Abstract
The dopamine transporter (DAT) plays a key role in the regulation of dopaminergic signaling wherein it controls both the spatial and temporal actions of dopamine. Here we evaluated the behavioral and neurochemical consequences of increased DAT function by generating DAT transgenic mice (DAT-tg) that overexpress the transporter. These mice were generated by pronuclear injection of a bacterial artificial chromosome containing the mouse DAT locus, yielding an anatomical expression pattern of DAT-tg identical to WT. In DAT-tg mice there is a 3-fold increase in the levels of total and membrane-expressed DAT, but synaptic plasma membrane fractions of DAT-tg mice show only a 30% increase in transporter levels. Functional studies reveal that in the DAT-tg animals there is a 50% increase in the rate of dopamine (DA) uptake resulting in extracellular levels of DA that are decreased by ≈40%. Behaviorally, DAT-tg animals display similar locomotor stimulation when treated with DAT blockers such as GBR12909, methylphenidate, and cocaine. However, these mice demonstrate markedly increased locomotor responses to amphetamine compared with WT animals. Furthermore, compared with controls, there is a 3-fold greater increase in the amount of DA released by amphetamine in DAT-tg mice that correlates with the 3-fold increase in protein expression. Finally, DAT-tg animals show reduced operant responding for natural reward while displaying preference for amphetamine at much lower doses (0.2 and 0.5 mg/kg) than WT mice (2 mg/kg). These results suggest that overexpression of DAT leads to a marked increase in sensitivity to psychomotor and rewarding properties of amphetamine.
Keywords: bacterial artificial chromosome transgenic, locomotion, addiction, ADHD
Dopamine (DA) is a key neurotransmitter regulating motivated behaviors such as food intake, locomotion, and reward, and its dysregulation is associated with a number of psychiatric and neurological disorders including schizophrenia, Parkinson's disease, drug addiction, attention deficit hyperactivity disorder (ADHD), and depression (1–3). A key step in the control of DA neurotransmission is the reuptake of DA into presynaptic neurons by the dopamine transporter (DAT) (4). DAT, as well as the serotonin transporter (SERT) and norepinephrine transporter (NET), belongs to the large family of Na+/Cl−-dependent transporters that also includes the transporters for glycine and GABA (5, 6). These transporters comprise 12 transmembrane domains and intracellular N- and C-terminal domains. Once at the plasma membrane, DAT cotransports two Na+, one Cl−, and one DA molecule from the extracellular space into the cytosolic compartment of the neuron.
Much insight regarding how DAT affects DA homeostasis has been gained from the study of mice lacking the DAT (DAT knockout; DAT-KO) (4, 7). In these animals, there is a 5-fold increase in the extracellular concentration of DA (8). The crucial role of DAT in determining the duration of action of extracellular DA has also been demonstrated in these animals. Using cyclic voltametry, it was shown that there is a 300-fold increase in the lifetime of extracellular DA in DAT-KO animals compared with their WT littermates (7, 8). In addition to the changes observed in the extracellular dynamics of DA, profound alterations in both the pre- and postsynaptic neurons of DAT-KO animals have also been documented. In the nigrostratial dopaminergic projections there is a 95% decrease in the content of DA despite a 1.5- to 2-fold increase in DA synthesis (8). Behaviorally, the absence of DAT translates in a marked increase in locomotor activity in a novel environment, disturbances in the prepulse inhibition of startle reflex (PPI), and multiple behavioral abnormalities related to cognitive inflexibility (4). In addition to its role as a key regulator of DA homeostasis in the brain, DAT is also a major target of psychostimulants such as amphetamine. It is believed that amphetamine can enter DA neurons through DAT and/or passive transmembrane diffusion. Once inside the terminal, amphetamine acts at DA containing vesicles and dissipates the proton gradient, resulting in the redistribution of DA from its vesicular localization to the cytoplasm (9). Ultimately, the increased cytoplasmic concentration of DA and the direct action of amphetamine on DAT lead to the reversal of the transporter and DA efflux from the intracellular to the extracellular space (9). The elevated DA in the dorsal and ventral striatum is thought to be a critical determinant of the psychomotor, rewarding, and addictive properties of amphetamine. Amphetamine also has a long history of therapeutic application in ADHD patients for the treatment of symptoms such as hyperactivity, impulsivity, and inattention (10). It has been postulated that, at therapeutic doses used in patients, amphetamine treatment results in enhanced DA transmission, leading to the hypothesis that there is a hypofunction of the DA system in ADHD (11). This hypothesis has been further encouraged by the observation that there is a 70% increase in the levels of DAT in ADHD subjects (12), but this still remains controversial (13).
