Abstract
Malaria kills >1 million people each year, in particular in sub-Saharan Africa. Although asexual forms are directly responsible for disease and death, sexual stages account for the transmission of Plasmodium parasites from human to the mosquito vector and therefore the spread of the parasite in the population. Development of a malaria vaccine is urgently needed to reduce morbidity and mortality. Vaccines against sexual stages of Plasmodium falciparum are meant to decrease the force of transmission and consequently reduce malaria burden. Pfs48/45 is specifically expressed in sexual stages and is a well established transmission-blocking (TB) vaccine candidate. However, production of correctly folded recombinant Pfs48/45 protein with display of its TB epitopes has been a major challenge. Here, we show the production of a properly folded Pfs48/45 C-terminal fragment by simultaneous coexpression with four periplasmic folding catalysts in Escherichia coli. This C-terminal fragment fused to maltose binding protein was produced at medium scale with >90% purity and a stability over at least a 9-month period. It induces uniform and high antibody titers in mice and elicits functional TB antibodies in standard membrane feeding assays in 90% of the immunized mice. Our data provide a clear perspective on the clinical development of a Pfs48/45-based TB malaria vaccine.
Malaria parasites are spread in the population by Plasmodium-infected Anopheles mosquitoes. Successful transmission of malarial parasites from humans to mosquitoes depends on the presence and infectiousness of gametocytes in the peripheral blood and the number of Anopheles mosquitoes in the area. Transmission of Plasmodium falciparum can be blocked inside the mosquito by antibodies that have been ingested together with the gametocytes as part of a blood meal, interrupting the sporogonic cycle inside the mosquito (1).
Pfs48/45 is a transmission-blocking (TB) target protein expressed by gametocytes (2–4) and present on the surface of the sporogonic (macrogametes) stages of the malaria parasites. Pfs48/45 plays a key role in parasite fertilization (5) and antibodies that exclusively target conformational epitopes of Pfs48/45 protein prevent fertilization (6, 7). Furthermore, anti-Pfs48/45 antibodies are present in human sera from endemic areas (8) and correlate with TB activity (8–10). The induction of antibodies after natural infection as observed in the field creates the highly beneficial potential of vaccine boosting in the endemic setting. TB vaccines might be applied alone or more likely as part of a combination vaccine or package of control measures depending on the intensity of malaria transmission (11).
A strategy for vaccine development requires the production of correctly folded recombinant Pfs48/45 protein. Proper folding of many cysteine-rich proteins, including Pfs48/45, depends on correct formation of disulphide bridges. In eukaryotes the oxidizing environment of the endoplasmic reticulum (ER) provides a milieu for disulphide bonds formation. Plasmodium parasites are one of the few eukaryotes that lack the N-linked glycosylation machinery, and many Plasmodium proteins contain multiple potential glycosylation sites that are aberrantly glycosylated when expressed in any of the available eukaryotic hosts. On the other hand, prokaryotic expression systems such as Escherichia coli, which lacks N-glycosylation also lack the sophisticated ER machinery of disulphide bond formation. In E. coli correct disulphide bonds are formed in the periplasmic space and catalyzed by a set of periplasmic oxidoreductases, termed Dsb (12, 13). These proteins function in two separate pathways: (i) oxidation by DsbA/DsbB, responsible for introducing S-S bonds, and (ii) reduction and isomerization of aberrant disulfide bonds by DsbC/DsbD. Previous studies (12) have shown that overproduction of the enzymes DsbA and DsbC greatly improve proper disulfide bond formation in cysteine-rich proteins.
Another well known rate-limiting step of the folding of proteins in vivo is the cis/trans isomerization of prolyl-iminopeptide bonds that is catalyzed by peptidyl-prolyl cis/transisomerases (PPIases). The actions of PPIases such as FkpA and SurA have already been shown to improve the production of recombinant proteins in the periplasm of E. coli. Previously, the genes for the oxidoreductase, PPIase, and the general chaperone activities of DsbA, DsbC, FkpA, and SurA have been combined on a expression plasmid called pTUM4 (14). Coexpression of the periplasmic folding catalysts was shown to improve the folding of two recombinant proteins carrying several disulfide bonds and showing poor folding efficiency in the periplasm of E. coli.
