Abstract
Human sarcomas are rare but diverse malignant tumors derived from mesenchymal tissue. Clinical response to therapy is currently determined by the modified World Health Organization (WHO) criteria or the Response Evaluation Criteria in Solid Tumors (RECIST), but these standards correlate poorly with sarcoma patient outcome. We introduced ligand-directed particles with elements of AAV and phage (AAVP) to enable integration of tumor targeting to molecular imaging. We report drug-response monitoring and prediction in a nude rat model of human sarcoma by AAVP imaging. As a proof-of-concept, we imaged Herpes simplex thymidine kinase in a clinic-ready setting with PET to show that one can a priori predict tumor response to a systemic cytotoxic. Given the target expression in patient-derived sarcomas, this platform may be translated in clinical applications. Sarcoma-specific ligands and promoters may ultimately lead to an imaging transcriptome.
Human sarcomas are rare yet heterogeneous malignant tumors from mesenchymal tissues (1). Monitoring drug responses in soft-tissue sarcoma has long been clinically problematic. Currently, responses are determined by the modified World Health Organization (WHO) criteria (2–4) or the Response Evaluation Criteria in Solid Tumors (RECIST) (5–7), which require marked tumor size decrease for patients to be considered responding to therapy. A major assumption of these criteria is that a solid tumor volume is directly proportional to the cancer cell number. However, in soft-tissue sarcomas, there are reasons to challenge such an assumption (8). First, in addition to tumor cells, sarcomas contain nonmalignant stromal cells and extracellular matrix (ECM) that do not disappear, even if the malignant component is treated. Moreover, when cytotoxics are used against sarcomas, there is often associated necrosis resulting in reduced total cell number but not necessarily overall tumor size changes. Finally, even if a soft-tissue sarcoma is predominantly or entirely composed of cancer cells, its remnant composition may not be fully eliminated when tumor cells are destroyed by therapy, because myxoid-type degeneration is not always promptly removed. Ultimately, the modified WHO criteria and RECIST correlate poorly with drug response and outcome in patients with soft-tissue sarcomas; validation in this setting is sporadic and restricted. In another level of complexity, drug responses in patients with soft-tissue sarcoma are determined through standard methods, such as CT, MRI, or PET scans. However, because systematic quantitative measurements have not been established because of the rarity and diversity of soft-tissue sarcomas, decreases in tumor size and/or density are not accepted as unequivocal evidence of response. Consequently, many conventional soft-tissue sarcoma responses in individual patients are evaluated qualitatively. Thus, new or alternative quantitative imaging criteria to improve management and follow-up of responses were proposed in ref. 9.
We have introduced a hybrid vector that enables convergence of ligand-directed targeting and molecular imaging (10–12); such vector incorporates genomic cis-elements of adeno-associated virus (AAV) and of an M13-derived (13) phage [AAV phage (AAVP)]. Our prototype displays CDCRGDCFC (RGD-4C) to target αv integrins (14, 15) and systemically deliver transgenes to tumors (10–12).
The present work offers insight into the use of systemically administered RGD-4C AAVP as a targeted imaging tool to monitor—but also to possibly predict—drug response in soft-tissue sarcoma. We used a model of athymic rnu/rnu (nude) rats bearing human soft-tissue sarcoma xenografts (16). We show that targeted AAVP (i) allows ligand-directed targeting of human sarcoma xenografts in a preclinical setting; (ii) provides molecular imaging of reporters; (iii) is suitable for serial noninvasive monitoring of soft-tissue sarcoma; and, as a proof-of-concept, (iv) enables response prediction to a cytotoxic. We also show that αv integrins are expressed in vascular endothelium and tumor cells in human soft-tissue sarcomas. AAVP-mediated PET scan of HSVtk expression (12, 17–19) enables molecular prediction of drug response in a preclinical model of soft-tissue sarcoma. Translation of this potential strategy in sarcoma patients might be achievable.
Results
Targeted AAVP Binds to Sarcoma Cell Surfaces and Mediates Transduction.
We reported the efficacy of a targeted AAVP vector (10–12) in which a CMV promoter drives a mammalian expression cassette containing a reporter transgene, and systemic tumor homing is ligand-directed through an RGD-4C peptide that binds to αv integrins within tumors (10, 14, 15).
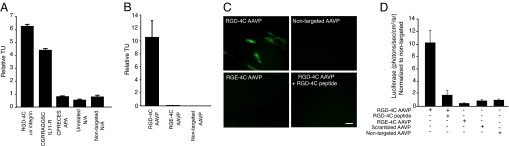
We first examined ligand binding to target sarcoma cells by comparing the binding capacity of tumor-homing peptides to human Sloan–Kettering Leiomyosarcoma-1 (SKLMS1) cells in an aqueous-to-organic phase separation assay (20). To determine the best homing, SKLMS1 cells were incubated with targeted phage displaying one of the following: RGD-4C (10, 14, 15), the interleukin-11 receptor (IL11R)-targeting peptide CGRRAGGSC (21), or the aminopeptidase A (APA)-targeting peptide CPRECES (22); insertless phage and phage displaying an unrelated peptides served as negative controls. RGD-4C had the highest binding to sarcoma cell surfaces (Fig. 1A). Next, we evaluated the magnitude and specificity of targeted RGD-4C AAVP binding compared with negative controls, including nontargeted (insertless) AAVP or mutant (Asp → Glu) RGE-4C AAVP. Under nonsaturated conditions, we found that the ratio of specific binding of RGD-4C AAVP to SKLMS1 cell surfaces (relative to negative controls) was ≈10-fold; controls had barely detectable cell binding (Fig. 1B). Finally, we analyzed SKLMS1 cell transduction by targeted AAVP vectors carrying two reporters: green fluorescence protein (RGD-4C AAVP-GFP) or Luciferase (RGD-4C AAVP-Luc). We observed GFP expression in cells incubated with RGD-4C AAVP-GFP, but no GFP was detected in cells incubated with negative controls (Fig. 1C). Similar results were obtained by RGD-4C AAVP-Luc transduction (Fig. 1D). Quantification of luciferase showed ≈10-fold more activity in cells treated with targeted RGD-4C AAVP-Luc compared with controls (Fig. 1D). We also showed that SKLMS1 cell transduction by RGD-4C AAVP-GFP and RGD-4C AAVP-Luc is specific because it is inhibited by the RGD-4C peptide (Fig. 1 C and D); negative control peptides had no detectable inhibitory effect.