To directly assess the role of increased DAT levels in the physiological functions and responses to amphetamine, we generated DAT transgenic (DAT-tg) animals by pronuclear injection of a bacterial artificial chromosome (BAC) containing the mouse DAT locus and 80 kb of upstream and downstream genomic sequence. We report here that DAT-tg animals have a 3-fold increase in the expression levels of DAT with a consequent 40% reduction in the concentration of extracellular DA in the striatum. These animals display increased behavioral responses to the stimulant and rewarding effects of amphetamine, indicating that DAT levels are critical for these properties of amphetamine. Furthermore, these data suggest that hypofunction of the DA system through increased DAT expression does not recapitulate important endophenotypes related to ADHD, such as hyperactivity and the seemingly paradoxical inhibitory responses to amphetamine.
Results
Generation of Transgenic Animals Overexpressing DAT.
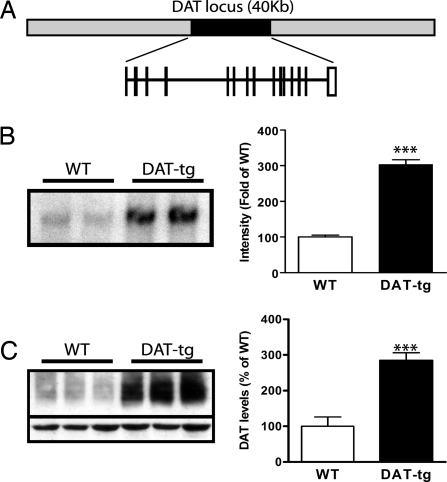
Because the promoter region of the DAT is not well characterized (14), a BAC transgenic approach was used for the overexpression of DAT. The BAC chosen for the generation of the transgene in this study contains the murine DAT locus (40 kb) and 80 kb of up and downstream genomic sequence surrounding the DAT locus (Fig. 1A). A BAC transgenic founder line was generated by pronuclear injection and characterized for the extent of DAT overexpression achieved. First, the number of transgene copies integrated in the genome of the mice was estimated by Southern blot analysis using a probe specific for DAT. As shown in Fig. 1B, there is an increase in the intensity of the genomic fragment from DAT-tg on the Southern blot. Densitometric analysis shows that there is a 3-fold increase in the levels of genomic DAT in the transgenic line compared with the WT, indicating that there are four copies of transgene integrated in the genome, bringing the total number of DAT copies to six in the DAT-tg animals. The observation of simple Mendelian inheritance of the transgene indicates that the four transgene copies have integrated in tandem presumably at a single locus. In accordance with the increased genomic copies of DAT, there is a 3-fold increase in the levels of DAT protein in the transgenic animals compared with WT controls as measured by Western blot of striatal preparations (Fig. 1C).
Fig. 1.
Generation of DAT-tg animals. (A) Schematic representation of the BAC clone used for the generation of the DAT-tg animals. (B) Representative DAT Southern blot analysis from WT and DAT-tg animals. Data are means ± SEM (four per group). (C) Representative DAT Western blot analysis of striatal tissue from WT and DAT-tg animals. Data are means ± SEM (five per group). ***, P < 0.001.
