Abstract
Primary cilia are ubiquitous cellular appendages that provide important yet not well understood sensory and signaling functions. Ciliary dysfunction underlies numerous human genetic disorders. However, the precise defects in cilia function and the basis of disease pathophysiology remain unclear. Here, we report that the proteins disrupted in the human ciliary disorder Bardet–Biedl syndrome (BBS) are required for the localization of G protein-coupled receptors to primary cilia on central neurons. We demonstrate a lack of ciliary localization of somatostatin receptor type 3 (Sstr3) and melanin-concentrating hormone receptor 1 (Mchr1) in neurons from mice lacking the Bbs2 or Bbs4 gene. Because Mchr1 is involved in the regulation of feeding behavior and BBS is associated with hyperphagia-induced obesity, our results suggest that altered signaling caused by mislocalization of ciliary signaling proteins underlies the BBS phenotypes. Our results also provide a potential molecular mechanism to link cilia defects with obesity.
Keywords: melanin-concentrating hormone receptor 1, neuronal cilia, obesity, somatostatin receptor 3, type III adenylyl cyclase
Cilia are microtubule-based appendages that extend from the basal bodies of cells and are classified as either motile or primary. Motile cilia and flagella are responsible for generating flow or movement. Primary cilia are generally immotile solitary organelles that are present on almost all human cell types (1). It is generally accepted that primary cilia serve important specialized signaling functions (2–5). Photoreceptors, which are modified primary cilia, sense and respond to light. Specialized olfactory cilia detect odors and initiate signaling cascades in olfactory neurons. Primary cilia on epithelial cells in the kidney act as mechanosensors to detect and respond to fluid flow (6, 7). The significance of primary cilia is exemplified by the fact that defects in cilia formation or function cause diseases, including renal cystic disease, retinal degeneration, liver fibrosis, anosmia, ataxia, cardiac defects, and situs inversus (8, 9). Primary cilia also serve important roles in the patterning of tissues during development (5, 9), and primary cilia dysfunction is thought to underlie the etiology of numerous human genetic disorders (10). Yet, the specific role of primary cilia on the vast majority of cells is unknown.
Bardet–Biedl syndrome (BBS) is a rare human genetic disorder characterized by obesity, retinal dystrophy, renal anomalies, hypogenitalism, polydactyly, and cognitive deficits (11). BBS is a heterogeneous disorder, and 12 causative genes (BBS1–12) have been identified (11). Although the precise functions of the BBS proteins are still unresolved, numerous studies in diverse model systems have implicated the BBS proteins in cilia function (11, 12). A recent study provides important insight into how the BBS proteins mediate cilia function in mammalian cells. Seven of the most evolutionarily conserved BBS proteins (BBS1, 2, 4, 5, 7, 8, and 9) form a stable complex, called the BBSome, that may mediate vesicular transport to the cilium (13).
Defects in neuronal signaling are likely components of many of the BBS phenotypes, including obesity, hypogenitalism, and cognitive deficits. It has been known for >40 years that neurons in the brain possess primary cilia (14), but the specific functions of these organelles remain unknown. The G protein-coupled receptors (GPCRs) somatostatin receptor 3 (Sstr3) (15) and serotonin receptor 6 (16, 17), specifically localize to neuronal cilia, suggesting a role for cilia in signaling on neurons. The functional importance of these cilia is suggested by the fact that several human ciliary disorders, including BBS, Joubert syndrome, and Meckel syndrome, have prominent functional and structural CNS phenotypes (18).
Here, we investigate the hypothesis that BBS proteins are required for assembly or function of primary cilia on central neurons. We show that neurons both in vivo and in vitro from mice lacking either the Bbs2 or Bbs4 protein possess apparently normal primary cilia but lack ciliary localization of Sstr3 or melanin-concentrating hormone receptor 1 (Mchr1). Remarkably, the lack of ciliary localization can be corrected in BBS neurons by heterologous expression of the missing BBS protein. Our studies indicate that the BBS proteins are required for the localization of GPCRs to cilia on central neurons and suggest that some BBS phenotypes are the result of altered signaling caused by ciliary GPCR mislocalization.
Results
BBS Mutant Mice Lack Sstr3-Positive Cilia.