Fig. 1.
Ligand-directed binding and gene transduction by targeted AAVP. (A) Binding of targeted phage displaying tumor-homing peptides or negative controls (as indicated) to the surface of SKLMS1 cells in aqueous-to-organic phase separation assays (20) was performed. (B) Binding of RGD-4C AAVP or negative controls (nontargeted AAVP or mutant RGE-4C AAVP) to the surface of SKLMS1 cells. (C) Targeted gene transduction mediated by RGD-4C AAVP-GFP to SKLMS1 cells relative to negative controls; inhibition by soluble RGD-4C peptide confirms specificity. (Scale bar, 10 μm.) (D) Quantitative analysis of cell transduction by targeted or several nontargeted AAVP-Luc controls. AAVP-Luc vectors were incubated with tumor cells. Error bars represent the SD of the means. Each experiment was repeated twice with similar results. In each case, a representative experiment is shown.
AAVP Targeting Sarcoma Xenografts.
We next attempted to determine the ability of RGD-4C AAVP to target a model of soft-tissue sarcoma in vivo. We systemically (tail vein) administered RGD-4C AAVP or controls to nude rats bearing SKLMS1 sarcoma xenografts in the right hind limbs. At 9 h after vector administration, sarcoma-bearing nude rats were killed, and tumors and control organs were collected. Relative targeted AAVP homing was quantified (11, 23) by recovery from tissue homogenates, bacterial infection, and counting transducing units (TU).
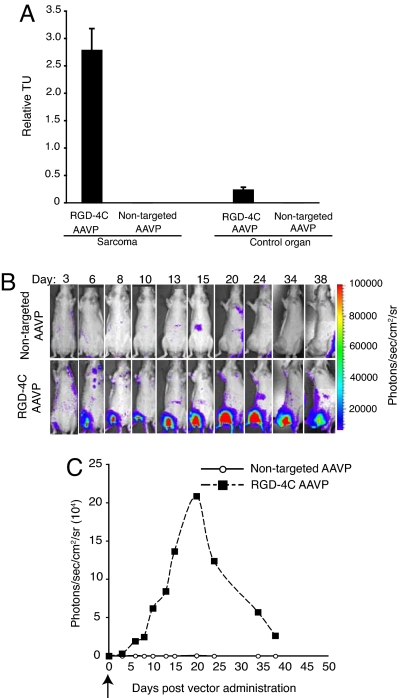
We observed marked enrichment of RGD-4C AAVP in SKLMS1 sarcoma xenografts. Specifically, we found ≈25-fold enrichment compared with homing of nontargeted AAVP control to size-matched tumors and ≈10-fold compared with control organs (Fig. 2A). Dose-dependent inhibition of homing to SKLMS1 sarcomas by RGD-4C peptide—but not by control peptides—was observed. These data establish that RGD-4C AAVP targets SKLMS1 xenografts in nude rats upon systemic administration.
Fig. 2.
Targeting soft-tissue sarcoma xenografts and spatial–temporal dynamics of reporter transgene expression. (A) Systemic homing ability of the RGD-4C AAVP in nude rats bearing SKLMS1 xenografts was evaluated after i.v. administration of targeted AAVP or control. Nude rats (n = 2) bearing established and size-matched SKLMS1 xenografts (≈200 mm3) in the right hind limbs received a single dose (3 × 1012 TU) of RGD-4C AAVP or nontargeted AAVP. After 9 h, sarcoma-bearing rats were killed, and targeted and control AAVP was recovered from tumors and control normal tissues (shown is pancreas). Experiments were repeated twice. A representative experiment is shown. Error bars represent the SD of the means. (B) BLI of luciferase expression after systemic delivery of targeted AAVP or control AAVP. Nude rats bearing size-matched SKLMS1 sarcoma xenografts (≈200 mm3) received a single dose (3 × 1012 TU) of RGD-4C AAVP-Luc or negative control (nontargeted) AAVP-Luc. Serial temporal and spatial monitoring of luciferase (Luc) gene expression was determined by repetitive BLI at different days after AAVP administration as indicated. A standard calibration scale is provided. Experiments were repeated twice. A representative experiment is shown. (C) Serial real-time quantification of Luc expression in the animals depicted above. Arrow (day 0) indicates systemic administration of a single dose of targeted AAVP or control.
Targeted Transgene Delivery.