In this study, we investigated the effect of coexpression of the four periplasmic folding catalysts on the folding, yield, and immunogenicity of the recombinant Pfs48/45 protein and fragments thereof. Our results demonstrate that the yield of recombinant Pfs48/45 protein is significantly improved as compared with the recombinant 10C as described (15). A Pfs48/45 fragment of 10C cysteines retained the highest stability in terms of conformation and resistance to proteases and elicited high titers of functional TB antibodies. Our data provide an efficient production and rapid purification of properly folded Pfs48/45–10C and a clear perspective on the clinical development of a Pfs48/45-based TB malaria vaccine.
Results and Discussion
Effect of Folding Catalysts on the Yield of Correctly Folded Pfs48/45.
We investigated the effect of coexpression of the protein folding catalysts DsbA, DsbC, FkpA, and SurA on the yield and conformation of recombinant Pfs48/45 protein and fragments thereof in the periplasm of E. coli. Previous unpublished results had indicated that Pfs48/45 fused to the PelB leader peptide could not be detected in E. coli periplasm and there was little effect of the coexpression of chaperones. To attain periplasmic localization Pfs48/45 full-length (16C) or C-terminal (10C) (residues 26–428 and 159–428, respectively) were fused to a periplasmic maltose binding protein (MBP) as a carrier molecule. As shown in Fig. 1B, both proteins M-Pfs16C and M-Pfs10C were detected in the periplasmic extracts of E. coli at low levels. Thus, MBP was an efficient vehicle in targeting Pfs48/45 to the periplasm. Furthermore, pTUM4 encoded periplasmic chaperones accumulated at high levels in the periplasmic fraction and had a profound effect of at least 10-fold enhancement on protein accumulation and epitope recognition of both M-Pfs16C and M-Pfs10C. Note that in addition to the full-length M-Pfs16C and M-Pfs10C we observed a degradation product with apparent mobility of ≈43–45 kDa (≈43 kDa in the case of M-Pfs16C and ≈45 kDa for the M-Pfs10C) that reacted to the MBP antibody (data not shown) in the Coomassie-stained SDS/PAGE. Thus the Pfs48/45 part of the MBP fusion degraded rapidly in the E. coli periplasm, and the protease-resistant MBP part accumulated as a prominent product. Coexpression of the chaperones increased significantly the amount of full-length M-Pfs16C and M-Pfs10C with an concomitant increase of reactivity with the conformation-dependent mAb (Fig. 1B). Apparently, proper folding of the Pfs48/45 protein is essential for its stability and accumulation.
Fig. 1.
Effect of chaperones expression from the pTUM4 plasmid on the MBP-fused Pfs48/45 full-length (M-Pfs16C) and 10C fragment (M-Pfs10C). (A) A schematic representation of the Pfs48/45 protein with three putative domains recognized by different TB mAb as described (15). The coding sequence contains a total of 448 aa. Bars indicate the relative position of cysteine residues. (B) Periplasmic E. coli extracts were screened with mAbs against epitope I, IIb, III, and V or Coomassie blue. Arrows indicate the position of M-Pfs16C (16C) and M-Pfs10C (10C) proteins. Samples were mixed with SDS sample buffer without reducing agent and separated on SDS/PAGE. Note that the blot with the mAb against epitope I was overexposed to show the expression in the lanes without chaperones.
Purification and Primary Characterization of M-Pfs16C and M-Pfs10C.