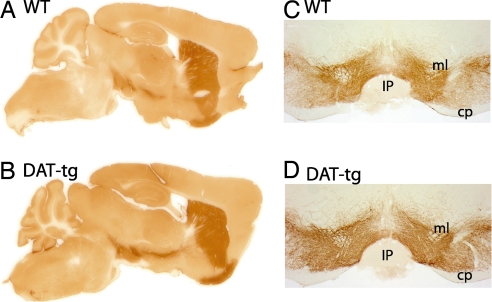
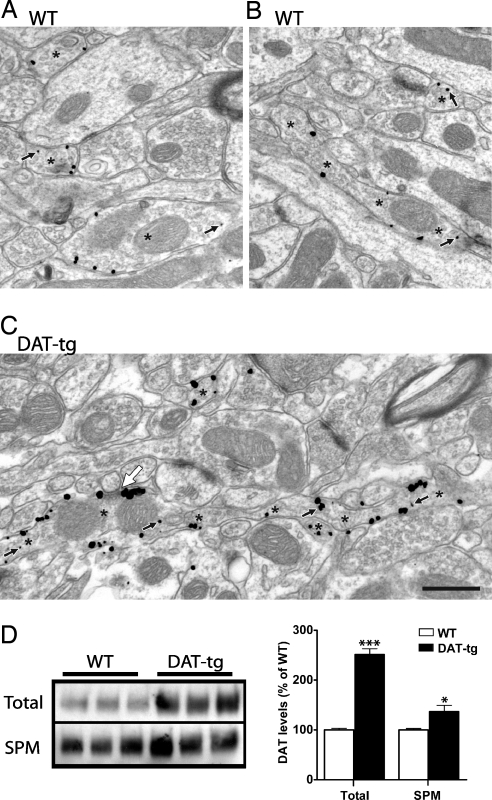
Next, the expression pattern of DAT within the transgenic line was examined by immunohistochemistry. As shown in Fig. 2, in both WT and DAT-tg animals DAT antibodies label dopaminergic neurons that originate in substantia nigra and ventral tegmental area with major projections to the striatum. In the DAT-tg animals the DA axon tracts are more apparent than in the WT animals (Fig. 2 A and B). Fig. 2 C and D shows coronal sections at the level of the DA cell bodies from WT and DAT-tg demonstrating the fidelity of the transgene expression. The proper expression of DAT in the DAT-tg mice was further demonstrated by detailed immunhistochemical analysis of coronal sections through the telencephalon, diencephalon, and mesencephalon of these mice as well as double labeling with tyrosine hydroxylase antibody [supporting information (SI) Figs. 7 and 8]. The subcellular localization of DAT was characterized by using electron microscopy and is shown in Fig. 3. In agreement with prior ultrastructural descriptions in the rat (15–17), immunoreactivity for DAT in both WT and DAT-tg mice is localized exclusively to small-caliber axons and axon varicosities that occasionally form short symmetric synapses on distal dendrites and spines (Fig. 3). Most of the gold–silver particles for DAT are associated with the plasma membrane; however, DAT is also present within intracellular compartments in both WT and DAT-tg animals (Fig. 3C, small arrows). In striatal sections from DAT-tg mice, the density of gold–silver labeling per axonal profile is increased, both on the plasma membrane and in the cytoplasm. Consequently, the overall proportion of gold–silver particles that are distributed to the plasmalemma is roughly the same in DAT-tg (82.5 ± 22.9%, mean ± SD; n = 499 profiles in four mice) and WT mice (81.0 ± 22.4%; n = 1,332 profiles in four mice). According to a generalized linear mixed model (see SI Text), the odds ratio of gold particles representing DAT being observed on the plasma membrane for DAT-tg versus WT mice was 0.98 with 95% confidence intervals of 0.70–1.36. These results are consistent with a nearly symmetrical distribution (i.e., no preferred direction) of ≈1.0, indicating no detectable difference between groups. A secondary two-sample t test confirmed the lack of detectable difference between groups (t6 = −0.30, P = 0.775), again with a nearly symmetrical distribution of confidence intervals (−0.102–0.080). Where immunoreactive axons form synapses, DAT labeling appears to be distributed mainly to the edges of the junction in a perisynaptic location (white arrow in Fig. 3C), consistent with prior observations in the rat striatum (16). To further investigate the subcellular localization of DAT, membrane fractionation experiments were carried out on striatal tissues from WT and DAT-tg animals. As shown in Fig. 3D, there is a 2.5-fold increase in the levels of DAT in the total membrane fraction from DAT-tg animals compared with their WT littermates; however, when synaptic plasma membrane (SPM) fractions are isolated from the total fraction, the increase in the levels of DAT observed in the DAT-tg samples is only 1.3-fold of the WT. This observation suggests that perhaps some specific cellular components required for the proper SPM targeting of DAT may be limiting in the DAT-tg animals.
Fig. 2.
Immunohistochemical localization of DAT. Saggital sections (A and B) and coronal sections (C and D) of WT and DAT-tg show the expression pattern of DAT by immunoperoxidase labeling. cp, cerebral peduncle; IP, intrapeduncular nucleus; ml, medial lemniscus.
Fig. 3.