Having shown that RGD-4C AAVP specifically transduces human SKLMS1 cells and homes to SKLMS1 xenografts in nude rats, we set out to evaluate the systemic delivery efficacy of targeted reporter transgenes. We assessed bioluminescence imaging (BLI) and PET imaging with [18F]-FEAU for noninvasive monitoring of spatial diversity and temporal dynamics of Luc and HSVtk expression in nude rats bearing SKLMS1 xenografts after RGD-4C AAVP administration. We first used a laboratory setup for in vivo imaging of Luc transgene in sarcoma-bearing rats. We selected BLI of Luc expression as a scout reporter because it is a simple, cost-effective, and sensitive technique for gene expression monitoring in rodents with virtually no unspecific background (17, 24). On day 0 of a typical repetitive BLI experiment, rats bearing SKLMS1 sarcomas in the right hind limb received RGD-4C AAVP-Luc or nontargeted AAVP-Luc. BLI corresponding to Luc expression started at day 3 after vector delivery and was serially monitored for 5–6 weeks, at which time the experiments were terminated and sarcoma-bearing rats killed (days 38–40); specific expression of Luc was detected in sarcoma xenografts of rats receiving RGD-4C AAVP-Luc at day 3 after systemic delivery. From day 3 after targeted vector delivery, BLI yielded a gradual increase in the levels of Luc expression in tumors during the initial week after administration of RGD-4C AAVP-Luc [Fig. 2B and supporting information (SI) Fig. 6]. Thereafter, two general outcomes were observed. In some cohorts of sarcoma-bearing rats, a gradual decrease of gene expression was found, ending with nonimageable low levels of reporter transgene expression within the tumors by experimental day 24 (SI Fig. 6). In other cohorts, Luc expression in the tumors continued to increase and achieved a very high-level peak by day 20 (the maximum Luc expression, coincidentally at approximately the half time point of the experiment), followed by a gradual decrease in gene expression until the experiment termination (Fig. 2B). In contrast, no specific BLI signals could be observed in control sarcoma-bearing rats receiving nontargeted AAVP-Luc. Administration of any AAVP did not result in reporter transgene expression in normal organs such as liver, spleen, or kidneys (Fig. 2B) as evident by the absence of specific BLI signals. These results show that targeted RGD-4C AAVP specifically and efficiently transduces sarcoma xenografts. Transduction of sarcoma-derived xenografts in rats with RGD-4C AAVP appears to be heterogeneous within tumors (up to ≈13-fold differential in Luc expression levels and ≈2.5-fold differential in Luc expression timelines (Fig. 2C and SI Fig. 6B). Tumor-specific expression of Luc was detected and monitored in follow-up of SKLMS1 sarcomas in rats receiving RGD-4C AAVP-Luc; in contrast, tumor-associated BLI signals were not observed in control sarcoma-bearing rats (Fig. 2B). No BLI was observed in normal control organs with any AAVP.
Experimental Modeling of Drug Response.
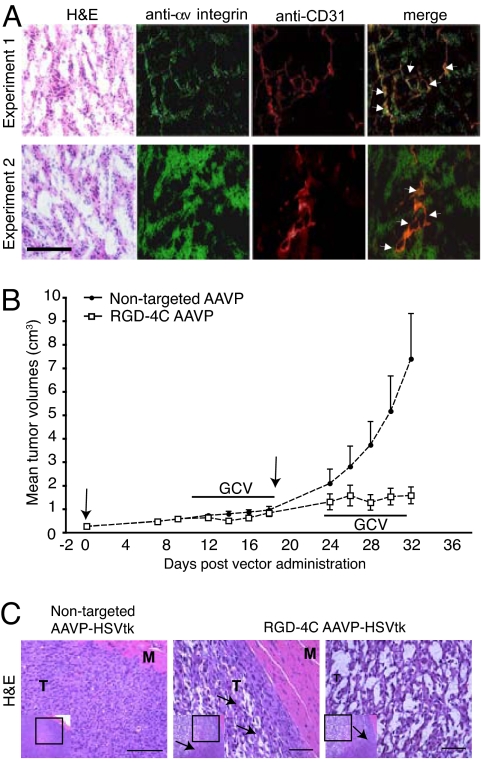
To confirm target presence in this system, we examined αv integrin expression in SKLMS1 xenografts. Immunofluorescence analysis showed strong expression in SKLMS1 xenografts (Fig. 3A, anti-αv integrin). Double-staining with anti-CD31 antibody (Fig. 3A, anti-CD31) showed that αv integrins also localized in tumor blood vessels (Fig. 3A, merge). We noticed differences in αv integrin expression among various tumor-bearing rat cohorts (Fig. 3A), consistently with—and likely accounting for—the serial BLI variability (Fig. 2 and SI Fig. 6).
Fig. 3.
Drug response to targeted AAVP-mediated systemic cytotoxic therapy. (A) Immunofluorescence analysis of αv integrin in frozen serial sections of SKLMS1 xenografts in nude rats. Representative H&E staining of the tumor sections are shown (far left). Antibodies anti-αv integrins and anti-CD31 antibody for blood vessel staining were used. Merge images (far right) confirm αv integrin expression in the vascular and tumor components. Arrows indicate αv integrins in the tumor blood vessels. (B) Cohorts of nude rats (n = 8) bearing established, size-matched (≈200 mm3) human SKLMS1-derived sarcomas received two i.v. doses (3 × 1012 TU per dose, indicated by arrows at days 0 and 19) of targeted RGD-4C AAVP-HSVtk or control nontargeted AAVP-HSVtk followed by systemic GCV therapy as indicated. Therapeutic tumor responses to two serial drug treatment cycles (first cycle, days 10–19; second cycle, days 24–31) were evaluated by serially measuring sarcoma xenografts. Shown are the mean tumor volumes ± SD. (C) Posttreatment histological evaluation of tumors by H&E staining of SKLMS1 sarcoma xenografts. Nontargeted AAVP-HSVtk-treated tumors (Left) and targeted RGD-4C AAVP-HSVtk-treated tumors (Right) are shown as high-magnification views from the low-magnification inserts of serial tumor sections. Arrows point to the border between the viable tumor rim and central necrotic area in the targeted images. M, muscle; T, tumor. (Scale bars, 25 μm.)
