Abstract
Lipid homeostasis and inflammation are key determinants in atherogenesis, exemplified by the requirement of lipid-laden, foam cell macrophages for atherosclerotic lesion formation. Although the nuclear receptor PPARδ has been implicated in both systemic lipid metabolism and macrophage inflammation, its role as a therapeutic target in vascular disease is unclear. We show here that orally active PPARδ agonists significantly reduce atherosclerosis in apoE−/− mice. Metabolic and gene expression studies reveal that PPARδ attenuates lesion progression through its HDL-raising effect and anti-inflammatory activity within the vessel wall, where it suppresses chemoattractant signaling by down-regulation of chemokines. Activation of PPARδ also induces the expression of regulator of G protein signaling (RGS) genes, which are implicated in blocking the signal transduction of chemokine receptors. Consistent with this, PPARδ ligands repress monocyte transmigration and macrophage inflammatory responses elicited by atherogenic cytokines. These results reveal that PPARδ antagonizes multiple proinflammatory pathways and suggest PPARδ-selective drugs as candidate therapeutics for atherosclerosis.
Keywords: inflammation, ligand, mouse, nuclear, receptor
Extensive research implicates inflammation associated with lipid dysregulation in the pathogenesis of atherosclerosis (1, 2). A network of proinflammatory cytokines, including interleukin-1β (IL-1β), tumor necrosis factor α (TNFα), and IFNγ, trigger chemokine production, leukocyte recruitment, smooth muscle cell migration, and ultimately, plaque rupture. The crucial role for chemokines in this process is exemplified by studies demonstrating that mice lacking monocyte chemoattractant protein-1 (MCP-1/CCL2) or its receptor CCR2 are highly protected against atherosclerosis (3–6). Thus, therapeutic manipulation to improve serum lipid profiles or attenuate inflammation is expected to control the progression of this disease.
Peroxisome proliferator-activated receptors (PPARs) are nuclear receptors activated by dietary fats and have critical functions in lipid homeostasis (7, 8). PPARα is the target of the fibrate class of lipid-lowering drugs (9–11). It is most highly expressed in the liver where it regulates fatty acid β-oxidation (8, 12). PPARγ is essential for adipocyte differentiation and fat storage (13–16). It is also the molecular target of the thiazolidinedione (TZD) class of insulin-sensitizing drugs (17, 18). PPARδ (also known as PPARβ) has the broadest expression of the PPAR isotypes, and its biological function has recently been examined through genetic manipulation (19–21). Restricted activation of this receptor in either muscle or adipose tissue results in a lean phenotype because of increased fatty acid β-oxidation (22, 23). Long-term treatment with a PPARδ investigational ligand GW501516 causes dramatic weight loss accompanied by improvements in lipoprotein profiles (23). Recently, we have shown that ligand treatment reduces hyperglycemia and improves insulin sensitivity in db/db mice (24). In addition, PPARδ has been implicated in keratinocyte homeostasis and hepatic lipoprotein production (25–27).
Based on their functions in lipid metabolism and inflammation, the potential for PPARs to modulate atherosclerosis has been explored. Several studies have demonstrated that PPARγ agonists reduce vascular lesion size, in part, by activating the LXRα-ABCA1 pathway and directly regulating ABCG1 in the macrophage to promote cholesterol efflux (28–30). A high-affinity PPARδ agonist GW501516 has been shown to up-regulate ABCA1 expression in human monocytic cell lines and increase high-density lipoprotein cholesterol (HDL-c) in monkeys (31), suggesting that PPARδ may suppress atherogenesis through a similar mechanism. However, our previous study using both loss- and gain-of-function approaches and work by others indicates that PPARδ does not directly regulate cholesterol efflux in the mouse macrophage (30, 32). Instead, it regulates the metabolism of very-low-density lipoprotein-derived fatty acid and is capable of down-regulating inflammatory mediators including IL-1β and MCP-1 (32, 33). Recently, two independent studies examined the effect of a less characterized PPARδ agonist GW0742 on lesion progression in fat- and cholesterol-supplemented Ldlr−/− mice and produced divergent results (30, 34). In the first study, GW0742 treatment did not affect the total lesion area after 14 weeks of drug treatment. In the second study, GW0742 reduced the lesion size after 10 weeks of treatment but only through a more aggressive dosing regimen. Neither study, however, detected the increase in HDL-c associated with GW501516 treatment.