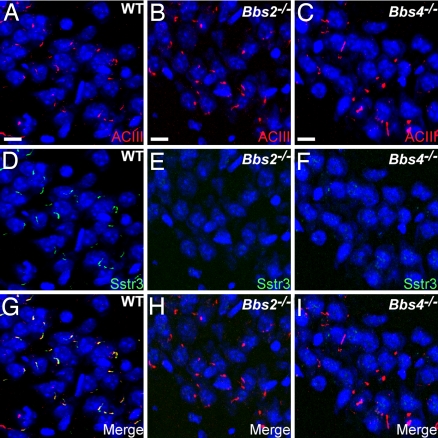
To investigate whether the BBS proteins are required for assembly of primary cilia on central neurons, we colabeled brain sections from adult wild-type (WT), Bbs2-null (Bbs2−/−), and Bbs4-null (Bbs4−/−) mice with antibodies to Sstr3 and type III adenylyl cyclase (ACIII). We recently reported that ACIII is a prominent marker of primary cilia throughout the mouse brain (19), and in some brain regions, such as the hippocampus, a subset of these cilia is also positive for Sstr3 (20). Examination of the distribution and abundance of ACIII-immunoreactive cilia throughout the brains of WT, Bbs2−/−, and Bbs4−/− mice revealed no obvious differences (Fig. 1A–C), indicating that Bbs2 and Bbs4 are not required for ciliogenesis in the brain. In the hippocampus, a significant proportion of the ACIII-positive cilia in the WT sections were also positive for Sstr3 (Fig. 1 A, D, and G). Interestingly, we failed to detect any Sstr3-immunoreactive cilia in the hippocampus of Bbs2−/− or Bbs4−/− sections (Fig. 1 E, F, H, and I). We reasoned that the absence of Sstr3-positive cilia in BBS mice could be the result of one or more of the following: a loss of Sstr3 expression, a loss of neurons that normally localize Sstr3 to the cilium, or a failure of Sstr3 to localize to cilia. To test whether Sstr3 is expressed in BBS animals, we analyzed Sstr3 protein levels in the hippocampus of adult WT, Bbs2−/−, and Bbs4−/− mice by immunoblotting [supporting information (SI) Fig. 5]. We found similar Sstr3 protein levels in all genotypes, indicating that the lack of Sstr3-positive cilia in BBS mice is not caused by a deficiency in Sstr3 expression.
Fig. 1.
BBS mice possess neuronal primary cilia in the brain but lack Sstr3-positive cilia. (A–C) Representative images of the CA3 region of the hippocampus in adult WT (A), Bbs2−/− (B), and Bbs4−/− (C) mice (n = 9) showing labeling for ACIII (red). Nuclei are stained with DRAQ5 (blue). The appearance and distribution of ACIII-positive cilia are similar among all genotypes. (D–F) The identical fields showing labeling for Sstr3 (green) reveals Sstr3-positive cilia in the WT (D) section but a complete lack of Sstr3-positive cilia in the Bbs2−/− (E) or Bbs4−/− (F) sections. (G–I) The merged images showing colocalization of ACIII and Sstr3 to cilia in the WT (G) section and no Sstr3 labeling of cilia in the Bbs2−/− (H) or Bbs4−/− (I) sections. (Scale bars, 10 μm.)
Bbs2 and Bbs4 Are Required for Sstr3 Ciliary Localization.