Although serial BLI of Luc expression provides a cheap strategy to initially analyze the specificity, temporal dynamics, and spatial heterogeneity of reporters, BLI is unlikely to be applicable in patients. In contrast, AAVP-mediated delivery of HSVtk has been established in preclinical tumor targeting and molecular imaging studies. A potential advantage of a targeted HSVtk-based methodology is that it serves as a suicide gene strategy [if combined with ganciclovir (GCV)] or as a reporter for imaging (if combined with HSVtk-specific radiolabeled nucleoside analogues). Thus, we reasoned that a targeted AAVP might become translatable as a clinically applicable PET imaging modality in human soft-tissue sarcomas. To evaluate responses by tumor measurements during physical examination (Fig. 3B) and histopathology (Fig. 3C), we delivered HSVtk by targeted RGD-4C AAVP or controls to cohorts of rats with SKLMS1 sarcomas. We treated both groups with systemic GCV and compared responses by HSVtk imaging (Figs. 3 and 4).
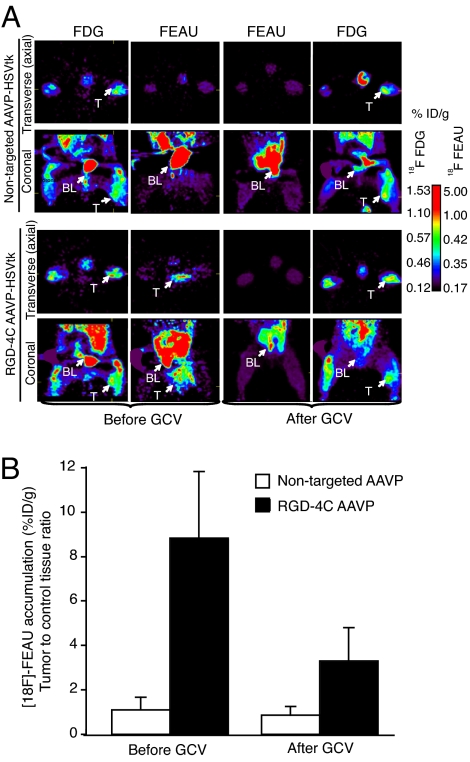
Fig. 4.
Predicting and monitoring drug response in a preclinical model of human sarcoma. PET imaging of HSVtk transgene expression was performed in sarcoma-bearing rats after i.v. delivery of RGD-4C targeted AAVP or nontargeted control. The first GCV treatment cycle (see Fig. 3) was initiated at 24 h after [18F]-FEAU administration and imaging to enable the molecular–genetic imaging of the corresponding drug response. (A) Cohorts of nude rats bearing human SKLMS1-derived xenografts (n = 8) received a single i.v. dose (3 × 1012 TU) of RGD-4C AAVP-HSVtk or control nontargeted AAVP-HSVtk. PET imaging of [18F]-FEAU was performed after AAVP administration (day 9) and then again after drug treatment with GCV (day 15). PET imaging of [18F]-FDG was performed (day 8) and then again after the second [18F]-FEAU (day 16). PET imaging with [18F]-FDG and with [18F]-FEAU are presented (before and after treatment with GCV) as indicated. Transverse (axial) and coronal sections are shown. A standard calibration scale is provided, and correspondence of [18F]-FDG and [18F]-FEAU PET imaging is indicated. (B) Relative sarcoma expression of HSVtk as assessed by repetitive PET imaging with [18F]-FEAU before and after initiation of cytotoxic drug treatment with GCV. A mesenchymal-derived normal tissue (muscle) served to normalize the tumor-to-control reporter transgene expression ratio.
Predicting and Monitoring Sarcoma Response.
The HSVtk gene was used as a reporter in combination with a radiolabeled nucleoside analogue 2′-[18F]-fluoro-2′-deoxy-1-β-d-arabino-furanosyl-5-ethyl-uracil ([18F]-FEAU), a radiolabeled substrate for the HSVtk enzyme; biochemical attributes, such as its high uptake rate and selectivity, render FEAU a suitable PET tracer for HSVtk expression (12, 25, 26). Nude rats with SKLMS1 xenografts received targeted or control AAVP-HSVtk intravenously. PET imaging with [18F]-fluorodeoxyglucose (FDG) was performed at day 8 after AAVP administration to evaluate tumor viability, and [18F]-FEAU PET was performed 24 h afterward (day 9) to assess localization and magnitude of HSVtk expression. [18F]-FDG images (day 8) demonstrated metabolically viable tumors located in the right hind limb of each sarcoma-bearing rat with similar levels of activity of tumors in rats receiving RGD-4C AAVP-HSVtk or nontargeted AAVP-HSVtk (Fig. 4A). In contrast, PET imaging with [18F]-FEAU showed HSVtk transgene reporter expression specifically in sarcoma xenografts in rats receiving RGD-4C AAVP-HSVtk (Fig. 4A). No HSVtk expression was detected in tumors in rats receiving nontargeted AAVP-HSVtk (Fig. 4A). As before, PET with [18F]-FEAU revealed intratumoral heterogeneity and interanimal variability of HSVtk transgene expression, consistent with our BLI results. GCV was initiated at day 10 after AAVP administration in these same animals, whereas sarcoma growth was monitored in each rat by caliper serial measurements (SI Fig. 7). After 5 days of systemic GCV treatment, PET imaging of [18F]-FEAU showed marked decrease in the levels of HSVtk expression (expressed as percentage of administered i.v. dose per gram of tissue) in sarcoma xenografts from the RGD-4C AAVP-HSVtk-treated rat cohort (Fig. 4). Moreover, suppression of sarcoma growth and even regression of primary tumors (in some animals) were observed with GCV (Fig. 3B). However, all tumors eventually recurred (Fig. 3B) with confirmatory analysis of viability with [18F]-FDG PET (Fig. 4A). To assess the potential of repetitive AAVP-mediated gene transfer, a second RGD-4C AAVP-HSVtk dose was administered to sarcoma-bearing rats and GCV was initiated 5 days later. Again, regression of the SKLMS1 sarcomas in rats was observed between days 26 and 30, followed by tumor regrowth (Fig. 3B); tumors were recovered and processed for analysis. Hematoxylin and eosin (H&E) staining revealed destruction of the central area of tumor tissue after treatment with targeted RGD-4C AAVP-HSVtk plus GCV (Fig. 3C). Notably, a viable peripheral rim was found in these tumors. In contrast, nontargeted AAVP-HSVtk plus GCV had no such effect (Fig. 3C). These results are consistent with reports in other cell type or species (10, 22).
