal target population for mitotic recombination. We speculated that the Pkg promoter might not be strong enough to provide high levels of Cre expression in the lymphoid lineages for efficient mitotic recombination among lymphoid progenitor cells. To further test mitotic recombination in the lymphoid system, we used Lck-Cre transgenic mice to provide Cre expression in the early phase of T cell development (13). Mitotic recombination, represented by the hCD2-negative cells, was detected among CD4 thymocytes (≈0.25–0.29%) and peripheral CD4 (≈0.25–0.28%) and CD8 (≈0.27–0.35%) T cells but not B cells (Fig. 3c). In contrast to the fluctuating rate of mitotic recombination induced by the Pgk-Cre transgene, the frequency of mitotic recombinant induced by Lck-Cre in each T cell subset fell in a narrow range. This result indicates that T lineage-specific expression of Cre is sufficient to induce mitotic recombination in the T cell lineage.
Effects of Tandem LoxP Sites on Mitotic Recombination.
The more accurate readout of mitotic recombination frequency with Lck-Cre allowed us to compare the frequency of mitotic recombination involving different LoxP alleles. The LPL allele contains two loxP sites separated by a 700-bp sequence, whereas the CD5 allele and the CD2 allele contain a single loxP site (Fig. 1a). The mitotic recombinant frequency for CD2/LPL;Lck-Cre mice was found to be ≈0.35–0.45% for CD4 single-positive thymocytes, ≈0.37–0.46% for lymph node CD4 T cells, and ≈0.39–0.54% for lymph node CD8 T cells (Fig. 3d), which were reproducibly higher than the numbers of mitotic recombinants obtained from CD2/CD5 mice. This result is consistent with an earlier report (9) that tandem LoxP sites could enhance mitotic recombination frequency.
Test of Recombination Mediated by the Flp/FRT System.
Finally, the efficiency of using the Flp/FRT system to induce mitotic recombination was tested with the Actin-Flpe transgene, which drives Flpe recombinase expression in both germ-line and somatic tissues. First, we examined Actin-Flpe-mediated recombination in the germ line. A cross between CD2/CD5;Actin-Flpe with CD2/CD5 was set up for the germ-line recombination test. We screened the progeny for recombination by using the PCR strategy described in Fig. 2. Two of 38 offspring (5.3%) were found to contain a recombinant chromosome resulting from Flpe-mediated recombination between the homologous chromosomes (SI Appendix). Sequencing analysis of the recombination site confirmed that a single FRT site remained in the recombinant chromosome after the recombination. We then evaluated mitotic recombination in lymphocytes by using CD2/CD5;Actin-Flp mice. With the exception of one mouse, which showed >0.2% detection frequency, the majority of mice did not show any significant sign of mitotic recombination based on the loss of hCD2 expression (Fig. 3e). To further test Actin-Flpe activity in somatic tissues, we evaluated deletion between the tandem FRT sites on the same chromosome. Actin-Flpe-mediated intrachromosomal deletion was readily detected in thymocyte or tail DNA, indicating that Actin-Flpe is fully functional in somatic tissues (SI Appendix). Therefore, this particular Actin-Flpe transgene appears to be inefficient in driving mitotic recombination in somatic cells even though it works well in germ lines.
Discussion
Our study has established an effective recombination system for mouse chromosome 17. Germ-line recombination between homologous chromosomes was achieved at a frequency of 9.2% and 5.3% with the Cre/LoxP and the Flp/FRT systems, respectively. The germ-line recombination may occur during mitotic division of germ cells or during meiosis. We speculate that chromosomal paring during meiosis should greatly facilitate Cre/LoxP- or Flp/FRT-mediated recombination between homologous chromosomes and thus contributes to most of the germ-line recombination. This interpretation is consistent with the fact that mitotic recombination with the same Cre or Flpe transgenes in somatic tissues exhibited much lower rate of recombination than in germ line. However, we cannot rule out the possibility that mitotic recombination in germ cells is intrinsically higher than in somatic cells. Although the exact timing of recombination in germ cells remains to be determined, the high efficiency of site-specific recombination between homologous chromosomes in the germ line should provide a practical means for genetic analysis of germ cells and maternal contributions to early embryogenesis.