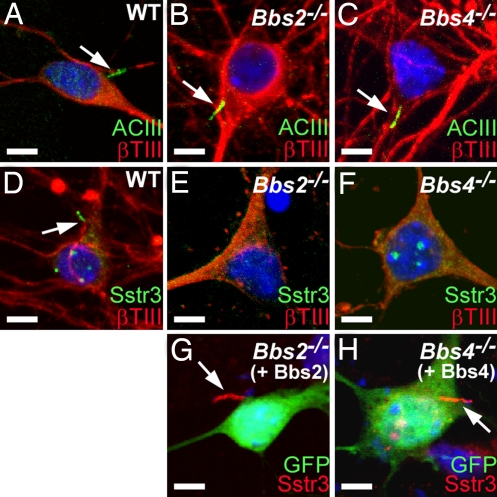
To investigate further the role of the BBS proteins in neuronal cilia, we cultured hippocampal neurons from newborn WT, Bbs2−/−, and Bbs4−/− mice. We reported that hippocampal neurons cultured from postnatal mice possess primary cilia that can be visualized with antibodies to ACIII and Sstr3 (20). Colabeling of cells after 7 days in culture with antibodies to ACIII and β-tubulin type III (βTIII), which is a marker of neurons, revealed the presence of neuronal cilia in all genotypes (Fig. 2A–C). No obvious differences in cilia length or structure were detected, and quantification of the percentage of neurons and the percentage of neurons possessing an ACIII-positive cilium indicated no differences among WT, Bbs2−/−, or Bbs4−/− cultures (SI Table 1). These results confirm that Bbs2 and Bbs4 are not required for neuronal ciliogenesis. In WT cultures, some cilia were also positive for Sstr3 (Fig. 2D and SI Table 2). However, we never detected Sstr3-immunolabeled cilia in Bbs2−/−or Bbs4−/− cultures (SI Table 2). Rather, we consistently observed apparent cell membrane labeling in a subset of the Bbs2−/− and Bbs4−/− neurons (Fig. 2 E and F), suggesting that the receptor was expressed in BBS neurons but failed to localize to cilia. The fact that there were no differences in the percentages of neurons or neurons possessing an ACIII-positive cilium among WT, Bbs2−/−, and Bbs4−/− cultures indicates that the lack of Sstr3-positive cilia in BBS cultures is not caused by the loss of a subpopulation of neurons. To test whether Sstr3 ciliary localization could be restored, we heterologously expressed Bbs2 and Bbs4 in cultured Bbs2−/− and Bbs4−/− hippocampal neurons, respectively. Notably, Sstr3 ciliary localization was observed on transfected Bbs2−/− and Bbs4−/− neurons (Fig. 2 G and H). Quantification of these neurons revealed that Sstr3 ciliary localization was restored to a frequency equivalent to that of WT neurons (SI Table 3). Overall, these results indicate that the BBS proteins are required for the localization of Sstr3 to cilia on hippocampal neurons.
Fig. 2.
Sstr3 ciliary localization can be restored in vitro. (A–C) Coimmunolabeling of day 7 hippocampal neurons from WT (A), Bbs2−/− (B), and Bbs4−/− (C) mice with antibodies to ACIII (green) and βTIII (red) shows the presence of cilia (arrows) in all three genotypes. (D–F) Coimmunolabeling of day 7 hippocampal neurons from WT (D), Bbs2−/− (E), and Bbs4−/− (F) mice with antibodies to Sstr3 (green) and βTIII (red) shows the presence of cilia (arrow) only on the WT neuron. Note the apparent punctate Sstr3 labeling in the Bbs2−/− (E) and Bbs4−/− (F) neurons. (G and H) Immunolabeling of day 7 hippocampal neurons from Bbs2−/− (G) and Bbs4−/− (H) mice for Sstr3 (red) 2 days posttransfection with an expression vector encoding Bbs2 and Bbs4, respectively, and a vector expressing enhanced green fluorescence protein (GFP; green) as a transfection marker. Note that heterologous expression of BBS proteins restores Sstr3 ciliary labeling (arrows). Nuclei are stained with DRAQ5 (blue). (Scale bars, 5 μm.)
Bbs2 and Bbs4 Are Required for Ciliary Localization of Mchr1.