Both M-Pfs16C and M-Pfs10C were extracted from the E. coli periplasm and purified on a DEAE FF column. Although the M-Pfs10C remained soluble after purification, the M-Pfs16C showed a strong tendency to aggregate upon storage for 1 week at 4°C especially at protein concentrations of ≈0.25 mg/ml or more (Fig. 2A). Further purification of the M-Pfs16C and M-Pfs10C on Superdex 75 yielded >95% pure protein preparations as judged from a nonreduced SDS/PAGE (Figs. 2A and 3A). In a two-site capture ELISA, purified M-Pfs10C showed similar epitope recognition as native gametocyte-derived Pfs48/45 protein (Fig. 3 B and C), whereas the recognition of the M-Pfs16C was much weaker (Fig. 2 B and C). Different combinations of Pfs48/45-specific mAbs for capture and detection gave similar results (data not shown). Thus, we obtained a >90% properly folded homogeneous M-Pfs10C preparation and a less well folded M-Pfs16C preparation. Because of poor solubility and much weaker epitope recognition of the M-Pfs16C preparations, we focused for further analysis mainly at the M-Pfs10C protein preparation. The final yield of this correctly folded M-Pfs10C protein was ≈1 mg/liter. Purified M-Pfs10C protein was stable at 4°C for at least 9 months, i.e., repetitive conformation-dependent two-site capture ELISA and mobility on a nonreduced SDS/PAGE yielded nearly identical results (data not shown). A second batch of M-Pfs10C was produced with similar yields and ELISA values.
Fig. 2.
Purification and characterization of M-Pfs16C. (A) Coomassie-stained SDS/PAGE analysis after DEAE column purification of the M-Pfs16C protein. Lane 1, aggregated insoluble M-Pfs16C after 1 week at 4°C; lane 2, broad-range protein marker; lane 3, remaining soluble M-Pfs16C; lane 4, soluble M-Pfs16C purified over Superdex 75 column. (B and C) Two-site ELISA experiment using rat mAbs against epitopes III for capture and peroxidase-conjugated mAb against epitope I (B) or IIb (C) for detection. Gametocyte extracts (gct) with known concentration of the Pfs48/45 protein were used as a positive control.
Fig. 3.
Immunocharacterization of M-Pfs10C. (A) Coomassie-stained SDS/PAGE analysis of the purified M-Pfs10C protein. (B and C) Two-site ELISA experiment using rat mAbs against epitopes III for capture and peroxidase-conjugated mAb against epitope I (B) or IIb (C) for detection. Gametocyte extracts with known concentration of the Pfs48/45 protein were used as a positive control.
Immunogenicity of Recombinant M-Pfs10C.
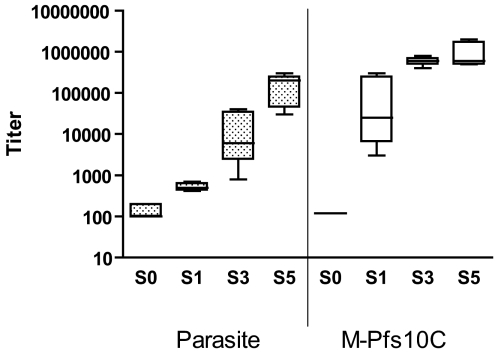
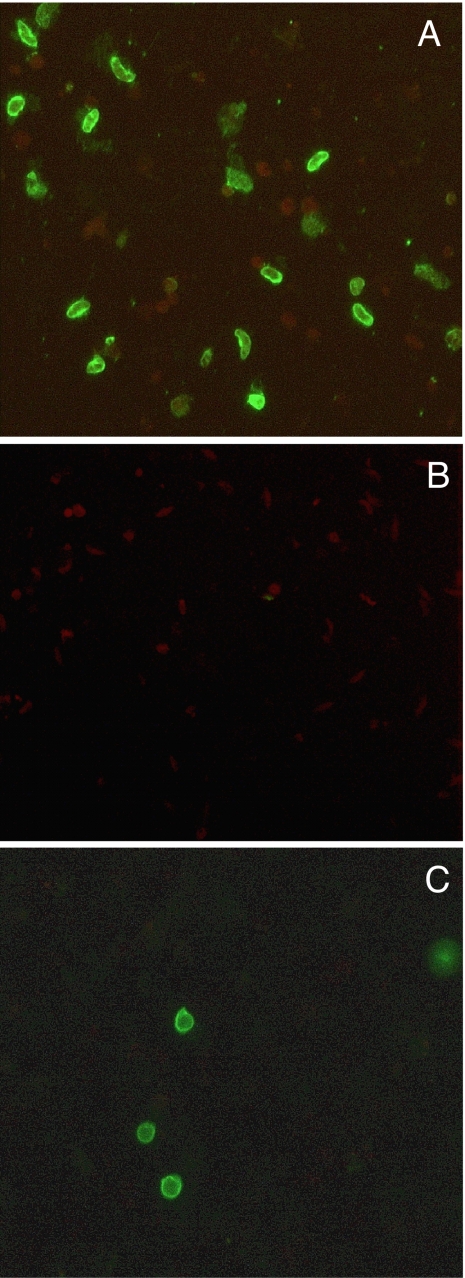