Target Analysis in Human Sarcomas.
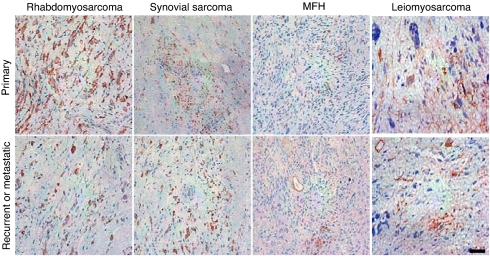
To begin to translate this strategy into clinical applications, we analyzed the presence of αv integrins in a patient-derived sarcoma set (n = 28). This panel of tumors (SI Table 1) included primary, recurrent, and metastatic soft-tissue sarcomas of many subtypes. We found that expression of αv integrins in sarcomas is consistently positive within the vascular and tumor compartments. These data suggest that this cell surface target may be suitable for translation into patient applications. Expression of αv integrins in representative specimens is shown (Fig. 5).
Fig. 5.
Analysis of αv integrin expression in human soft-tissue sarcomas. Shown is αv integrin expression in paraffin-embedded sections from a panel of the following patient-derived soft-tissue sarcoma specimens: rhabdomyosarcomas, synovial sarcomas, and malignant fibrous histiocytoma (MFH). Representative images of primary, recurrent, or metastatic tumors are shown as indicated. (Scale bar, 25 μm.)
Discussion
Reliable assessment of tumor response in human soft-tissue sarcoma remains a challenge since the report of a “meaningful” response classification by Karnofsky (26) nearly half-century ago. Despite sequential improvements, modifications, and revisions over the ensuing decades, the problem is still unsolved. Currently, tumor response evaluation is established by criteria originally developed in ref. 2 and subsequently modified in refs. 3 and 4 by the WHO and, since the year 2000, by the RECIST methodology (5–7). A recent prospective trial suggested that RECIST is equivalent to the modified WHO criteria in soft-tissue sarcoma patients (6). However, the unprecedented development of imaging methodology in cancer medicine has led to clinical settings in which current guidelines, with predetermined evaluation criteria for anti-tumor drug activity, might no longer be adequate (19, 27–29).
Conventionally, RECIST requires a 30% decrease in the sum of the longest dimension of tumors to be considered a partial response to therapy (5). Historically, one can trace the origins of this arbitrary standard to >30 years ago, when physical examination was the predominant methodology available to determine tumor response to chemotherapy (30). However, in soft-tissue sarcoma patients, RECIST may be too stringent. First, anatomically based imaging measurements are rarely used to determine drug responses today because of the increased sophistication in imaging (27). Moreover, in soft-tissue sarcoma patients, RECIST does miss “true” (i.e., biopsy-proven) pathological responses; consequently, anything less than RECIST-defined progression may represent a tumor response (8). Although contemporary imaging allows accurate sarcoma size measurement, marked required decreases in tumor diameter have been questioned on the empirical basis that any sarcoma size reduction—or even tumor growth stabilization or decreasing tumor density (indicating necrosis or degeneration)—in response to therapy might be clinically significant. Choi et al. (9) have proposed that a 10% tumor size decrease or 15% tumor density decrease (the so-called “Choi criteria”) in contrast enhanced-CT imaging is evidence of response in patients with soft-tissue sarcomas (8, 9, 29). Finally, RECIST does not adequately evaluate activity of targeted agents that may be predominantly cytostatic rather than cytotoxic. Brennan and colleagues have reported a postoperative algorithm for sarcoma-specific death (31); in addition to this valuable tool, earlier biological endpoints other than patient survival might be desirable. Because functional imaging (FDG PET) has shown clinical promise to monitor disease in soft-tissue sarcoma (27, 32), we reasoned that a PET-based system could also be potentially developed to predict tumor response.