Because it remains unclear whether PPARδ drugs could modulate atherosclerosis, we examined the effect of GW501516 (GW) on lesion development in apoE−/− mice. Low doses of the PPARδ agonist GW501516 significantly reduced atherosclerotic lesions and increased HDL-c, although they had no effect on total cholesterol levels. Expression profiling of the aortas of treated mice suggested that multiple chemokine-mediated cell migration pathways are down-regulated by ligand treatment. Consistent with this observation, activation of PPARδ represses the expression of chemoattractants MCP-1, MCP-3, and MCP-5 induced by IL-1β and IFNγ in cultured macrophages. In addition, monocytes pretreated with GW501516 exhibit reduced transendothelial migration. These results provide molecular targets through which PPARδ suppresses atherogenic inflammation and substantiate PPARδ-selective drugs as potential therapeutics to treat atherosclerosis.
Results
PPARδ Inhibits Atherosclerotic Lesion Formation.
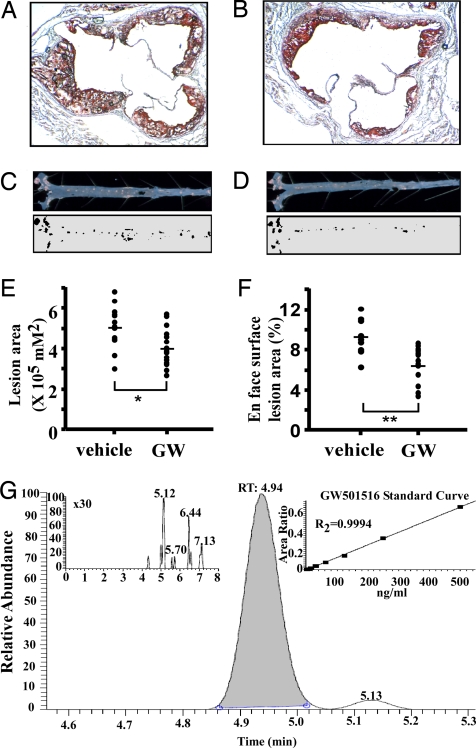
A high-affinity PPARδ agonist, GW501516 (GW), has been reported to increase HDL-c and reduce circulating triglycerides in monkeys and humans (31, 35). To determine whether PPARδ could modulate atherosclerotic lesion progression, we examined the effect of GW on lesion development in apoE−/− mice. Ten-week-old male apoE−/− mice were placed on a high-fat (HF) diet and treated with either vehicle or GW (n = 15 for each group) at 2 mg·kg−1·day−1 for 8 weeks. Atherosclerotic lesions were subsequently determined by two methods: cross-sectional analysis of the aortic valves and en face analysis of the aorta. Examination of the oil red O-stained area of the aortic valves revealed fewer lesions in GW-treated mice (Fig. 1 A and B). Quantitative analyses further suggested that ligand treatment reduced aortic valve lesions by 20% (Fig. 1E; control, 504,904.2 ± 33,357.3; GW, 403,632.2 ± 2,5261.1 μm2, P = 0.016). Similarly, GW decreased the number of fatty streaks throughout the aorta (Fig. 1 C and D) with a 30% reduction in total lesion area (control, 9.25 ± 0.55; GW, 6.53 ± 0.46%, P = 0.002) (Fig. 1F). Serum GW501516 concentrations were ≈106 nM in drug-treated mice and undetectable in controls, as determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS) (Fig. 1G). The former concentrations of GW exceed its reported EC50 for murine PPARδ (20 nM) but are far below the EC50 for PPARα (2.5 μM) or PPARγ (1 μM), indicating that specific activation of PPARδ inhibits lesion formation (31, 36).
Fig. 1.
PPARδ activation protects against atherosclerosis. (A and B) Representative oil red O-stained sections of aortic valves from vehicle (A) or GW501516 (GW)-treated (B) apoE−/− mice. Ten-week-old male apoE−/− mice on an atherogenic diet were gavaged daily with either vehicle or GW, a high-affinity PPARδ agonist, at 2 mg·kg−1·day−1 for 8 weeks. (C and D) En face lesion area in representative vehicle (C) or GW-treated (D) aortas. (E and F) Quantitative analysis of the lesion size in aortic valves (E) and aortas (F) (n = 15). GW treatment significantly reduced lesion sizes. *, P < 0.05; **, P < 0.005 Mann–Whitney U test. (G) LC-MS/MS quantitation of GW drug levels in pooled serum from vehicle (Inset Left) versus drug-treated mice. Standard curve for quantitation of GW levels (Inset Right).
PPARδ Increases HDL-c in apoE−/− Mice.