Immunogenicity of two independently produced batches of the purified M-Pfs10C protein with >90% proper folding was assessed in BALB/c mice. Fig. 4 shows that serum of M-Pfs10C (batch 1)-immunized mice showed antibody reactivity in an ELISA using whole gametocyte extract with increasing titers after each subsequent boost. Reactivity against the M-Pfs10C was already apparent after the first immunization. In the final bleed (S5), the M-Pfs10C fragment induced titers of 1/165,000 (SD = 1/101,143; range 1/30,000 to 1/300,000) when tested in the Gametocyte-ELISA using enriched gametocyte extracts and up to 1/1,000,000 in the M-Pfs10C ELISA. Control sera from the two protein batches were negative in the Gametocyte-ELISA. Sera tested in an ELISA using MBP gave similar results as compared with the M-Pfs10C ELISA. Sera from the immunization experiment with batch 2 of M-Pfs10C yielded similar results (data not shown). The ability of the antiserum to recognize native sexual stage protein was further assessed by immunofluorescence assays (IFAs) of air-dried sexual stage parasites and live intact macrogametes/zygotes in suspension IFA (SIFA). All sera reacted specifically with the antigen present in air-dried gametocytes (Fig. 5A) and on the surface of live intact gametes and zygotes of P. falciparum (Fig. 5C) but not against Pfs48/45 knockout parasites (Fig. 5B) or asexual stage parasites.
Fig. 4.
Immunogenicity of sera from mice immunized with M-Pfs10C. Antibody reactivity of sera from six mice immunized with recombinant M-Pfs10C from the first immunization experiment in the gametocyte-ELISA (Left) and M-Pfs10C ELISA (Right). S0, S1, S3, and S5 indicate preimmune sera, day 14, day 56, and day 98 of the time course of the immunization, respectively; see Materials and Methods. Boxes extend from the 25th and 75th percentiles with the median value of the six mice sera.
Fig. 5.
IFA and SIFA analysis of sera from mice immunized with M-Pfs10C. (A and B) Immunofluorescence microscopy with anti-M-Pfs10C mouse sera on P. falciparum gametocytes air-dried on a multispot slide (IFA) with wild-type parasites (A) and Pfs48/45 knockout parasites (B). (C) SIFA using live intact macrogametes/zygotes. (Magnification: ×400.)
To test for recognition of TB epitopes, M-Pfs10C immune sera (batches 1 and 2) were used in a two-site competition ELISA with a fixed amount of peroxide-conjugated rat anti-epitope I and III mAbs for binding to native Pfs48/45 (Fig. 6 and data not shown). Sera from batch 1 competed effectively for epitope I and III of Pfs48/45 at serum dilutions from 1/20 to 1/160. Ten of 12 sera of M-Pfs10C-immunized mice competed >50% with conjugated anti-epitope I and III mAbs, whereas no competition was found with adjuvant-immunized control sera or preimmune sera. Competition with nonconjugated anti-epitope I or III Pfs48/45 mAb was used as positive control and for quantification (data not shown). Estimates based on unlabeled mAb added to the assay revealed concentrations of anti-epitope I and anti-epitope III antibodies in the sera of the M-Pfs10C-immunized mice between 20 and 100 μg/ml, which is in the range in which TB activity can be expected (15). Briefly, a fixed amount of labeled mAb competed for Pfs48/45 binding with either test serum or a known concentration range of the same but unlabeled mAb. An estimate of anti-epitope-specific antibodies in the test serum was made based on comparison of OD values between test serum and labeled mAb.
Fig. 6.