Subcellular localization of DAT by electron microscopy and membrane fractionation. Dorsal striatum of WT (A and B) and DAT-tg (C) mice show the density of Immunogold–silver labeling for DAT. Immunoreactive axons are identified by asterisks, with two profiles cut longitudinally indicated by multiple asterisks (B and C). Small black arrows indicate cytoplasmic gold particles; all other gold particles are associated with the plasma membrane. The large white arrow shows a synapse formed by the longitudinally sectioned axon most likely onto the neck of a spine. Immunoreactivity for DAT occurs in a perisynaptic location. (Scale bar: 500 nm.) (D) Representative DAT Western blot analysis of total or synaptic plasma membrane fractions from WT and DAT-tg animals. Data are means ± SEM (six per group in duplicates). *, P < 0.05; ***, P < 0.001.
DAT Function in DAT-tg Animals.
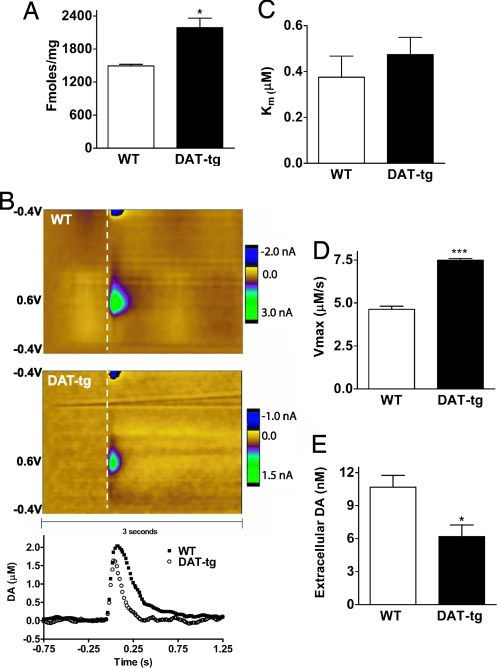
As a first step to assess the amount of functional DAT in the DAT-tg mice we assessed its levels by 3[H]WIN 35428 radioligand binding. Fig. 4A shows that there is a 38% increase in the measurable levels of DAT in DAT-tg mice (2,190 ± 170 fmol/mg) compared with the WT (1,490 ± 30 fmol/mg) in agreement with the increases observed in the SPM samples. We next evaluated the clearance of DA in these animals. In DAT-KO animals DA clearance is essentially reduced to the rate of diffusion (8); thus, it would be expected that, in DAT-tg animals expressing increased levels of DAT, DA clearance should be faster and kinetics of DA uptake should be increased. To evaluate these parameters, fast scan cyclic voltametry (FSCV) on striatal slices of WT and DAT-tg animals was performed. As shown in Fig. 4B, the initial clearance of evoked DA (Vmax) is substantially faster in slice preparations from DAT-tg animals than in those from WT animals, confirming that there is a functional increase in the levels of DAT in the DAT-tg animals. Interestingly, the determined KM values for DA uptake are very similar in both WT (0.37 ± 0.09 μM) and DAT-tg (0.47 ± 0.07 μM) animals, indicating that the affinity of DAT for DA is unchanged in the DAT-tg animals (Fig. 4C). However, using a Michaelis–Menten-based kinetic analysis, we found that there is a 60% increase in the uptake rate for DA in DAT-tg animals (7.48 ± 0.10 μM/s) compared with WT animals (4.63 ± 0.18 μM/s), consistent with the notion of increased transporter activity and levels in DAT-tg animals (Fig. 4D). Furthermore, using a quantitative “low-perfusion-rate” microdialysis on freely moving animals we observed that there is there is a 40% reduction in the extracellular concentration of DA in the DAT-tg animals (6.2 ± 1.1 nM) compared with WT controls (10.7 ± 1.1 nM) (Fig. 4E). Together these observations indicate that the overexpressed transporter in DAT-tg animals is functional and that its activity leads to increased clearance and reduced extracellular levels of DA and thus to hypodopaminergia.
Fig. 4.
Analysis of DAT function. (A) Levels of DAT determined by 3[H]WIN 35428 radioligand binding. (B) Release and clearance of DA measured by FSCV in striatal slices from WT and DAT-tg animals after a single-pulse stimulation. The color plot topographically depicts the voltammetric data, with time on x axis, applied scan potential on the y axis, and background-subtracted current measured on the z axis in pseudocolor (27). The white dashed line denotes the location of the stimulation. (Bottom) Dopamine concentrations obtained every 16 ms over a 2-s interval where traces monitor the release (ascending curve) and reuptake (descending curve) of DA. (C) Km values of WT and DAT-tg animals for DA determined by FSCV. Data are means ± SEM (n = 4 per group). (D) Uptake rates (Vmax) of DAT for DA in WT and DAT-tg animals measured by FSCV. Data are means ± SEM (n = 4 per group). (E) Extracellular levels of DA in the striatum of freely moving WT (n = 4) and DAT-tg (n = 7) mice as determined by quantitative low-perfusion-rate microdialysis. Data are means ± SEM. *, P < 0.05; ***, P < 0.001.