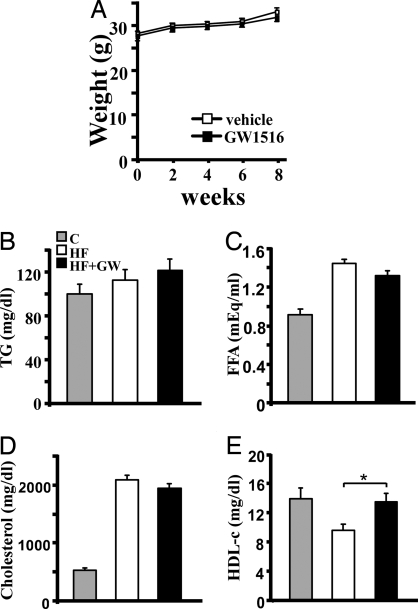
To determine the systemic effects of ligand administration, metabolic parameters were measured. Both control and ligand-treated groups consumed an equal amount of food and gained similar weight during the experimental period (Fig. 2A and data not shown). Because long-term treatment of GW compound at a higher dose causes weight loss (23, 37), this regimen minimized the indirect effects resulting from weight differences. Interestingly, circulating levels of glucose (control, 164.06 ± 3.65; GW, 155.73 ± 3.92 mg/dl), insulin (control, 0.71 ± 0.08; GW, 0.75 ± 0.13 ng/ml) and triglycerides (Fig. 2B) were not elevated on this diet, nor were they affected by GW treatment. GW also had no effect on levels of free fatty acid or cholesterol, which were increased significantly after HF challenge (Fig. 2 C and D). HDL-c, however, was lowered by HF and was restored by GW to levels similar to those of apoE−/− mice on a normal chow diet (Fig. 2E). This HDL-raising effect is consistent with results in monkeys but contrasts with previous studies in Ldlr−/− mice that used the alternative PPARδ agonist GW0742 (30, 34).
Fig. 2.
PPARδ increases HDL-c in apoE−/− mice. (A) GW does not affect weight gain. (B–D) Similar levels of serum triglyceride, free fatty acid (FFA), and cholesterol are observed between vehicle- and GW-treated groups. C, chow diet; HF, high-fat diet; HF+GW, high-fat diet with GW treatment. (E) An increase in HDL cholesterol occurs in GW-treated apoE−/− mice. *, P < 0.05.
Chemokine Signaling Pathways Are the Major Targets of PPARδ in Vivo.
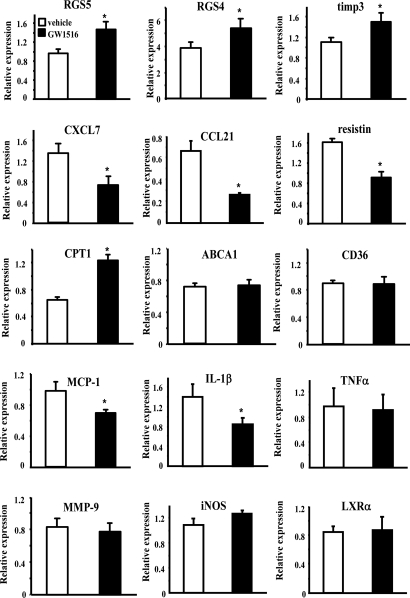
To understand how PPARδ modulates lesion progression, we isolated aortas from control and ligand-treated mice to compare the expression profiles at lesion sites by using DNA array analysis followed by real-time PCR verification. Consistent with our previous study, no cholesterol efflux genes were affected by ligand treatment. At this low dose, carnitine palmitoyltransferase 1 (CPT-1), a known PPARδ target gene regulating the rate-limiting step of fatty acid β-oxidation (22, 37), was the only verifiable gene related to lipid homeostasis identified in the array (Table 1 and Fig. 3). The majority of the regulated genes are involved in inflammatory signaling pathways (Table 1 and Fig. 3). Changes include the down-regulation of IFN-stimulated protein (Isg20), CXCL7, CCL21α, resistin, and chemokine-like factor superfamily 6 and up-regulation of regulator of G protein signaling (RGS) 4 and 5 and tissue inhibitor of metalloproteinase 3 (TIMP3). Among these, chemokines CXCL7 and CCL21α have been shown to induce neutrophil and T lymphocyte recruitment, respectively (38–41), whereas RGSs antagonize the signaling mediated by chemokine receptors, and TIMP3 inhibits smooth muscle cell migration and maintains lesion stability (42, 43).
Table 1.
PPARδ regulated genes identified by DNA array analysis
| Array* | Q-PCR* | |
|---|---|---|
| Inflammation | ||
| Regulator of G protein signaling 3 (Mm.286753†) | 1.5‡ | |
| Regulator of G protein signaling 4 (Mm.54164) | 1.4 | 1.4 |
| Regulator of G protein signaling 5 (Mm.20954) | 1.5 | 1.5 |
| Tissue inhibitor of metalloproteinase 3 (Mm.132958) | 1.4 | 1.4 |
| Interferon-stimulated protein (Isg20, Mm.19019) | −1.5 | −1.7 |
| CXCL7 (Mm.293614) | −2.5 | −1.8 |
| CCL21α (Mm.22085) | −1.4 | −1.7 |
| Resistin (Mm.1181) | −1.4 | −1.8 |
| Chemokine-like factor superfamily 6 (Mm.28858) | −1.6 | |
| Lipid oxidation | ||
| Carnitine palmitoyltransferase 1 (Mm.18522) | 1.8 | 1.9 |
*Minus sign indicates down-regulation.