Here, we report a targeted AAVP-based monitoring of response to a drug in a preclinical experimental model of human soft-tissue sarcoma xenografts in nude rats. Specifically, we demonstrated ligand-directed targeting, noninvasive serial imaging of reporter transgenes, and drug response prediction and monitoring in human xenografts established in nude rats with PET-based imaging of RGD-4C AAVP-delivered HSVtk incorporating specific radiotracers. We first sought to determine whether RGD-4C AAVP would bind to and transduce SKLMS1 cells. We showed that the RGD-4C peptide is a consistent and specific ligand in vitro. Next, we administered the RGD-4C AAVP or nontargeted controls to tumor-bearing nude rats to evaluate whether RGD-4C AAVP would home to SLMS1 xenografts. Again, we observed specific localization of RGD-4C AAVP to tumors. The magnitude and specificity of these results are consistent with in vitro and in vivo data in other experimental systems (10). We then used luciferase BLI to serially analyze the potential of RGD-4C AAVP for targeted delivery and to monitor the distribution and persistence of reporter expression. Our data show that systemic delivery of RGD-4C AAVP-Luc results in efficient and specific gene expression within human soft-tissue sarcoma xenografts but that the tumor transduction is heterogeneous, even in a carefully standardized experimental model. This result suggests that RGD-4C AAVP can be used to target gene therapy to distant tumors after systemic administration, a route that is applicable for localized and metastatic disease. Such diversity in sarcoma transduction could be due to many factors (i.e., heterogeneity in the expression and receptor accessibility to circulating ligands), resulting in variability in the magnitude of targeted delivery and gene transduction. These results and others (17) indicate that targeted molecular imaging is a promising approach to study the persistence of transgene expression in tumors after systemic administration of targeted AAVP, and also provides the ability to visualize and quantify not only the magnitude and spatial distribution of transgene expression, but also the temporal dynamics (with repetitive molecular imaging). In a previous report (10), we followed the levels of transgene expression of HSVtk by PET imaging of both human tumor xenografts and isogenic tumors in mice until day 10 (before GCV treatment) after i.v. AAVP delivery, and observed a stabilization between days 5–10 consistent with the possibility that proliferating cells continue to pass the transgene without a noticeable dilution effect in this period. In the current study, a biphasic and heterogeneous pattern of AAVP-mediated transgene expression emerged in growing sarcoma xenografts. BLI revealed a gradual, almost linear increase in BLI signal in sarcomas beginning 3 days after AAVP administration, reaching a plateau of transgene expression between days 5 and 10 that lasted only briefly, followed by a decrease in transgene expression. Thus, dynamics and magnitude of transgene expression may be variable with each tumor type. These observations could well be explained by dilution effect of proliferating transduced cells within a larger population of proliferating nontransduced cells. Indeed, in some animals, the decrease of luciferase expression in tumors coincided with a striking increase of tumor growth rate. However, the CMV promoter that drives transgene expression in the current version of the AAVP is notorious for silencing its own transcriptional activity. Any of these phenomena could have played a role in the occasional observed decrease of AAVP-Luc expression in sarcomas. These questions notwithstanding, our results are in agreement with other studies that have demonstrated transgene silencing with CMV promoters and reporters such as GFP or βgal. Although the CMV promoter is active in mammalian cells, it is susceptible to transcriptional inactivation because of DNA methylation and histone acetylation; the possibility of such inactivation in human soft-tissue sarcoma cells is an open question. Despite this potential limitation, BLI of the Luc reporter transgene provided the yield initial cost-effective scout imaging for 2D monitoring of targeted delivery, tumor expression levels, and longitudinal persistence needed to optimize the preclinical sarcoma model used here. Subsequently, we designed and developed a clinic-ready AAVP-based candidate approach for translation in human soft-tissue sarcomas: by using [18F]-FEAU PET imaging, we were able to repetitively monitor the location, magnitude, and temporal dynamics of HSVtk expression in nude rats bearing human sarcoma xenografts in vivo after i.v. administration of targeted AAVP-HSVtk. Such targeted molecular–genetic imaging provides a potential advantage because of its ability to monitor the efficiency and specificity of HSVtk delivery before and to predict tumor response after GCV. Our proof-of-concept results suggest that targeted AAVP can be integrated with PET imaging to predict sarcoma response.
In addition to SKLMS1, the susceptibility of other human sarcoma lines to the HSVtk plus GCV has been established. Based on these data, targeted AAVP particles may provide a systemic mean to deliver imaging reporters at sufficient expression levels to accomplish imaging of tumor response to drugs in soft-tissue sarcomas patients.
A few translational aspects merit comment. First, discovery of sarcoma-specific cell surface ligands and sarcoma-specific (or radiation-induced) promoters may improve the efficacy of this system; genetic targets, such as PAX3-FKHR, EWS-FLI1, and SYT-SSX1 fusion proteins (33), may provide imaging opportunities. Second, in addition to reporters, targeted delivery of therapeutic transgenes may also be feasible. Third, both SKLMS1 sarcoma cells and the tumor blood vessels express αv integrins; the relative tumor targeting versus vascular targeting will depend on the relative levels of receptor expression and accessibility (10). Consistently, αv integrins are expressed in the tumor vascular endothelium and in sarcoma cells in patient-derived specimens (Fig. 5 and SI Table 1).
The results reported here serve as a preclinical blueprint for design of translational clinical trials with targeted AAVP-mediated molecular imaging in human soft-tissue sarcoma; if so, targeted AAVP molecular imaging applications in patients with soft sarcoma may eventually become possible.
Materials and Methods
Cell Culture.
SKLMS1 cells were grown at 37°C in 5% CO2 in DMEM supplemented with 10% FCS, penicillin, and streptomycin. A model of nude rat-bearing SKLMS1-derived xenografts is described in ref. 16.
Targeted Phage and AAVP.
αv integrin-binding (10, 14, 15), IL11R-binding (21), and APA-binding (22) phage were described; the insertless fd-tet phage (13) or phage displaying unrelated peptide sequences served as controls. Design and genetic construction of targeted AAVP-based vectors is detailed in ref. 11. Vectors were purified from MC1061 Escherichia coli, resuspended in PBS and recentrifuged to remove residual debris. Supernatant containing AAVP was titrated by k91Kan E. Coli infection. Serial dilutions were plated on LB agar plates with tetracycline and kanamycin. TU were determined by colony counting.
Cell Surface Binding Assay.
We used the biopanning and rapid analysis of selective interactive ligands (BRASIL) methodology (20) to evaluate sarcoma cell binding. SKLMS1 cells were detached by EDTA and resuspended in DMEM containing 1% BSA at 4 × 106 cells per ml. SKLMS1 cell suspensions (50 μl each) were incubated with 109 TU of RGD-4C AAVP or controls. After 2 h, the admixture was transferred to the top of a nonmiscible organic phase and centrifuged. Binding clones were recovered by k91Kan E. coli infection (20).
Sarcoma Cell Transduction.
SKMLS1 cell transduction of was carried out as reported in ref. 11. Cells (4 × 104) were cultured on 24-well plate, and the culture medium was replaced by 200 μl of serum-free DMEM and 106 TU per cell of targeted AAVP and controls. Vectors were incubated with cells followed by medium change to DMEM containing 10% FCS. For binding inhibition, SKLMS1 cells were incubated with peptides in normal growth medium. Then, cells were washed with PBS and incubated with AAVP for transduction. Cells were analyzed for gene expression after transduction. d-Luciferin (Invivogen) was added to transduced cells at 20 μg/ml and activity was quantified with the BLI system 200 (Xenogen); luciferase activity was measured as photons per sec per cm2 per sr and normalized to activity in cells treated with controls.