Increased Locomotor Response to Amphetamine in DAT-tg Mice.
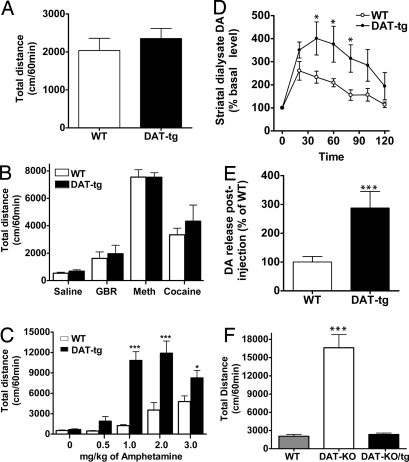
To evaluate how reduced levels of DA in DAT-tg animals would affect their locomotion, basal and psychostimulant-induced locomotor activity of WT and DAT-tg animals was examined. As shown in Fig. 5A, there is no significant difference in the basal locomotion of WT and DAT-tg animals for total distance traveled during 60 min. Likewise, the DAT inhibitors GBR12909, methylphenidate, and cocaine induce similar locomotor activities in WT and DAT-tg animals (Fig. 5B). However, upon injection with amphetamine (0.5, 1, 2, and 3 mg/kg), there is a remarkable increase in locomotor activity (shown as total distance) in DAT-tg animals compared with their WT littermates (Fig. 5C). This increase in locomotor activity is presumably due to a marked increase in extracellular levels of DA after amphetamine treatment of DAT-tg animals compared with WT mice as measured by conventional microdialysis experiments performed on freely moving animals (Fig. 5 D and E). Interestingly, the increase in DA release in the DAT-tg animals after amphetamine is approximately three times higher than that of their WT littermates (Fig. 5E), in agreement with the increased levels of total DAT protein in the Tg animals (Fig. 1C). Importantly, although DAT-tg animals do not display any discernable basal locomotor phenotype compared with their WT littermates, crossing the DAT-tg to DAT-KO animals completely abolishes the novelty-induced hyperactivity observed in DAT-KO animals (Fig. 5F), indicating that the transgenic expression of DAT in the DAT-tg animals can fully replace and rescue the function of the endogenous DAT allele inactivated in DAT-KO animals. Furthermore, it should be noted that increased responses to amphetamine were also observed in another line of transgenic mice overexpressing an HA-tagged DAT. These mice were generated by using the same BAC construct described above in addition to harboring an HA epitope (data not shown). These results indicate that the effects described here are unlikely to result from a founder/insertion effect.
Fig. 5.
Locomotor analysis. (A) Basal locomotion of WT and DAT-tg animals as measured by total distance over a 60-min period. Data are means ± SEM (n = 8 per group). (B) Total distance traveled during 60 min after i.p. injection of GBR12909 (10 mg/kg), methylphenidate (20 mg/kg), and cocaine (20 mg/kg) to WT and DAT-tg animals that were habituated for 60 min. Data are means ± SEM (n = 8 per group). (C) Total distance traveled during 60 min after i.p. injection of amphetamine to WT and DAT-tg animals that were habituated for 60 min. Data are means ± SEM (n = 8 per group). (D) Extracellular striatal DA dynamics in WT and DAT-tg mice as determined by conventional microdialysis after i.p. injection of amphetamine (3 mg/kg) to animals. Data are presented as percentage of average level of DA measured in at least three samples collected before the drug administration. Data are means ± SEM (n = 5–8 per group). (E) Calculation of the fold increase in DA release after amphetamine injection in WT and DAT-tg animals. Data are means ± SEM (n = 5–8 per group). (F) Basal locomotor activity of WT, DAT-KO, and crosses between DAT-KO and DAT-tg animals (DAT-KO/tg) as measured by total distance over a 60-min period in the open field. Data are means ± SEM (n = 5–8 per group). *, P < 0.05; ***, P < 0.001.
Increased Rewarding Properties of Amphetamine but Not Natural Reward in DAT-tg Mice.