†UniGene number.
‡Fold changes comparing ligand treated to the control group.
Fig. 3.
Real-time PCR analyses of potential PPARδ target genes in the aorta. Verification of PPARδ regulated genes identified by DNA array experiments and examination of additional atherosclerosis-related genes by using whole aorta RNA from vehicle- or GW-treated apoE−/− mice. Results are presented as mean (of three mice) ± SEM. *, P < 0.05.
We also examined the expression of other factors critical for lesion development, which were either not included in the gene collection of the array or did not show differences by the analysis. Consistent with our previous results in cultured macrophages, real-time PCR demonstrated that GW suppressed the expression of MCP-1 and IL-1β but not TNFα or MMP-9 (Fig. 3), whereas IFNγ was undetectable (data not shown). Known PPARγ targets ABCA1 (29), CD36 (44), iNOS (45), and LXRα (29) (Fig. 3) were not altered, nor was there a difference in the expression of PPARδ or PPARγ (data not shown). These data implicate PPARδ and PPARγ in distinct transcriptional programs to ameliorate atherogenesis and suggest that chemokine-mediated cell migration is a major target of PPARδ in the aorta. Because leukocyte recruitment plays a critical role in atherogenesis, this regulatory mechanism may account for the reduced lesion area by GW, in addition to its HDL-raising effect.
PPARδ Suppresses Chemokine Expression in Vitro.
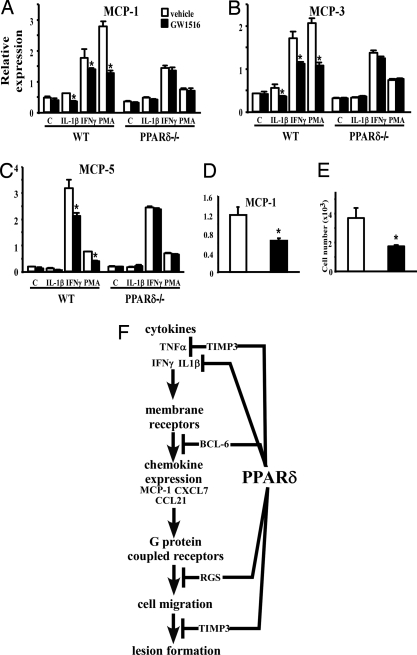
The ability of GW to down-regulate the expression of several chemokines prompted us to investigate whether PPARδ does so by attenuating signaling pathways initiated by proatherogenic cytokines. Expression analysis showed that GW treatment significantly down-regulated MCP-1, MCP-3, and MCP-5 expression by macrophages stimulated with IL-1β, IFNγ, and phorbal 12-myristate 13-acetate (PMA). This was abolished in PPARδ−/− cells, indicating a receptor-dependent mechanism (Fig. 4A–C). Consistent with this, MCP-1 expression was reduced by 50% in primary macrophages derived from GW-treated apoE−/− mice compared with vehicle controls (Fig. 4D). To determine whether this regulation was of functional consequence, we examined the effect of GW on transmigration. Monocytic THP-1 cells were preincubated with either vehicle or GW and subjected to an MCP-1 gradient compartmented by an endothelial cell monolayer. GW treatment significantly reduced the number of THP-1 monocytes that migrated through the endothelial layer (Fig. 4E). Collectively, these results suggest that PPARδ can attenuate multiple inflammatory signaling pathways to inhibit cell migration and lesion progression.
Fig. 4.
Activation of PPARδ attenuates chemoattractant signaling. (A–C) Real-time PCR demonstrating ligand treatment suppresses chemokine expression induced by IL-1β, IFNγ, and PMA in a receptor-dependent manner. Thioglycollate-elicited peritoneal macrophages were isolated from wild-type (wt) or PPARδ−/− mice and stimulated with the indicated stimulant in the presence or absence of ligand. (D) Reduced MCP-1 expression in peritoneal macrophages isolated from ligand treated apoE−/− mice. (E) PPARδ ligand treatment inhibits monocyte transmigration. THP-1 monocytes pretreated with vehicle or GW were examined for their ability to migrate through an endothelial cell monolayer along an MCP-1 gradient. (F) Schematic demonstrating that PPARδ inhibits inflammatory signaling through multiple downstream effectors. We have previously shown that PPARδ regulates the expression of the cytokine IL-1β and ligand-bound PPARδ releases the transcriptional repressor BCL-6 to repress the expression of chemokines such as MCP-1. In this study, RGSs and TIMP-3 are shown to be up-regulated by PPARδ activation. RGSs block chemokine receptor signal transduction, whereas TIMP-3 inhibits TNFα shedding and cell migration. *, P < 0.05.