Nude Rat Model of Human Sarcoma.
The Institutional Animal Care and Use Committee approved all experimentation. Rats were anesthetized by gas (2% isoflurane and 98% O2) inhalation. To establish human sarcoma xenografts, 106 SKLMS1 cells were suspended in Matrigel and implanted in the right hind limb of 8-week-old female nude rats. When size-matched tumors reached ≈200 mm3 (day 0), tumor-bearing rats received a single i.v. dose (3 × 1012 TU per rat) of RGD-4C AAVP-HSVtk or control. After 9 h, xenografts and control organs were removed and vectors recovered. GCV (100 mg·kg−1·day−1) was administered i.p. as indicated. Tumor volumes were determined as described in refs. 10, 14, and 15.
BLI of Sarcoma-Bearing Rats.
To image the Luc expression, rats received a dose (150 mg/kg) of d-luciferin. Photonic emission was imaged after i.v. administration of targeted RGD-4C AAVP-Luc or control. BLI parameters are described in ref. 10.
PET Scanning.
Sarcoma-bearing nude rats were imaged at 1 h after i.v. administration of [18F]-FDG (PETNet) at 200 μCi/rat. To image HSVtk expression, PET scans were performed at 2 h after i.v. administration of the radiolabeled nucleoside analogue [18F]-FEAU. A microPET R4 (Concorde Microsystems) equipped with a computer-controlled positioning bed in a 10.8-cm transaxial and 8-cm axial field of view with no septa and operating in 3D list mode was used (10).
Radiolabeled Substrate Synthesis.
Radiolabeled [18F]-FEAU was synthesized to radiochemical purity >99% by using 5-ethyluracil-2,5-bis-trimethylsilyl ether as the pyrimidine base for condensation with 1-bromo-2-deoxy-2-[18F]-fluoro-3,5-di-O-benzoyl-α-d-arabinofuranose (12, 34). To quantitate [18F]-FEAU or [18F]-FDG radioactivity, regions of interest were drawn on images and the measured values converted from nCi/mm3 into percentage of injected dose per gram [%ID/g (18, 30)]. Repetitive [18F]-FEAU PET imaging scans were performed after administration of targeted AAVP carrying the HSVtk gene or controls. PET imaging was performed at 24 h after GCV dosing to allow for sufficient elimination of GCV.
Immunohistochemistry.
αv integrin expression in SKLMS1 xenografts in rats was analyzed in frozen samples. Cryosections were fixed in cold acetone for 10 min, washed with PBS, and blocked with 5% normal horse serum and 1% normal goat serum in PBS. Tissue sections were then incubated with a polyclonal antibody against αv integrin (AB1930; Chemicon) at 1:400 dilution ON at 4°C. Sections were rinsed with PBS, blocked, and incubated for 1 h at RT with a goat anti-rabbit Alexa488 secondary antibody. Tumor vascularization was assessed on frozen sections with a rat anti-mouse CD31 antibody (BD PharMingen). Sections were stained with a goat anti-rat Alexa Fluor 594 secondary antibody. A nuclear counter stain with Hoechst 33342 was applied. Analysis of αv integrin expression in human specimens was carried out on paraffin-embedded sections with a mouse anti-integrin monoclonal (MAB 1976; Chemicon). Sections were deparaffinized, rehydrated in PBS, and incubated ON at 4°C with the primary antibody at 1:100. A peroxidase-conjugated goat anti-mouse IgG (Jackson ImmunoResearch) was added for 1 h at RT. Sections were counterstained with hematoxylin.
Supplementary Material
Acknowledgments.
We thank Johanna Lahdenranta and Michael Ozawa for assistance in initial phase of this project. This work was supported by National Cancer Institute and Department of Defense grants and by a Gillson-Longenbaugh Foundation award (to W.A. and R.P.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712184105/DC1.
References
- 1.Kotilingam D, Lev DC, Lazar AJ, Pollock RE. Staging soft tissue sarcoma: Evolution and change. CA Cancer J Clin. 2006;56:282–291. doi: 10.3322/canjclin.56.5.282. [DOI] [PubMed] [Google Scholar]
- 2.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Van Glabbeke M, van Oosterom AT, Steward W, Verweij J, Mouridsen H. Selection of large and objectively measurable target lesions in EORTC phase II trials: Impact on recruitment and response rate. EORTC Soft Tissue and Bone Sarcoma Group (STBSG). Eur J Cancer. 1993;29:1943–1947. doi: 10.1016/0959-8049(93)90449-p. [DOI] [PubMed] [Google Scholar]
- 4.Van Glabbeke M, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: An analysis of 2,185 patients treated with anthracycline-containing first-line regimens—a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group study. J Clin Oncol. 1999;17:150–157. doi: 10.1200/JCO.1999.17.1.150. [DOI] [PubMed] [Google Scholar]
- 5.Therasse P, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 6.Therasse P, Le Cesne A, Van Glabbeke M, Verweij J, Judson I. RECIST vs. WHO: Prospective comparison of response criteria in an EORTC phase II clinical trial investigating ET-743 in advanced soft tissue sarcoma. Eur J Cancer. 2005;41:1426–1430. doi: 10.1016/j.ejca.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: A review of validation studies on tumour assessment. Eur J Cancer. 2006;42:1031–1039. doi: 10.1016/j.ejca.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin R. Measuring drug response in sarcoma. Clin Adv Hematol Oncol. 2006;4:513–514. [PubMed] [Google Scholar]