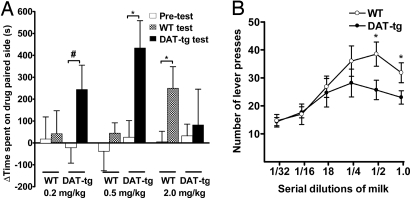
Next we evaluated whether the DAT-tg animals also showed an increased sensitivity to the rewarding effects of amphetamine. For this, conditioned place preference (CPP) experiments were carried out testing the rewarding effects of amphetamine in WT and DAT-tg animals. As shown in Fig. 6A, DAT-tg animals display CPP at lower doses of amphetamine (0.2 and 0.5 mg/kg) compared with the WT animals. In fact, at amphetamine concentrations of 2 mg/kg, where there is a modest CPP in WT animals, DAT-tg animals no longer display place preference. The shift of the CPP response to lower doses of amphetamine suggests that DAT-tg animals are more sensitive to the rewarding effects of amphetamine.
Fig. 6.
Responsiveness of DAT-tg animals to psychostimulant and natural rewards. (A) Amphetamine-induced CPP in WT and DAT-tg animals. CPP was performed as described in Materials and Methods. Data are shown as the difference in the time spent in the amphetamine-associated chamber vs. the saline-associated chamber on the test day and the preconditioning day. Data are means ± SEM (n = 7–12 animals). *, P < 0.05; #, P < 0.062. (B) Results of operant responding of WT and DAT-tg animals for sweet liquid reward. The experiments were conducted as described in Materials and Methods. Shown are the number of lever presses during the first 15 min for WT and DAT-tg animals (six per group) for different dilutions of sweetened condensed milk.
Because DAT-tg mice display enhanced sensitivity to the rewarding effects of amphetamine, we also examined their responsiveness to natural reward. For this, an operant behavioral paradigm was designed to measure the propensity of WT and DAT-tg animals to lever-press for different dilutions of sweetened condensed milk. As shown in Fig. 6B, WT animals show a bell-shaped dose–response curve of lever presses for the dilutions of sweetened condensed milk, with maximal lever pressing seen at the 1/2 dilution milk concentration. Although DAT-tg animals show a similar bell-shaped curve, they lever-press significantly less than the WT animals for two of the concentrations tested, indicating that DAT-tg animals exhibit reduced operant responding for this natural reward compared with WT animals. This observation is consistent with the notion that DA is a central determinant involved in natural reward and that animals with reduced levels of extracellular DA would be expected to show a reduced response for the tested natural food reward. Furthermore, the increased amphetamine-mediated reward observed in the CPP experiments occurs despite reduced responsiveness of DAT-tg animals to natural reward.
Discussion
In this study we describe a genetically modified mouse line in which four additional copies of the DAT gene have been incorporated. These mice show a 3-fold increase in DAT protein expression with an ≈50% increase in DAT functional activity. This increase in DAT activity leads to a 40% reduction of extracellular DA; however, no change in the basal locomotor phenotype of the transgenic mice is observed. A striking observation made with the DAT-tg mouse is their increased responsiveness to the locomotor and rewarding effects of amphetamine.
The mouse line described in this study was generated by BAC transgenic technology, using a clone of the complete DAT locus (40 kb) flanked by 80 kb of upstream and downstream genomic sequence. Our results indicate that the essential elements required for the proper spatial expression of DAT are located within this sequence and specify expression in dopaminergic neurons, reaffirming prior observations that BAC transgenesis recapitulates the native expression pattern of the gene of interest (18). This was of particular importance because previously reported transgenic animals expressing DAT under the control of the tyrosine hydroxylase promoter region had only a modest overexpression of DAT in several brain regions that is not restricted to dopaminergic neurons (19).
The fraction of total membrane expressed DAT in the DAT-tg animals mirrors that observed in WT animals, where the majority of the protein is localized at the plasmalemma membrane with some transporters detected in intracellular compartments. Despite the fact that there is a 3-fold increase in both the DAT protein and amphetamine-induced DA efflux in DAT-tg animals, there is only a 30% increase in the levels of DAT in the SPM fraction of DAT-tg animals, which leads to a 38% increase in 3[H]WIN 35428 binding. Consequently, there is a 60% increase in DA uptake in striatal tissues that is accompanied by a 40% reduction of extracellular brain DA concentration in these animals compared with WT. These observations are quite intriguing because the global membrane localization of the DAT in DAT-tg animals seems to be very similar to what is observed in WT, whereas its subcellular SPM localization is not increased to the same level. These results suggest that the fraction of DAT involved in DA uptake and 3[H]WIN 35428 binding is the one localized to the SPM, whereas all membrane localized DAT can be substrate for amphetamine-induced DA efflux. This in turn suggests that other regulatory mechanisms, besides transporter levels, have to be considered for uptake and binding activity. Indeed, it has been reported that interaction of DAT with several cytosolic proteins can influence both its membrane localization and its uptake activity (20). It is possible that levels of these proteins expressed in dopaminergic neurons could become limiting and allow only an ≈30–50% increase in DAT uptake activity despite a 300% increase in protein expression. Alternatively, the perisynaptic compartment where DAT proteins are localized could itself be limiting in allowing increases of DAT incorporation, which may explain the relatively minor (30%) increases observed in synaptic fractions. Further studies will be required to elucidate what other mechanisms, beyond transporter levels, could limit the transporter activity in the DAT-tg animals.