Discussion
Significant efforts have focused on the role of PPARδ in lipid homeostasis because of its ability to increase fatty acid oxidation. This study uncovers regulatory pathways for PPARδ to limit atherosclerosis by improving systemic lipid profiles and repressing inflammation. HDL-c is believed to be protective against atherosclerosis. Synthetic ligands of PPARδ have been shown to increase HDL-c in monkeys and rodents and thus may have therapeutic potential to treat this disease (31). We have also observed increased HDL-c in GW-treated apoE−/− mice. The molecular mechanism underlying this observation remains to be determined, given that PPARδ does not regulate genes involved in cholesterol uptake or efflux in aortas. It is unlikely that the atheroprotective effects of PPARδ are contributed solely by its modest enhancement of HDL-c, however, and the anti-inflammatory aspects of PPARδ function may play a more dominant role.
Consistent with this notion, our data suggest that the anti-inflammatory aspects of the PPARδ function may have an important role in inhibiting leukocyte recruitment and thus lesion progression by down-regulating the expression of chemokines, attenuating chemokine receptor signaling, and inhibiting matrix metalloproteinases (Fig. 3). Indeed, expression analysis demonstrates multitiered suppression of chemoattractant signaling by PPARδ (Fig. 4F). First, it down-regulates the expression of chemokines including MCP-1, CXCL7, and CCL21, which mediate the recruitment of macrophages, neutrophils, and T lymphocytes, respectively. In vitro studies suggest that PPARδ does so by suppressing inflammatory responses elicited by cytokines such as IL-1β and IFNγ (Fig. 4 A–C). In addition, PPARδ may attenuate chemokine receptor signaling by the induction of RGS proteins, which interact with Gα subunits to accelerate GTP hydrolysis and terminate G protein signals (42). Although the functions of these family members are not well defined, several of them, including RGS4, have been shown to antagonize chemokine receptor-mediated lymphocyte migration when overexpressed (46). RGS5, however, is a marker for arterial smooth muscle cells. Its expression is down-regulated in atherosclerotic plaques, whereas its up-regulation correlates with statin-induced atheroprotection in apoE−/− mice (47–49). In independent studies, we have identified RGS genes 1, 10, 16, and 18 as PPARδ-regulated targets in the macrophage, suggesting modulation of G protein-coupled pathways as a common regulatory mechanism of this receptor (data not shown). Last, PPARδ may block cell migration through TIMP-3, which inhibits the activity of matrix metalloproteinases (MMPs), thereby maintaining integrity of the extracellular matrix. TIMP-3 can also inhibit TNFα-converting enzyme (TACE), resulting in reduced TNF shedding, indicating that PPARδ may suppress TNFα signaling posttranslationally (50).
The basis for the discrepant effects of PPARδ ligands on atherosclerosis observed in this versus two prior studies (30, 34), which used a less characterized PPARδ agonist GW0742, is unclear. Several lines of evidence suggest that chemically distinct PPARδ agonists exhibit different biological activities. For instance, at high doses, GW501516 causes weight loss, decreases serum TGs, and increases HDL-c, whereas GW0742 increased weight gain and has weak effects on TGs and no effects on HDL-c (34). We show that treatment with GW501516 at 2 mg·kg−1·day−1 significantly reduces lesions in 8 weeks, whereas GW0742 is effective only at 60 mg·kg−1·day−1 for 10 weeks. In the latter case, drug administration resulted in signs of severe liver enlargement and serum drug levels up to 21 μM, which is 420- and 700-fold higher than the reported EC50 for mouse and human PPARδ, respectively, raising questions about drug efficacy and specificity in vivo. We have recently demonstrated that GW501516 increases insulin sensitivity in a receptor-dependent manner in obese mice dosed with a similar drug regimen (24). Moreover, the ≈106 nM serum concentrations of GW501516 detected with drug treatment in this study indicate that the atheroprotection herein observed is very likely mediated by PPARδ.
Besides distinctions in the chemical properties and biological activities of PPARδ drug compounds, the mouse models and diets used distinguish the studies to date. Hypercholesterolemia is clearly essential for atherogenesis to proceed because there are no animal models without it, and the degree of atherosclerosis is related to the degree of hypercholesterolemia. However, inflammation is also a dominant factor promoting lesion formation, and although likely proportional to hypercholesterolemia, it is influenced by many other factors. In the study by Li et al. (30), in which no atheroprotection for the PPARδ drug GW0742 was observed, it is possible that the extensive, 1.25% dietary cholesterol content, versus 0.25% in Graham et al. (34) or 0.15% cholesterol in this study, produced a model in which the hyperlipidemic factor overshadowed other inflammatory components. With the exception of patients with homozygous familial hypercholesterolemia, such hypercholesterolemia is not likely to occur in humans.