Two-site competition ELISAs. mAb against epitope IIb (85RF45.2b) was used to capture Pfs48/45 molecule from gametocyte extract. Percentage competition of sera from mice immunized with the M-Pfs10C protein (batch 1) was calculated by using a fixed amount of peroxidase-conjugated mAb anti-epitope I (Ep I) or anti-epitope III (Ep III) at serum dilutions [ranging from 1/20 to 1/160 (5–0.62% vol/vol)]. Data are presented as mean percentage competition ± 1 SD of the six mice.
TB Activity Induced by M-Pfs10C.
Individual sera of mice immunized with M-Pfs10C (batches 1 and 2) after bleed 5 (S5) were analyzed for TB activity in the standard membrane-feeding assay (SMFA). Table 1 shows a near-complete blockade of transmission in 11/12 sera at a standard dilution of 1:10 (Mann–Whitney test P < 0.0001). Sera of control mice immunized with PBS and MBP alone showed no reducing activity in the SMFA. Finally, a clear correlation was observed between TB activity of the sera from M-Pfs10C-immunized mice including results obtained with a recombinant 10C fragment without the MBP fusion (15) and the percentage of competition in the epitope I competition ELISA (r2 = 0.873) (Fig. 7). The curve shows that >90% inhibition in the SMFA correlated with >40% competition for epitope I.
Table 1.
TB activity of sera from mice immunized with M-Pfs10C
| Sera | AM, mean no. | TN, n | TBA, % |
|---|---|---|---|
| Immunization batch 1 | |||
| 1A1 | 0 | 0 | 100 |
| 1A2 | 0.05 | 1 | 99.6 |
| 1A3 | 0.30 | 6 | 97.4 |
| 1A4 | 0 | 0 | 100 |
| 1A5 | 0.75 | 15 | 94.5 |
| 1A6 | 0 | 0 | 100 |
| Control | 13.6 | 263 | |
| Immunization batch 2 | |||
| 2A1 | 30.7 | 614 | 27.1 |
| 2A2 | 0 | 0 | 100 |
| 2A3 | 5.1 | 91 | 88.0 |
| 2A4 | 0.05 | 1 | 99.9 |
| 2A5 | 0.15 | 3 | 99.6 |
| 2A6 | 0 | 0 | 100 |
| Control | 42 | 843 | |
Sera (1:10 diluted) were tested in the SMFA. Seven days after membrane feed, the number of infected mosquitoes and oocyst density were determined. Sera, mouse serum from M-Pfs10C-immunized group; Control, mean of five feeders with serum from control immunization; AM, arrhythmic mean oocyst number in 20 dissected mosquitoes; TN, total number of oocysts in 20 dissected mosquitoes; TBA, percentage of TB activity (19).
Fig. 7.
TB activity correlates with the concentration of the anti-epitope I antibodies. Percentage inhibition of oocysts are related to the percentage competition of the anti-epitope I antibodies in the sera from mice after five immunizations with the rec10C (□) (15) and M-Pfs10C (♦) protein as calculated by using a fixed amount of peroxidase-conjugated mAb anti-epitope I (85RF45.1) at a dilution of 1/20 of the serum. The line represents the regression of results by use of a polygon equation (y = −1E − 06x5 + 0.0003x4 − 0.0322x3 + 1.2884x2 − 17.369x + 43.997; R value of 0.93).
Concluding Remarks.
Our data show that an N-terminally truncated Pfs48/45 protein fused to MBP, coined M-Pfs10C, and coexpressed with four periplasmic folding catalysts in the periplasm of E. coli yielded a properly folded homogeneous Pfs48/45 protein that is stable over for at least 9 months. The two independent batches of M-Pfs10C protein elicited functional TB antibodies (transmission reducing activity >90%) in >90% of the mice.
Clinical development of malaria vaccines has accelerated over recent years with a clear increase in the portfolio of candidates. Although most efforts are concentrated on preeythrocytic and blood-stage vaccines, progress in TB vaccine development has lagged behind and was basically limited to two postfertilization proteins, P25 and P28 (16).