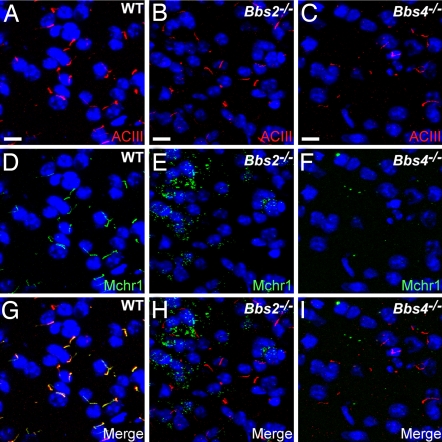
Defects in the localization of ciliary signaling proteins could be the basis for the BBS phenotypes. Therefore, we asked whether other GPCRs, and specifically GPCRs whose functions are consistent with the BBS phenotypes, fail to localize to cilia in BBS animals. We recently found that Mchr1, which is involved in the regulation of feeding and energy balance (21), localizes to cilia in regions of the brain involved in feeding and reward pathways, including the olfactory bulb, olfactory tubercle, nucleus accumbens, and hypothalamus (43). To test whether the BBS proteins are required for Mchr1 ciliary localization, we colabeled WT, Bbs2−/−, and Bbs4−/− brain sections with anti-ACIII and anti-Mchr1. In WT brains, we observed cilia that were positive for both ACIII and Mchr1 in the olfactory tubercle, hypothalamus, and nucleus accumbens (Fig. 3A, D, and G). Remarkably, we did not detect any Mchr1-positive cilia in these regions in Bbs2−/− or Bbs4−/− mice (Fig. 3 E, F, H, and I), but instead we observed obvious punctate labeling. Immunoblotting of proteins from the nucleus accumbens/olfactory tubercle and hypothalamus of WT, Bbs2−/−, and Bbs4−/− brains confirmed that Mchr1 is expressed in BBS brains at levels comparable with WT brains (SI Fig. 6). To investigate further the localization of the punctate Mchr1 labeling in BBS mice, we colabeled Bbs2−/− and Bbs4−/− brain sections with anti-Mchr1 and anti-γ-aminobutyric acid (GABA), a marker of GABAergic neurons that primarily labels the cell body. In Bbs2−/− and Bbs4−/− brain sections, we consistently observed colocalization of Mchr1-positive puncta with GABA labeling (SI Fig. 7), suggesting that in the absence of ciliary localization Mchr1 accumulates in cytoplasmic puncta in BBS neurons.
Fig. 3.
BBS mice lack Mchr1 ciliary labeling in the brain. (A–C) Representative images of the nucleus accumbens in adult WT (A), Bbs2−/− (B), and Bbs4−/− (C) mice (n = 9) showing labeling for ACIII (red). The appearance and distribution of ACIII-positive cilia are similar among all genotypes. (D–F) The identical fields showing labeling for Mchr1 (green) reveals Mchr1-positive cilia in the WT (D) section but a complete lack of Mchr1-positive cilia in the Bbs2−/− (E) or Bbs4−/− (F) sections. Note the presence of increased punctate labeling in the Bbs2−/− (E) and Bbs4−/− (F) sections. (G–I) The merged images showing colocalization of ACIII and Mchr1 to cilia in the WT (G) section and no Mchr1 labeling of cilia in the Bbs2−/− (H) or Bbs4−/− (I) sections. Nuclei are stained with DRAQ5 (blue). (Scale bars, 10 μm.)
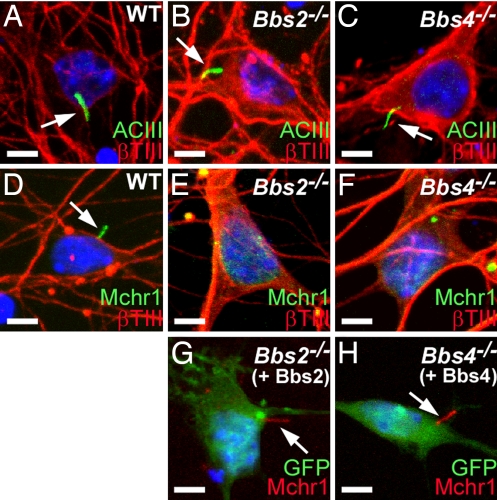
We then generated primary cultures enriched for neurons from the nucleus accumbens/olfactory tubercle from newborn WT, Bbs2−/−, and Bbs4−/− mice. Colabeling the cells after 7 days in culture with anti-ACIII and anti-βTIII revealed the presence of ACIII-positive neuronal cilia in all genotypes (Fig. 4A–C). However, colabeling the cells with anti-Mchr1 and anti-βTIII revealed the presence of Mchr1-positive cilia exclusively in the WT cultures (Fig. 4D). Similar to our results for Sstr3 in hippocampal neurons, a subset of Bbs2−/− and Bbs4−/− nucleus accumbens/olfactory tubercle-enriched neurons displayed punctate Mchr1 labeling (Fig. 4 E and F), and we were able to restore Mchr1 ciliary localization in BBS hypothalamic neurons by heterologous expression of BBS protein (Fig. 4 G and H). Thus, BBS proteins are required for proper localization of both Sstr3 and Mchr1 to neuronal cilia.
Fig. 4.