Because the evidence is that the activity of PPARδ is predominantly anti-inflammatory, human atherogenesis, typified by more modest hypercholesterolemia, or murine models of atherogenesis in which inflammation is amplified, are predicted to be more amenable to therapeutic intervention by PPARδ drugs. Together with its functions in fatty acid and HDL-c metabolism, PPARδ represents an attractive therapeutic target for drug development to treat atherosclerosis.
Materials and Methods
Animal Experiments.
PPARδ knockout mice were generated as described in ref. 19. Ten-week-old male apoE−/− mice under C57 background were purchased from The Jackson Laboratory (stock no. 002052) and challenged with an atherogenic diet (0.15% cholesterol and 21% fat, TD88137, Harlan Teklad) for 8 weeks. These animals were divided into two groups (15 mice per group); one was given GW501516 at 2 mg·kg−1·day−1 daily by gavage and the other was given vehicle control. GW501516 was dissolved in DMSO and suspended in 0.5% carboxymethylcellulose. All animal procedures were approved by and carried out under the guidelines of the Institutional Animal Care and Use Committee of the Salk Institute.
Lesion Analysis.
Mice were killed and perfused with 4% paraformaldehyde. Atherosclerotic lesions at aortic valves were identified by oil red O staining. The mean lesion area for each animal was determined by five sections taken every 40 μm. To examine lesions in the aorta, aortas were dissected out and stained with Sudan IV. The extent of lesion formation is expressed as the percentage of the total aortic surface area covered by lesions. Images were taken by a video camera and lesion area was determined by using Adobe Photoshop and National Institutes of Health Scion Image software. Statistics were performed by using the Mann–Whitney U test.
Liquid Chromatography-Tandem Mass Spectrometry.
Solid-phase extraction on Strata-X columns (Phenomenex) was used to prepare 100-μl samples of pooled serum containing 100 ng of internal standard (GW0742) for LC-MS/MS quantitation. The treatment drug and internal standard were subsequently resolved on a 2 × 50 mm Synergi Polar-RP column by using an acetonitrile/0.1% formic acid gradient and detected by selective reaction monitoring (452/394, GW1516; 470/412, and GW0742) on a Thermo LTQ-XL mass spectrometer. A standard curve was constructed by using drug-spiked mouse serum to facilitate quantitation.
Metabolic Measurement.
Serum and plasma were collected after 6 h of fasting. Total cholesterol, triglyceride, and free fatty acids were determined by using enzymatic reactions (Thermo DMA; free fatty acid, Wako Chemicals). HDL cholesterol was determined by using a HDL cholesterol dextran sulfate-precipitating reagent set (Pointe Scientific). Blood glucose was measured through the tail tip by using the OneTouch (Lifescan) glucose-monitoring system. Insulin was measured by using RIA kits (Linco). Statistics were performed by using Student's t test. Values are presented as means ± SEM. Significance was established at P < 0.05.
Gene Expression Analysis.
RNA was isolated by using TRIzol reagent (Invitrogen). For DNA array analysis, aorta RNA samples from three mice of each group were amplified and hybridized to GeneChip 430A 2.0 (Affymetrix). Target genes were determined by using both ANOVA (P < 0.05) and fold change (>1.4-fold) with the assistance of the GeneSpring software (Silicon Genetics) and the Bullfrog program. The results were confirmed by real-time PCR. For real-time PCR, 1 μg of RNA was DNase-treated and reverse-transcribed by using oligo(dT) (SuperScript II kit; Invitrogen) and then treated with RNase. Samples were run in triplicate with SYBR Green (Applied Biosystems) and compared with levels of 36B4 as a control.
Macrophage Studies.
Resident macrophages were isolated from the peritoneum of vehicle- or GW-treated apoE−/− mice before collecting their aortas to analyze the expression of MCP-1 in vivo. For in vitro studies, peritoneal macrophages were isolated 3 days after thioglycollate challenge and cultured in RPMI, 10% FBS. Cells were treated with the indicated stimulant in the presence or absence of ligand for 18 h before harvesting. Concentrations used were: GW501516, 0.1 μM; IL-1β, 1 ng/ml; IFNγ, 100 units/ml; and PMA, 25 ng/ml. Cytokines and chemokines were purchased from BD Biosciences (PharMingen).
For the transmigration assay, THP-1 cells (1 × 105) were pretreated with GW501516 overnight and added to a human umbilical vein endothelial cell monolayer covering a gelatin-coated membrane (8 μm, Transwell, Costar). Migration was induced by the addition of human monocyte chemoattractant protein-1 (MCP-1; 100 ng/ml) to the lower compartment. Migrated cells were determined after 30 min.