From a biological perspective and supported by experimental data, Pfs48/45 has been for a long time considered as an attractive prefertilization target for inhibition of sporogonic development. However, technological constraints related to protein folding have hampered its development for many years. The high TB activity of the C-terminal fragment of Pfs48/45 described here provides a solid basis for the clinical development of a prefertilization TB malaria vaccine.
Materials and Methods
Construct Preparation.
A synthetic Pfs48/45 gene optimized for expression in E. coli (GenBank accession no. EU366251) encoding wild-type Pfs48/45 protein (17) was designed and used throughout this study. Fragments corresponding to processed Pfs48/45 protein residues 26–428 (16C) and N-terminally truncated (10C) residues 159–428 were cloned into pMAL-p2x (New England Biolabs), resulting in N-terminal MBP fusions. The pTUM4 plasmid has been described (14).
Expression and Purification of M-Pfs10C.
Expression and preparations of periplasmic fractions were performed essentially as described (14). The recombinant proteins were purified on a DEAE FF (Amersham Biosciences) column equilibrated in 20 mM Tris·HCl, pH 8.6. The column was washed with 50 mM NaCl, and bound proteins were eluted with a linear gradient of 50–400 mM NaCl in the same buffer. Fractions containing the M-Pfs10C protein as analyzed by Coomassie staining and ELISA were concentrated on a Vivaspin 20, 30-kDa molecular mass cut-off utrafiltration unit (Vivascience). M-Pfs10C protein was further purified over a Superdex 75 HR 10/30 column (Amersham Biosciences) in PBS that contained 0.01% N-dodecyl-N-N-dimethyl-3-ammonio-1-propane sulfonate.
Parasites.
Mature P. falciparum gametocytes (NF54 strain) were produced in an automated static culture system as described (7, 18), isolated (19), and stored at −70°C until used. NF54 gametocytes were extracted in 25 mM Tris·HCl (pH 8.0) supplemented with 150 mM NaCl, 1.0% sodium desoxycholate, and 1 mM phenylmethylsulphonyl fluoride. Insoluble debris was pelleted by centrifugation (13,000 × g for 5 min at room temperature); the supernatant provided antigen for Western blot analysis and ELISAs.
Antibodies.
To detect epitopes of Pfs48/45, various mAbs of mouse origin, 32F5 (epitope I) and 32F1 (epitope IIb) (6, 20), or rat origin, 85RF45.1 (epitope I), 85RF45.2b (epitope IIb), 85RF45.3 (epitope III), and 85RF45.5 (epitope V) (7, 19), were used. HRP-conjugated anti-mouse IgG was purchased from Dako (P0161). Alexa Fluor488 anti-mouse IgG (A21200) was purchased from Molecular Probes.
Immunization of BALB/c Mice.
Groups (n = 6) of female BALB/c mice were immunized s.c. with 50 μg of recombinant M-Pfs10C fragment per mouse or with adjuvant alone (control, n = 5) as described (15). Briefly, mice were immunized with a total volume of 0.1 ml per mouse emulsified in complete Freund's adjuvant on day 1 and boosted with the same amount of antigen on 21, 42, 63, and 84 days with incomplete Freund's adjuvant. Blood was taken on days 0 (preimmune serum), 14, 35, 56, 77, and 98 and tested for specific antibody reactivity and TB activity in the SMFA.
IFA.
An indirect IFA was done with cultured sexual-stage parasites (NF54 isolate of P. falciparum) air-dried on multispot slides as described (7, 8). Briefly, parasites were incubated with a 1:100 dilution of the test sera in PBS, rinsed with PBS, and incubated with Alexa-conjugated goat-anti-mouse Ig (IgG). The cells were examined for clear sexual-stage parasite-specific green fluorescence. For SIFA analysis, gametocytes were allowed to undergo gametogenesis as described (7, 8, 19), and the cells were examined as above under IFA.
ELISAs.
All incubation steps, except antigen coating, were done at room temperature.
Detection of Pfs48/45.