Mchr1 ciliary localization can be restored in vitro. (A–C) Coimmunolabeling of day 7 nucleus accumbens/olfactory tubercle-enriched neurons from WT (A), Bbs2−/− (B), and Bbs4−/− (C) mice with antibodies to ACIII (green) and βTIII (red). Cilia (arrows) are present in all three genotypes. (D–F) Coimmunolabeling of day 7 nucleus accumbens/olfactory tubercle-enriched neurons from WT (D), Bbs2−/− (E), and Bbs4−/− (F) mice with antibodies to Mchr1 (green) and βTIII (red) shows the presence of cilia (arrow) only on the WT neuron. Note the punctate staining in E and F. (G and H) Immunolabeling of day 7 hypothalamic neurons from Bbs2−/− (G) and Bbs4−/− (H) mice for Mchr1 (red) 2 days posttransfection with an expression vector encoding Bbs2 and Bbs4, respectively, and a vector expressing enhanced green fluorescence protein (GFP; green) as a transfection marker. Note that heterologous expression of BBS proteins restores Mchr1 ciliary labeling (arrows). Nuclei are stained with DRAQ5 (blue). (Scale bars, 5 μm.)
Discussion
Our results suggest a unique function for the BBS proteins in the localization of GPCRs to cilia on central neurons, which is consistent with the previous findings suggesting that the BBSome mediates vesicular transport to the cilium (13) and further implicates the BBS proteins in the transport of specific signaling proteins to cilia. The fact that another membrane-bound ciliary protein, ACIII, localizes normally in Bbs2−/− and Bbs4−/− neurons indicates that the BBS proteins mediate ciliary localization of distinct signaling proteins. Several studies have found that defects in ciliary protein transport can be associated with mislocalization of some signaling proteins but not others, suggesting that there are multiple mechanisms for trafficking proteins to the cilium (22–25). Interestingly, hypomorphic mutations in CEP290/NPHP6, which are known to cause the early onset retinopathy Leber congenital amaurosis (26), are associated with defective ciliary localization of olfactory G proteins but not other olfactory signaling proteins, including odorant GPCRs and ACIII (25). It is possible that CEP290/NPHP6 and the BBS proteins are part of separate ciliary protein transport mechanisms in neurons, with the BBS proteins specifically mediating GPCR transport to cilia.
Loss of BBS proteins has been associated with defective protein transport in specialized sensory cilia. Rhodopsin (a GPCR) accumulates in the cell bodies of photoreceptors in Bbs2−/−, Bbs4−/−, and Bbs6−/− mice (27–30). Normally, rhodopsin is synthesized in the cell body and transported across the connecting cilium to the outer segment of the photoreceptor. However, in BBS mice, rhodopsin can be detected in the cell body of some photoreceptors before the onset of apoptosis. It is interesting that in BBS photoreceptors rhodopsin transport is not abrogated, and significant amounts of protein are properly localized in the outer segments, whereas we did not detect any Sstr3 or Mchr1 in cilia on BBS neurons. This finding may reflect differences in the mechanisms of ciliary protein trafficking between connecting cilia and cilia on central neurons or indicate that the precise functions of the BBS proteins vary between cell types.
Loss of olfactory neuronal cilia and mislocalization of ciliary signaling proteins, including ACIII, have been reported in Bbs1−/−, Bbs4−/−, and Bbs6−/− mice (30, 31). In this case, protein mislocalization was accompanied by disorganization of microtubules within the olfactory bulb, raising the possibility that the trafficking defects were the result of microtubule disruption. The BBS proteins have been implicated in microtubule stability (32), which could be an underlying mechanism in the mislocalization of ciliary signaling proteins. However, it is doubtful that alterations in microtubule structure are involved in the lack of Sstr3 or Mchr1 ciliary localization in BBS neurons. We found that ACIII localized to neuronal cilia on BBS neurons and the cilia were indistinguishable from cilia on WT neurons. Furthermore, β-tubulin III labeling did not reveal any alterations in microtubule structure in Bbs2−/− or Bbs4−/− neurons, which suggests that in some mammalian cells the function of the BBS proteins is to localize specific signaling proteins to cilia. A recent study showed altered distribution of the thermosensory channel TRPV1 and the mechanosensory channel STOML3 within the soma of peripheral neurons in BBS animals (33), suggesting that the BBS proteins may also contribute to trafficking of proteins within the cell body in some cell types.