We show that mitotic recombination in a lymphoid system can be achieved with either a nontissue-specific or tissue-specific Cre. The T cell-specific Cre transgene driven by the Lck proximal promoter induced mitotic recombination at a reproducible frequency in the T lineage cells. In contrast, the recombination frequency with the broadly expressed Pgk-Cre transgene was generally low and variable among individuals analyzed. This result implies that Pgk-Cre is less efficient than Lck-Cre in driving Cre expression in the T cell progenitors. The result further implies a general low Pgk-Cre activity among the ancestor cells that give rise to T lineage progenitors. Therefore, the rate of mitotic recombination may be further improved by increasing the expression level and duration of Cre recombinase.
Our assay detects half of the recombinant daughter progeny in the lymphoid system. The generation of each hCD2-negative cell by mitotic recombination should be accompanied by the production of a sibling hCD2 double-positive cell. If both populations of recombinants are included in the calculation, the actual frequency of Lck-Cre-mediated mitotic recombination should be 0.5–0.7% for the CD2/CD5 chromosomal pair and 0.8–1.0% for the CD2/LPL pair in the T cell lineage. It is estimated that each T cell progenitor goes through ≈10 cell cycles in the thymus before reaching the mature CD4 or CD8 T cell pool (14). Therefore, we estimate that the mitotic recombination frequency per cell cycle is 0.05–0.07% and 0.08–0.1% for CD2/CD5;Lck-Cre and CD2/LPL;Lck-Cre mice, respectively. A comparison of CD4 thymocytes and peripheral CD4 T cells showed identical frequency of mitotic recombinants. This result supports the notion that most mitotic recombination observed in our test system must have occurred early in T cell development when thymocytes undergo extensive proliferation. CD4 and CD8 single-positive thymocytes and naïve CD4 and CD8 T cells in peripheral lymphoid organs do not divide before antigen engagement. Lymphocytes may undergo additional phases of clonal expansion upon antigen stimulation. Therefore, antigen-mediated clonal expansion could provide another developmental window for a broader application of the mitotic recombination technique in lymphocytes.
Lymphocytes are constantly generated throughout life and can be easily analyzed in large quantities to allow accurate detection of relatively low recombination events. These features make the lymphoid system particularly suitable for immediate application of the mitotic recombination techniques. For example, our mitotic recombination system could be used in mosaic analysis of lymphocytes carrying homozygous mutations on chromosome 17. The homozygous clones can be easily identified and purified if needed for phenotypic and functional analyses. Furthermore, the use of surface markers in the analysis of mitotic recombination in the lymphoid organ provides an easy and standardized assay for general evaluation and improvement of mitotic recombination frequency in live animals in the future. To make the system generally applicable to a broader tissue types, we have incorporated additional markers, e.g., tyrosinase (the agouti marker in the LPL allele) as a marker for coat color and GFP (data not shown) as a general marker for live tissue imaging, in our recombination system. Although the feasibility of these other markers for detection of mitotic recombination in nonlymphoid tissues is still under investigation, the strategy used in building this mitotic recombination system for chromosome 17 should be generally applicable to building effective mitotic recombination systems for the other mouse chromosomes.
Materials and Methods
Gene Targeting and Animal Breeding.
Gene targeting experiments were performed with W4 ES cells (Taconic Transgenics). ES clones carrying targeting alleles were injected into C57BL/6 blastocysts, and the resulting chimeras were crossed with C57BL/6 mice for germ-line transmission. Offspring were screened for transmission of targeted alleles by PCR first and then by Southern blot analyses for final confirmation. The targeted alleles were maintained on a C57BL/6 and 129/sv mixed background. The Pgk-Cre transgenic line was established from ES cells transfected with a Pgk-Cre construct. The Lck-Cre strain was established in a previously published work (13). The Actin-Flpe strain was obtained from the Jackson Laboratory. Mouse breeding and experimental manipulation were carried out following the general guidelines published by The Association for Assessment and Accreditation of Laboratory Animal Care. Animal-related procedures were reviewed and approved by the Institute of Developmental Biology and Molecular Medicine Institutional Animal Care and Use Committee.
Histological Analysis.
Mouse tissues were embedded in OCT compound (Leica), sectioned, and stained following standard procedures. Images were captured with a Leica DM RXA2 microscope and a Leica DFC300 FX CCD camera.
FACS Analysis.