Pfs48/45-specific ELISA was performed by a two-site ELISA as described (7, 8, 15). Briefly, 96-well microtiter plates were coated with rat mAbs recognizing particular epitopes of Pfs48/45 (19). After blocking with 5% milk in PBS, the antigen (starting with 250,000 parasites per well or 20 μg/ml M-Pfs10C) was captured by incubation in a serial dilution with PBS, and the bound antigen was detected by HRP-conjugated rat mAbs.
Gametocyte and M-Pfs10C ELISA.
Serum antibody analysis was conducted by ELISA with whole gametocyte extracts or M-Pfs10C ELISA as described (15). Briefly, ELISA plates were coated with enriched gametocyte antigen extract (250,000 parasites per well) or 2 μg/ml M-Pfs10C diluted in PBS, stored at 4°C overnight, and then blocked with 5% skimmed milk/PBS. Diluted sera were added and incubated for 2 h, and bound antibodies were detected by HRP-conjugated goat anti-Mouse IgG.
Competition ELISA.
The competition ELISA was performed by a two-site ELISA as described (7, 8, 15, 19). Briefly, gametocyte extract was captured essentially as for the Pfs48/45-specific ELISA by using mAb 85RF45.2b. Competition of HRP-labeled mAb 85RF45.1 or 85RF.45.3 with sera antibodies from M-Pfs10C-immunized mice was performed by incubation of 30 μl of HRP-labeled mAb (2.5 μg/ml, diluted with PBS containing 1% milk) and 30 μl of mice serum (dilutions ranging from 1/20 to 1/160) or 30 μl of unlabeled mAb (5-fold serially diluted started at 5 μg/ml) for 2 h.
TB Assay.
Antisera obtained from mice immunized with the M-Pfs10C fragment were tested for their TB activities in a SMFA as described (15, 21, 22). Briefly, 27 μl of the mice sera was mixed with 63 μl of naïve human serum and 180 μl of in vitro gametocyte culture of P. falciparum (NF54 line). This mixture was fed to Anopheles stephensi (Nijmegen strain) mosquitoes through a membrane feeding apparatus, and 7 days later at least 20 mosquitoes (>90% survival) were dissected and oocysts were counted from extracted midguts of test and control mosquitoes.
Acknowledgments.
We thank Dr. Arne Skerra (Technische Universitat, Munich) for pTUM4 plasmid, Karina Teelen for technical assistance, and Rumyana Karlova for stimulating discussions. This work was supported by European Malaria Vaccine Initiative Grant 041222 and BioMalPar.
Footnotes
The authors declare no conflict of interest.
References
- 1.Bousema JT, Drakeley CJ, Sauerwein RW. Sexual-stage antibody responses to P. falciparum in endemic populations. Curr Mol Med. 2006;6:223–229. doi: 10.2174/156652406776055140. [DOI] [PubMed] [Google Scholar]
- 2.Carter R, Mendis KN, Miller LH, Molineaux L, Saul A. Malaria transmission-blocking vaccines: How can their development be supported? Nat Med. 2000;6:241–244. doi: 10.1038/73062. [DOI] [PubMed] [Google Scholar]
- 3.Stowers A, Carter R. Current developments in malaria transmission-blocking vaccines. Exp Opin Biol Ther. 2001;1:619–628. doi: 10.1517/14712598.1.4.619. [DOI] [PubMed] [Google Scholar]
- 4.Kaslow DC. Transmission-blocking vaccines. Chem Immunol. 2002;80:287–307. doi: 10.1159/000058850. [DOI] [PubMed] [Google Scholar]
- 5.van Dijk MR, et al. A central role for P48/45 in malaria parasite male gamete fertility. Cell. 2001;104:153–164. doi: 10.1016/s0092-8674(01)00199-4. [DOI] [PubMed] [Google Scholar]
- 6.Vermeulen AN, et al. Plasmodium falciparum transmission blocking monoclonal antibodies recognize monovalently expressed epitopes. Dev Biol. Stand. 1985;62:91–97. [PubMed] [Google Scholar]