We hypothesize that the fundamental mechanism underlying the pathophysiology of the pleiotropic BBS phenotypes involves mislocalization of cell type-specific ciliary signaling proteins and disruption of cellular signaling. Somatostatin regulates neurotransmission (34), and aberrant neuronal somatostatin levels are associated with CNS diseases and cognitive impairment (35). Thus, it is possible that lack of Sstr3 ciliary localization could disrupt neuronal signaling and contribute to cognitive deficits in BBS patients. Melanin-concentrating hormone (MCH) and its receptor, Mchr1, are important regulators of feeding and energy balance (21). Injection of MCH induces a rapid increase in feeding behavior (36), whereas injection of Mchr1 antagonists reduces feeding behavior (37). Further, transgenic mice overexpressing MCH are obese (38), and mice lacking expression of either MCH or Mchr1 are lean (39, 40). Our results showing that Mchr1 fails to localize to cilia in mouse models of obesity suggest that ciliary localization of Mchr1 is important for proper signaling. Interestingly, lack of cilia in the brain, and specifically on the proopiomelanocortin-expressing neurons of the hypothalamus, results in hyperphagia-induced obesity (41). Our findings now provide a potential molecular mechanism to link loss of cilia or cilia dysfunction with obesity. Overall, these results provide important insights into the pathophysiology of BBS and other ciliary disorders.
Materials and Methods
Additional procedures are described in SI Methods.
Mice and Tissue Preparation.
The generation and characterization of Bbs2- and Bbs4-null mice have been described (27, 42). All procedures were approved by the Institutional Animal Care and Use Committee at Ohio State University. WT, Bbs2−/−, and Bbs4−/− littermates were generated by intercrossing heterozygous animals. Animals were anesthetized by a 0.1 ml per 10 g of body weight i.p. injection of 2.5% tribromoethanol (Sigma–Aldrich), killed by cardiac puncture, and perfused with PBS followed by a 1:1 mixture of 4% paraformaldehyde:HistoChoice (Amresco). The brains were further fixed in 4% paraformaldehyde:HistoChoice for 16–24 h at 4°C followed by cryoprotection in 30% sucrose in PBS for 16–24 h. For immunofluorescence procedures, cryoprotected brains were embedded in Optimal Cutting Temperature compound (VWR) and sectioned in a cryostat at a thickness of 30 μm.
Neuronal Cell Culturing and Transfections.
Primary neurons from postnatal day 0–1 mouse pups were cultured as described in ref. 20. Primary neurons were transfected after 5 days in culture with Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions. The expression vectors used in these studies included pcDNA3.1 (Invitrogen) and pEGFP-N3 (Clontech). Bbs2−/− or Bbs4−/− neurons were cotransfected with pcDNA3.1 expressing either Bbs2 or Bbs4, respectively, and a vector expressing EGFP alone as a transfection marker. Neuronal cultures were fixed 48 h after transfection.
Immunofluorescence.
Day 7 neurons were fixed with a solution of 4% (weight/vol) paraformaldehyde and 10% (weight/vol) sucrose for 10 min at room temperature, followed by a 5-min PBS wash. Neurons were then postfixed with cold MeOH at −20°C for 15 min and permeabilized with 0.1% Triton X-100 in PBS for 7 min. After permeabilization, the cells were put in a blocking solution of PBS with 2% serum, 0.02% sodium azide, and 10 mg/ml BSA for ≈1 h at room temperature. All antibody incubations and washes were carried out in PBS with 2% serum, 0.02% sodium azide, and 10 mg/ml BSA. All primary antibody incubations were carried out for 16–24 h at 4°C. Primary antibodies included anti-ACIII (sc-588; Santa Cruz Biotechnology), anti-Sstr3 (ss-830; Gramsch), anti-Sstr3 (sc-11617; Santa Cruz Biotechnology), anti-Mchr1 (sc-5534; Santa Cruz Biotechnology), anti-βTIII (T-8660; Sigma–Aldrich), and anti-GABA (A-0310; Sigma–Aldrich). Secondary antibodies included Alexa Fluor 488- and 546-conjugated goat anti-mouse IgG, Alexa Fluor 488- and 546-conjugated goat anti-rabbit IgG, Alexa Fluor 488-conjugated donkey anti-goat IgG, Alexa Fluor 488-conjugated donkey anti-rabbit IgG, Alexa Fluor 488-conjugated donkey anti-mouse IgG (Invitrogen); Cy3-conjugated donkey anti-goat IgG and Cy3-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch). Nucleic acids were stained with DRAQ5 (Axxora). All samples were imaged on a Zeiss 510 META laser scanning confocal microscope at the Ohio State University Central Microscopy Imaging Facility. Multiple consecutive focal planes (Z-stack), spaced at 0.5-μm intervals, were captured. For all collected images, the brightness and contrast of each channel were adjusted by using the Zeiss LSM Image Browser program.