Although many studies have evaluated the effect of DA receptor antagonists in reward-related behaviors (21), our work is based on a genetic manipulation resulting in reduced levels of extracellular DA due to DAT overexpression. Our results indicate that the DAT-tg animals have a reduced propensity to lever-press for food reward. This does not seem to be due to a reduced ability of these animals to sense the food, because, like WT animals, they also show an inverted U-shaped curve for the dilutions of milk. It thus appears that the DAT-tg animals have a modest reduction in motivation to work for the specific natural reward tested here.
In contrast to this operant behavior, the DAT-tg animals show a remarkable increase in amphetamine-induced locomotor activation and an increased sensitivity to the rewarding effects of amphetamine. This increased responsiveness to amphetamine is in accord with the molecular mechanism of amphetamine action in which its DA-releasing activities are dependent on DAT (9). Indeed, we and others (22–24) have reported that the DAT heterozygous animals, or animals in which DAT expression has been reduced by 50% via siRNA treatment, demonstrate a commensurate reduction in amphetamine-induced locomotor activation. It thus seems clear that the locomotor-stimulating effects of amphetamine are absolutely dependent and directly proportional to the levels of DAT. In contrast, the locomotor stimulant effects of DAT blockers such as GBR12909, methylphenidate, and cocaine are similar in WT and DAT-tg animals. This indicates that elevations in DA levels resulting from inhibition of reuptake after neuronal activity-dependent vesicular DA release are substantially less susceptible to DAT overexpression than amphetamine-dependent changes in DA levels. It is also important to note that the basal locomotor activity of the DAT-tg animals is not different from that of the WTs, whereas DAT-KO or DAT knockdown (DAT-KD) animals show a significant increase in novelty-induced hyperlocomotion (7, 25). Furthermore, in DAT-KO or DAT-KD animals (hyperdopaminergic), amphetamine treatment results in a marked reduction of their locomotor behavior, whereas amphetamine treatment of DAT-tg animals results in a dramatic increase in the locomotor activity.
In conclusion, we show that mice with selective overexpression of DAT in DA neurons have a 40% functional reduction in extracellular brain DA concentration with no discernible effects on their basal locomotor phenotype. However, these animals display a marked increase in amphetamine-induced locomotion and reward. These observations indicate that the sensitivity of animals to the stimulant and rewarding effects of amphetamine seem to be directly proportional to the levels of DAT. At the same time, our results indicate that this mouse model of DAT overexpression does not recapitulate the hyperactivity endophenotype and responses to amphetamine seen in ADHD that have been linked to increased DAT expression (12). In fact, the observations presented in this study, as well as previous reports on DAT-KO and DAT-KD animals (22, 25), are more in line with a recent study that actually found modest decreases in DAT levels in drug-naïve adult ADHD patients rather than an overexpression of DAT (13), suggesting that symptoms of ADHD might be more consistent with loss rather than gain of DAT function in humans.
Methods
Animals.
For all experiments we used age- and gender-matched WT and DAT-tg mice (3–5 months old) maintained on a C57BL/6J genetic background. All behavioral experiments were performed during the light cycle. Experiments were conducted in accordance with the National Institutes of Health guidelines for the care and use of animals and an approved animal protocol from the Duke University Animal Care and Use Committee.
Generation of DAT-tg Mice.