Acknowledgments.
We thank Drs. J. Plutzky (Harvard Medical School), R. Tangirala [University of California, Los Angeles (UCLA)], W. Hsueh (UCLA), C. Glass [University of California at San Diego (UCSD)], and J. Witztum (UCSD) for valuable comments, G. Bradshaw for help in lesion analyses, and S. Ganley and E. Ong for administrative assistance. This work was supported by National Institutes of Health (NIH) National Research Service Award Grants T32 CA009370, T32 HL007770, and F32 DK071478 (to G.D.B.); by CMG training grant (Department of Biology, UCSD) (P.O.), the Chapman Fellowship and the Molecular Neurobiology grant at the Salk Institute (P.O.), and the Achievement Rewards for College Students Foundation (P.O.); MarineBio 21 (Ministry of Maritime Affairs and Fisheries, Korea) (H.J.K.); and by the American Heart Association and American Diabetes Association (C.H.L.) and the Howard Hughes Medical Institute, NIH Grant 5R37DK057978, UCSD Specialized Center for Research in Molecular Medicine and Atherosclerosis Grant 5P50HL56989, and the NIH Nuclear Receptor Signaling Atlas orphan receptor program Grant U19DK62434-01. R.M.E. is an investigator of the Howard Hughes Medical Institute at the Salk Institute for Biological Studies and March of Dimes Chair in Molecular and Developmental Biology.
Footnotes
The authors declare no conflict of interest.
References
- 1.Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 3.Gosling J, et al. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest. 1999;103:773–778. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu L, et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 5.Dawson TC, Kuziel WA, Osahar TA, Maeda N. Absence of CC chemokine receptor-2 reduces atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 1999;143:205–211. doi: 10.1016/s0021-9150(98)00318-9. [DOI] [PubMed] [Google Scholar]
- 6.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2-/- mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 7.Lee CH, Olson P, Evans RM. Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology. 2003;144:2201–2207. doi: 10.1210/en.2003-0288. [DOI] [PubMed] [Google Scholar]
- 8.Kersten S, et al. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci USA. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kliewer SA, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci USA. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krey G, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol. 1997;11:779–791. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- 12.Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci USA. 1999;96:7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He W, et al. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci USA. 2003;100:15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barak Y, et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 15.Kubota N, et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 16.Rosen ED, et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 17.Lehmann JM, et al. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 18.Forman BM, et al. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 19.Barak Y, et al. Effects of peroxisome proliferator-activated receptor delta on placentation, adiposity, and colorectal cancer. Proc Natl Acad Sci USA. 2002;99:303–308. doi: 10.1073/pnas.012610299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters JM, et al. Growth, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor beta(delta). Mol Cell Biol. 2000;20:5119–5128. doi: 10.1128/mcb.20.14.5119-5128.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barish GD, Narkar VA, Evans RM. PPAR delta: A dagger in the heart of the metabolic syndrome. J Clin Invest. 2006;116:590–597. doi: 10.1172/JCI27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y-X, et al. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2004;2:e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang YX, et al. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell. 2003;113:159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 24.Lee CH, et al. PPARdelta regulates glucose metabolism and insulin sensitivity. Proc Natl Acad Sci USA. 2006;103:3444–3449. doi: 10.1073/pnas.0511253103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michalik L, et al. Impaired skin wound healing in peroxisome proliferator-activated receptor (PPAR)alpha and PPARbeta mutant mice. J Cell Biol. 2001;154:799–814. doi: 10.1083/jcb.200011148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di-Poi N, Tan NS, Michalik L, Wahli W, Desvergne B. Antiapoptotic role of PPARbeta in keratinocytes via transcriptional control of the Akt1 signaling pathway. Mol Cell. 2002;10:721–733. doi: 10.1016/s1097-2765(02)00646-9. [DOI] [PubMed] [Google Scholar]
- 27.Akiyama TE, et al. Peroxisome proliferator-activated receptor beta/delta regulates very low density lipoprotein production and catabolism in mice on a Western diet. J Biol Chem. 2004;279:20874–20881. doi: 10.1074/jbc.M312802200. [DOI] [PubMed] [Google Scholar]