Supplementary Material
Acknowledgments.
We thank E. Fink, D. Lautenbach, K. Wolken, B. Kemmenoe, and R. Burry for technical assistance. We thank V. Sheffield and D. Nishimura (University of Iowa, Iowa City) for providing the Bbs2 mice. This work was supported in part by Research Grant 5-FY05-39 from the March of Dimes Birth Defects Foundation (to K.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711027105/DC1.
References
- 1.Wheatley DN, Wang AM, Strugnell GE. Expression of primary cilia in mammalian cells. Cell Biol Int. 1996;20:73–81. doi: 10.1006/cbir.1996.0011. [DOI] [PubMed] [Google Scholar]
- 2.Pan J, Wang Q, Snell WJ. Cilium-generated signaling and cilia-related disorders. Lab Invest. 2005;85:452–463. doi: 10.1038/labinvest.3700253. [DOI] [PubMed] [Google Scholar]
- 3.Singla V, Reiter JF. The primary cilium as the cell's antenna: Signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- 4.Marshall WF, Nonaka S. Cilia: Tuning in to the cell's antenna. Curr Biol. 2006;16:R604–R614. doi: 10.1016/j.cub.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol. 2007;23:345–373. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- 7.Nauli SM, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 8.Hildebrandt F, Otto E. Cilia and centrosomes: A unifying pathogenic concept for cystic kidney disease? Nat Rev Genet. 2005;6:928–940. doi: 10.1038/nrg1727. [DOI] [PubMed] [Google Scholar]
- 9.Davenport JR, Yoder BK. An incredible decade for the primary cilium: A look at a once-forgotten organelle. Am J Physiol. 2005;289:F1159–F1169. doi: 10.1152/ajprenal.00118.2005. [DOI] [PubMed] [Google Scholar]
- 10.Fliegauf M, Benzing T, Omran H. When cilia go bad: Cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- 11.Tobin JL, Beales PL. Bardet–Biedl syndrome: Beyond the cilium. Pediatr Nephrol. 2007;22:926–936. doi: 10.1007/s00467-007-0435-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blacque OE, Leroux MR. Bardet–Biedl syndrome: An emerging pathomechanism of intracellular transport. Cell Mol Life Sci. 2006;63:2145–2161. doi: 10.1007/s00018-006-6180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nachury MV, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 14.Dahl HA. Fine structure of cilia in rat cerebral cortex. Z Zellforsch Mikrosk Anat. 1963;60:369–386. doi: 10.1007/BF00336612. [DOI] [PubMed] [Google Scholar]
- 15.Handel M, et al. Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience. 1999;89:909–926. doi: 10.1016/s0306-4522(98)00354-6. [DOI] [PubMed] [Google Scholar]
- 16.Hamon M, et al. Antibodies and antisense oligonucleotide for probing the distribution and putative functions of central 5-HT6 receptors. Neuropsychopharmacology. 1999;21:68S–76S. doi: 10.1016/S0893-133X(99)00044-5. [DOI] [PubMed] [Google Scholar]
- 17.Brailov I, et al. Localization of 5-HT(6) receptors at the plasma membrane of neuronal cilia in the rat brain. Brain Res. 2000;872:271–275. doi: 10.1016/s0006-8993(00)02519-1. [DOI] [PubMed] [Google Scholar]
- 18.Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: An emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 19.Bishop GA, Berbari NF, Lewis JS, Mykytyn K. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol. 2007;505:562–571. doi: 10.1002/cne.21510. [DOI] [PubMed] [Google Scholar]
- 20.Berbari NF, Bishop GA, Askwith CC, Lewis JS, Mykytyn K. Hippocampal neurons possess primary cilia in culture. J Neurosci Res. 2007;85:1095–1100. doi: 10.1002/jnr.21209. [DOI] [PubMed] [Google Scholar]
- 21.Pissios P, Bradley RL, Maratos-Flier E. Expanding the scales: The multiple roles of MCH in regulating energy balance and other biological functions. Endocr Rev. 2006;27:606–620. doi: 10.1210/er.2006-0021. [DOI] [PubMed] [Google Scholar]