Mice used in FACS analysis were 1–1.5 months old. Single-cell suspensions of lymphocytes from the thymus, spleen, bone marrow, and peripheral lymph nodes were prepared in ice-cold PBS supplemented with 5% bovine calf serum. Approximately 1 × 106 cells were used immediately for staining with antibodies and analyzed on a FACSCalibur cell sorter (BD Biosciences). Each staining contained a FITC antibody, a phycoerythrin-conjugated (PE) antibody, an allophycocyanin-conjugated (APC) antibody, and 7-amino actinomycin D (7AAD). The antibodies (all purchased from Caltag Laboratories) included the FITC- or PE-conjugated anti human CD2 (clone S5.5), the FITC- or PE-conjugated anti human CD5 (clone CD5–5D7), APC-conjugated anti-mouse CD4 (clone CT-CD4), PE-conjugated anti mouse CD8β (clone CT-CD8b), FITC-conjugated anti-mouse IgM (clone II/41), APC-conjugated B220 (clone RA3–6B2), PE-conjugated Mac-1 α (clone M1/70.15) and PE-conjugated Ter-119. 7AAD staining was used to exclude dead and damaged cells from the analysis.
Supplementary Material
Acknowledgments.
We thank Yanfeng Tan, Yanling Yang, Fang Wang, and Chunyan Gao for technical assistance and Mary Elizabeth Jones for reading and critiquing the manuscript. This study was supported by Chinese Key Projects for Basic Research (973) Grant 2006CB806700, Hi-tech Research and Development Project (863) Grant 2007AA022101, National Natural Science Foundation of China Grant 30228014, Shanghai Pujiang Program Grant 05PJ14024, and the 211 and 985 projects of the Chinese Ministry of Education.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800798105/DC1.
References
- 1.Collins FS, Rossant J, Wurst W. A mouse for all reasons. Cell. 2007;128:9–13. doi: 10.1016/j.cell.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Nord AS, et al. The International Gene Trap Consortium Website: A portal to all publicly available gene trap cell lines in mouse. Nucleic Acids Res. 2006;34:D642–D648. doi: 10.1093/nar/gkj097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell KJ, et al. Functional analysis of secreted and transmembrane proteins critical to mouse development. Nat Genet. 2001;28:241–249. doi: 10.1038/90074. [DOI] [PubMed] [Google Scholar]
- 4.Skarnes WC, et al. A public gene trap resource for mouse functional genomics. Nat Genet. 2004;36:543–544. doi: 10.1038/ng0604-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun LV, et al. PB mice: An integrated database system of piggyBac (PB) insertional mutations and their characterizations in mice. Nucleic Acids Res. 2007;36:D729–D734. doi: 10.1093/nar/gkm790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keng VW, et al. Region-specific saturation germ-line mutagenesis in mice using the Sleeping Beauty transposon system. Nat Methods. 2005;2:763–769. doi: 10.1038/nmeth795. [DOI] [PubMed] [Google Scholar]
- 7.Golic KG. Site-specific recombination between homologous chromosomes in Drosophila. Science. 1991;252:958–961. doi: 10.1126/science.2035025. [DOI] [PubMed] [Google Scholar]
- 8.Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 9.Liu P, Jenkins NA, Copeland NG. Efficient Cre-loxP-induced mitotic recombination in mouse embryonic stem cells. Nat Genet. 2002;30:66–72. doi: 10.1038/ng788. [DOI] [PubMed] [Google Scholar]
- 10.Zong H, Espinosa JS, Su HH, Muzumdar MD, Luo L. Mosaic analysis with double markers in mice. Cell. 2005;121:479–492. doi: 10.1016/j.cell.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Muzumdar MD, Luo L, Zong H. Modeling sporadic loss of heterozygosity in mice by using mosaic analysis with double markers (MADM). Proc Natl Acad Sci USA. 2007;104:4495–4500. doi: 10.1073/pnas.0606491104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Warren M, Bradley A. Induced mitotic recombination of p53 in vivo. Proc Natl Acad Sci USA. 2007;104:4501–4505. doi: 10.1073/pnas.0607953104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan L, Hanrahan J, Li J, Hale LP, Zhuang Y. An analysis of T cell intrinsic roles of E2A by conditional gene disruption in the thymus. J Immunol. 2002;168:3923–3932. doi: 10.4049/jimmunol.168.8.3923. [DOI] [PubMed] [Google Scholar]
- 14.Penit C, Lucas B, Vasseur F. Cell expansion and growth arrest phases during the transition from precursor (CD4−8−) to immature (CD4+8+) thymocytes in normal and genetically modified mice. J Immunol. 1995;154:5103–5113. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.