- 9.Choi H, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 10.Hajitou A, et al. A Hybrid vector for ligand-directed tumor targeting and molecular imaging. Cell. 2006;125:385–398. doi: 10.1016/j.cell.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 11.Hajitou A, et al. Design and construction of targeted AAVP vectors for mammalian cell transduction. Nat Protoc. 2007;2:523–531. doi: 10.1038/nprot.2007.51. [DOI] [PubMed] [Google Scholar]
- 12.Soghomonyan S, et al. Molecular Pet imaging of HSV1-tk reporter gene expression using 18F-FEAU. Nat Protoc. 2007;2:416–423. doi: 10.1038/nprot.2007.49. [DOI] [PubMed] [Google Scholar]
- 13.Zacher AN, III, Stock CA, Golden JW, II, Smith GP. A new filamentous phage cloning vector: fd-tet. Gene. 1980;9:127–140. doi: 10.1016/0378-1119(80)90171-7. [DOI] [PubMed] [Google Scholar]
- 14.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 15.Ellerby HM, et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nat Med. 1999;5:1032–1038. doi: 10.1038/12469. [DOI] [PubMed] [Google Scholar]
- 16.Hannay J, et al. Isolated limb perfusion: A novel delivery system for wild-type p53 and fiber-modified oncolytic adenoviruses to extremity sarcoma. Gene Ther. 2007;14:671–681. doi: 10.1038/sj.gt.3302911. [DOI] [PubMed] [Google Scholar]
- 17.Gross S, Piwnica-Worms D. Spying on cancer: Molecular imaging in vivo with genetically encoded reporters. Cancer Cell. 2005a;7:5–15. doi: 10.1016/j.ccr.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Tjuvajev JG, et al. Imaging Herpes virus thymidine kinase gene transfer and expression by positron emission tomography. Cancer Res. 1998;58:4333–4341. [PubMed] [Google Scholar]
- 19.Tjuvajev JG, et al. Imaging adenoviral-mediated herpes virus thymidine kinase gene transfer and expression in vivo. Cancer Res. 1999;59:5186–5193. [PubMed] [Google Scholar]
- 20.Giordano RJ, Cardó-Vila M, Lahdenranta J, Pasqualini R, Arap W. Biopanning and rapid analysis of selective interactive ligands. Nat Med. 2001;11:1249–1253. doi: 10.1038/nm1101-1249. [DOI] [PubMed] [Google Scholar]
- 21.Arap W, et al. Steps toward mapping the human vasculature by phage display. Nat Med. 2002;8:121–127. doi: 10.1038/nm0202-121. [DOI] [PubMed] [Google Scholar]
- 22.Marchiò S, et al. Aminopeptidase A is a functional target in angiogenic blood vessels. Cancer Cell. 2004;5:151–162. doi: 10.1016/s1535-6108(04)00025-x. [DOI] [PubMed] [Google Scholar]
- 23.Pasqualini R, Arap W, Rajotte D, Ruoslahti E. In: Phage Display: A Laboratory Manual. Barbas CF III, Burton DR, Scott JK, Silverman GJ, editors. New York: Cold Spring Harbor Press; 2001. pp. 22.1–22.24. [Google Scholar]
- 24.Gelovani-Tjuvajev J, Blasberg RG. In vivo imaging of molecular-genetic targets for cancer therapy. Cancer Cell. 2003;3:327–332. doi: 10.1016/s1535-6108(03)00082-5. [DOI] [PubMed] [Google Scholar]
- 25.Serganova I, et al. Molecular imaging of temporal dynamics and spatial heterogeneity of hypoxia-inducible factor-1 signal transduction activity in tumors in living mice. Cancer Res. 2004;64:6101–6108. doi: 10.1158/0008-5472.CAN-04-0842. [DOI] [PubMed] [Google Scholar]
- 26.Karnofsky DA. Meaningful clinical classification of therapeutic responses to anti-cancer drugs. Clin Pharmacol Ther. 1961;2:709–712. doi: 10.1002/cpt196126709. [DOI] [PubMed] [Google Scholar]
- 27.Jaffe CC. Measures of response: RECIST, WHO, and new alternatives. J Clin Oncol. 2006;24:3245–3251. doi: 10.1200/JCO.2006.06.5599. [DOI] [PubMed] [Google Scholar]
- 28.Michaelis LC, Ratain MJ. Measuring response in a post-RECIST world: From black and white to shades of grey. Nat Rev Cancer. 2006;6:409–414. doi: 10.1038/nrc1883. [DOI] [PubMed] [Google Scholar]
- 29.Tuma RS. Sometimes size doesn't matter: Reevaluating RECIST and tumor response rate endpoints. J Natl Cancer Inst. 2006;98:1272–1274. doi: 10.1093/jnci/djj403. [DOI] [PubMed] [Google Scholar]
- 30.Moertel CG, Hanley JA. The effect of measuring error on the results of therapeutic trials in advanced cancer. Cancer. 1976;38:388–394. doi: 10.1002/1097-0142(197607)38:1<388::aid-cncr2820380156>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 31.Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. 2002;20:791–796. doi: 10.1200/JCO.2002.20.3.791. [DOI] [PubMed] [Google Scholar]
- 32.Schuetze SM. Utility of positron emission tomography in sarcomas. Curr Opin Oncol. 2006;18:369–373. doi: 10.1097/01.cco.0000228744.49294.12. [DOI] [PubMed] [Google Scholar]
- 33.Helman LJ, Meltzer P. Mechanisms of sarcoma development. Nat Rev Cancer. 2003;3:685–694. doi: 10.1038/nrc1168. [DOI] [PubMed] [Google Scholar]
- 34.Alauddin MM, Fissekis JD, Conti PS. A general synthesis of 2′-deoxy-2′-([18F]fluoro-5-methyl-1-β-d-arabinofuranosyluracil and its 5-substituted nucleosides. J Labelled Compds Radiopharm. 2003;46:285–289. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.