- 7.Roeffen W, et al. Plasmodium falciparum: Production and characterization of rat monoclonal antibodies specific for the sexual-stage Pfs48/45 antigen. Exp Parasitol. 2001;97:45–49. doi: 10.1006/expr.2000.4586. [DOI] [PubMed] [Google Scholar]
- 8.Roeffen W, et al. A comparison of transmission-blocking activity with reactivity in a Plasmodium falciparum 48/45-kDa molecule-specific competition enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 1995;52:60–65. doi: 10.4269/ajtmh.1995.52.60. [DOI] [PubMed] [Google Scholar]
- 9.Bousema JT, et al. Rapid onset of transmission-reducing antibodies in javanese migrants exposed to malaria in Papua, Indonesia. Am J Trop Med Hyg. 2006;74:425–431. [PubMed] [Google Scholar]
- 10.Bousema JT, et al. A longitudinal study of immune responses to Plasmodium falciparum sexual stage antigens in Tanzanian adults. Parasite Immunol. 2007;29:309–317. doi: 10.1111/j.1365-3024.2007.00948.x. [DOI] [PubMed] [Google Scholar]
- 11.Sauerwein RW. Malaria transmission-blocking vaccines: The bonus of effective malaria control. Microbes Infect. 2007;9:792–795. doi: 10.1016/j.micinf.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Frand AR, Cuozzo JW, Kaiser CA. Pathways for protein disulphide bond formation. Trends Cell Biol. 2000;10:203–210. doi: 10.1016/s0962-8924(00)01745-1. [DOI] [PubMed] [Google Scholar]
- 13.Baneyx F, Mujacic M. Recombinant protein folding and misfolding in Escherichia coli. Nat Biotechnol. 2004;22:1399–1408. doi: 10.1038/nbt1029. [DOI] [PubMed] [Google Scholar]
- 14.Schlapschy M, Grimm S, Skerra A. A system for concomitant overexpression of four periplasmic folding catalysts to improve secretory protein production in Escherichia coli. Protein Eng Des Sel. 2006;19:385–390. doi: 10.1093/protein/gzl018. [DOI] [PubMed] [Google Scholar]
- 15.Outchkourov N, et al. Epitope analysis of the malaria surface antigen pfs48/45 identifies a subdomain that elicits transmission-blocking antibodies. J Biol Chem. 2007;282:17148–17156. doi: 10.1074/jbc.M700948200. [DOI] [PubMed] [Google Scholar]
- 16.Epstein JE, Giersing B, Mullen G, Moorthy V, Richie TL. Malaria vaccines: Are we getting closer? Curr Opin Mol Ther. 2007;9:12–24. [PubMed] [Google Scholar]
- 17.Kocken CH, et al. Cloning and expression of the gene coding for the transmission blocking target antigen Pfs48/45 of Plasmodium falciparum. Mol Biochem Parasitol. 1993;61:59–68. doi: 10.1016/0166-6851(93)90158-t. [DOI] [PubMed] [Google Scholar]
- 18.Ponnudurai T, Lensen AH, Leeuwenberg AD, Meuwissen JH. Cultivation of fertile Plasmodium falciparum gametocytes in semiautomated systems. 1. Static cultures. Trans R Soc Trop Med Hyg. 1982;76:812–818. doi: 10.1016/0035-9203(82)90116-x. [DOI] [PubMed] [Google Scholar]
- 19.Roeffen WF, et al. Recombinant human antibodies specific for the Pfs48/45 protein of the malaria parasite Plasmodium falciparum. J Biol Chem. 2001;276:19807–19811. doi: 10.1074/jbc.M100562200. [DOI] [PubMed] [Google Scholar]
- 20.Vermeulen AN, et al. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J Exp Med. 1985;162:1460–1476. doi: 10.1084/jem.162.5.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Kolk M, et al. Evaluation of the standard membrane feeding assay (SMFA) for the determination of malaria transmission-reducing activity using empirical data. Parasitology. 2005;130:13–22. doi: 10.1017/s0031182004006067. [DOI] [PubMed] [Google Scholar]
- 22.Lensen A, et al. Measurement by membrane feeding of reduction in Plasmodium falciparum transmission induced by endemic sera. Trans R Soc Trop Med Hyg. 1996;90:20–22. doi: 10.1016/s0035-9203(96)90464-2. [DOI] [PubMed] [Google Scholar]