- 22.Marszalek JR, et al. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–187. doi: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- 23.Dwyer ND, Adler CE, Crump JG, L'Etoile ND, Bargmann CI. Polarized dendritic transport and the AP-1 mu1 clathrin adaptor UNC-101 localize odorant receptors to olfactory cilia. Neuron. 2001;31:277–287. doi: 10.1016/s0896-6273(01)00361-0. [DOI] [PubMed] [Google Scholar]
- 24.Qin H, et al. Intraflagellar transport is required for the vectorial movement of TRPV channels in the ciliary membrane. Curr Biol. 2005;15:1695–1699. doi: 10.1016/j.cub.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 25.McEwen DP, et al. Hypomorphic CEP290/NPHP6 mutations result in anosmia caused by the selective loss of G proteins in cilia of olfactory sensory neurons. Proc Natl Acad Sci USA. 2007;104:15917–15922. doi: 10.1073/pnas.0704140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.den Hollander AI, et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet. 2006;79:556–561. doi: 10.1086/507318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishimura DY, et al. Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc Natl Acad Sci USA. 2004;101:16588–16593. doi: 10.1073/pnas.0405496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abd-El-Barr MM, et al. Impaired photoreceptor protein transport and synaptic transmission in a mouse model of Bardet–Biedl syndrome. Vision Res. 2007;47:3394–3407. doi: 10.1016/j.visres.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fath MA, et al. Mkks-null mice have a phenotype resembling Bardet–Biedl syndrome. Hum Mol Genet. 2005;14:1109–1118. doi: 10.1093/hmg/ddi123. [DOI] [PubMed] [Google Scholar]
- 30.Ross AJ, et al. Disruption of Bardet–Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 31.Kulaga HM, et al. Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat Genet. 2004;36:994–998. doi: 10.1038/ng1418. [DOI] [PubMed] [Google Scholar]
- 32.Kim JC, et al. The Bardet–Biedl protein BBS4 targets cargo to the pericentriolar region and is required for microtubule anchoring and cell cycle progression. Nat Genet. 2004;36:462–470. doi: 10.1038/ng1352. [DOI] [PubMed] [Google Scholar]
- 33.Tan PL, et al. From the cover: Loss of Bardet–Biedl syndrome proteins causes defects in peripheral sensory innervation and function. Proc Natl Acad Sci USA. 2007;104:17524–17529. doi: 10.1073/pnas.0706618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20:157–198. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 35.Weckbecker G, et al. Opportunities in somatostatin research: Biological, chemical and therapeutic aspects. Nat Rev Drug Discov. 2003;2:999–1017. doi: 10.1038/nrd1255. [DOI] [PubMed] [Google Scholar]
- 36.Qu D, et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 37.Borowsky B, et al. Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist. Nat Med. 2002;8:825–830. doi: 10.1038/nm741. [DOI] [PubMed] [Google Scholar]
- 38.Ludwig DS, et al. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest. 2001;107:379–386. doi: 10.1172/JCI10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, et al. Targeted disruption of the melanin-concentrating hormone receptor-1 results in hyperphagia and resistance to diet-induced obesity. Endocrinology. 2002;143:2469–2477. doi: 10.1210/endo.143.7.8903. [DOI] [PubMed] [Google Scholar]
- 41.Davenport JR, et al. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol. 2007;17:1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mykytyn K, et al. Bardet–Biedl syndrome type 4 (BBS4)-null mice implicate Bbs4 in flagella formation but not global cilia assembly. Proc Natl Acad Sci USA. 2004;101:8664–8669. doi: 10.1073/pnas.0402354101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berbari NF, Johnson AD, Lewis JS, Askwith CC, Mykytyn K. Identification of Ciliary Localization Sequences within the Third Intracellular Loop of G Protein-Coupled Receptors. Mol Biol Cell. 2008 doi: 10.1091/mbc.E07-09-0942. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.