- 28.Li AC, et al. Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2000;106:523–531. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chawla A, et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 30.Li AC, et al. Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARalpha, beta/delta, and gamma. J Clin Invest. 2004;114:1564–1576. doi: 10.1172/JCI18730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliver WR, Jr, et al. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci USA. 2001;98:5306–5311. doi: 10.1073/pnas.091021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CH, et al. Transcriptional repression of atherogenic inflammation: modulation by PPARdelta. Science. 2003;302:453–457. doi: 10.1126/science.1087344. [DOI] [PubMed] [Google Scholar]
- 33.Chawla A, et al. PPARdelta is a very low-density lipoprotein sensor in macrophages. Proc Natl Acad Sci USA. 2003;100:1268–1273. doi: 10.1073/pnas.0337331100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham TL, Mookherjee C, Suckling KE, Palmer CN, Patel L. The PPARdelta agonist GW0742X reduces atherosclerosis in LDLR(-/-) mice. Atherosclerosis. 2005;181:29–37. doi: 10.1016/j.atherosclerosis.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 35.Sprecher DL, et al. Triglyceride:high-density lipoprotein cholesterol effects in healthy subjects administered a peroxisome proliferator activated receptor delta agonist. Arterioscler Thromb Vasc Biol. 2007;27:359–365. doi: 10.1161/01.ATV.0000252790.70572.0c. [DOI] [PubMed] [Google Scholar]
- 36.Sznaidman ML, et al. Novel selective small molecule agonists for peroxisome proliferator-activated receptor delta (PPARdelta)—synthesis and biological activity. Bioorg Med Chem Lett. 2003;13:1517–1521. doi: 10.1016/s0960-894x(03)00207-5. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka T, et al. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci USA. 2003;100:15924–15929. doi: 10.1073/pnas.0306981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weninger W, et al. Naive T cell recruitment to nonlymphoid tissues: A role for endothelium-expressed CC chemokine ligand 21 in autoimmune disease and lymphoid neogenesis. J Immunol. 2003;170:4638–4648. doi: 10.4049/jimmunol.170.9.4638. [DOI] [PubMed] [Google Scholar]
- 39.Lo JC, et al. Differential regulation of CCL21 in lymphoid/nonlymphoid tissues for effectively attracting T cells to peripheral tissues. J Clin Invest. 2003;112:1495–1505. doi: 10.1172/JCI19188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schenk BI, Petersen F, Flad HD, Brandt E. Platelet-derived chemokines CXC chemokine ligand (CXCL)7, connective tissue-activating peptide III, CXCL4 differentially affect and cross-regulate neutrophil adhesion and transendothelial migration. J Immunol. 2002;169:2602–2610. doi: 10.4049/jimmunol.169.5.2602. [DOI] [PubMed] [Google Scholar]
- 41.Oda M, Haruta H, Nagao M, Nagata Y. Isolation and characterization of mouse homolog of the neutrophil activating peptide-2. Biochem Biophys Res Commun. 2002;290:865–868. doi: 10.1006/bbrc.2001.6279. [DOI] [PubMed] [Google Scholar]
- 42.Moratz C, Harrison K, Kehrl JH. Regulation of chemokine-induced lymphocyte migration by RGS proteins. Methods Enzymol. 2004;389:15–32. doi: 10.1016/S0076-6879(04)89002-5. [DOI] [PubMed] [Google Scholar]
- 43.Fabunmi RP, Sukhova GK, Sugiyama S, Libby P. Expression of tissue inhibitor of metalloproteinases-3 in human atheroma and regulation in lesion-associated cells: A potential protective mechanism in plaque stability. Circ Res. 1998;83:270–278. doi: 10.1161/01.res.83.3.270. [DOI] [PubMed] [Google Scholar]
- 44.Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 45.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 46.Bowman EP, et al. Regulation of chemotactic and proadhesive responses to chemoattractant receptors by RGS (regulator of G-protein signaling) family members. J Biol Chem. 1998;273:28040–28048. doi: 10.1074/jbc.273.43.28040. [DOI] [PubMed] [Google Scholar]
- 47.Adams LD, Geary RL, Li J, Rossini A, Schwartz SM. Expression profiling identifies smooth muscle cell diversity within human intima and plaque fibrous cap: Loss of RGS5 distinguishes the cap. Arterioscler Thromb Vasc Biol. 2006;26:319–325. doi: 10.1161/01.ATV.0000196647.45718.d6. [DOI] [PubMed] [Google Scholar]
- 48.Li J, et al. Regulator of G protein signaling 5 marks peripheral arterial smooth muscle cells and is downregulated in atherosclerotic plaque. J Vasc Surg. 2004;40:519–528. doi: 10.1016/j.jvs.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 49.Liu SL, et al. The effect of statin on the aortic gene expression profiling. Int J Cardiol. 2007;114:71–77. doi: 10.1016/j.ijcard.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 50.Mohammed FF, et al. Abnormal TNF activity in Timp3-/- mice leads to chronic hepatic inflammation and failure of liver regeneration. Nat Genet. 2004;36:969–977. doi: 10.1038/ng1413. [DOI] [PubMed] [Google Scholar]