A BAC containing the mouse DAT locus was obtained from Genome Sciences. The BAC4 clone was chosen for pronuclear injection because the DAT locus (40 kb) is flanked by 80 kb of downstream and upstream genomic sequences. The BAC DNA was isolated by using the Clontech BAC preparation kit. The isolated BAC DNA was resuspended at 2 ng/μl in injection buffer (0.03 mM spermine/0.07 mM spermidine). Pronuclear injections were carried out by the Duke Transgenic Mouse Facility using C57BL/6J embryos. One transgene positive founder was identified by PCR-based genotyping using oligos that recognize BAC vector sequences (forward, 5′-gcatcagccagcgcagaaatatttcc; reverse, 5′-gatacttcgttatcgacaccagctgc-3′). This founder was further bred for characterization of the transgene outlined in this study.
Southern Blot.
Genomic DNA was isolated from tail biopsy after Proteinase K digestion and isopropanol precipitation. A total of 8 μg of genomic DNA was used for overnight EcoRI digestion. Nitrocellulose membranes were hybridized in QuikHyb buffer (Stratagene) with a radiolabeled probe generated by PCR amplification of primers 5′-tggagctcatcttggtcaag-3′ and 5′-tacaccatgccctgcacaca-3′, which correspond to sequences within and 3′ of DAT exon 1, respectively.
Western Blot.
Brain samples were mechanically homogenized in RIPA buffer (50 mmol/liter Tris·HCl, pH 7.4/150 mmol/liter NaCl/1% Nonidet P-40/0.5% sodium deoxycholate/0.1% SDS; Sigma) plus protease inhibitor mixture (Roche 1873580). Protein concentration was measured by using a DC protein assay (Bio-Rad). Protein extracts (30 μg) were separated by 10% SDS/PAGE and transferred to nitrocellulose membranes. Blots were immunostained overnight at 4°C with anti-DAT (1:1,000 dilution; Chemicon MAB369) as a primary antibody and revealed with peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch) and a chemiluminescent reagent (SuperSignal West-Pico; Pierce). Densitometric analysis was carried out by scanning the film with a Fluor-S multiimager (Bio-Rad) and quantifying the bands using Quantity One software (Bio-Rad).
Microdialysis.
In vivo microdialysis measurements of striatal extracellular DA levels in freely moving mice were performed at least 24 h after implantation of a microdialysis probe as described previously (26). Dialysate samples were assayed for DA by using HPLC-EC. On day 1 of the experiment (24 h after operation), a quantitative low-perfusion-rate microdialysis approach with perfusion flow rate of 70 nl/min was applied to reliably establish basal extracellular levels of DA. On day 2 of the experiment (48 h after operation), a conventional microdialysis experiment (perfusion flow rate 1 μl/min) was performed to assess the effect of amphetamine on extracellular DA levels.
Locomotor Analysis.
Locomotor activity was assessed in automated locomotor activity monitors (Accuscan Instruments). Mice were initially placed into the activity monitor chamber (20 cm × 20 cm) for 60 min, then injected with vehicle (10 ml/kg of body weight i.p.) or the drug, returned to the chamber, and monitored for 60 min after injection. Locomotor activity was measured in terms of the total distance covered unless indicated otherwise.
Statistics.
Data are reported as means ± SEM. Statistical significance was evaluated by two-tailed t test or one- or two-way ANOVA, as appropriate.
Additional Details.
See SI Text for a description of 3[H]WIN 35428 binding, histology, FSCV, and CPP.
Supplementary Material
Acknowledgments.
We thank Katherine Clark for assistance with genotyping of mice. We thank Drs. Ramona Rodriguez and William Wetsel for access to CPP equipment. This work was supported in part by National Institutes of Health, National Institute on Drug Abuse Grants NS-19576 (to M.G.C.) and NS-38879 (to R.M.W.). A.S. was supported by a fellowship from the Canadian Institutes of Health Research. A.J.R. holds a National Institute on Drug Abuse K01 award (DA017703). K.M. holds a Howard Hughes Medical Institute scholarship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707646105/DC1.
References
- 1.Molinoff PB, Axelrod J. Biochemistry of catecholamines. Annu Rev Biochem. 1971;40:465–500. doi: 10.1146/annurev.bi.40.070171.002341. [DOI] [PubMed] [Google Scholar]
- 2.Hornykiewicz O. Biochemical aspects of Parkinson's disease. Neurology. 1998;51(2) Suppl 2:S2–S9. doi: 10.1212/wnl.51.2_suppl_2.s2. [DOI] [PubMed] [Google Scholar]
- 3.Carlsson A. A paradigm shift in brain research. Science. 2001;294:1021–1024. doi: 10.1126/science.1066969. [DOI] [PubMed] [Google Scholar]
- 4.Gainetdinov RR, Caron MG. Monoamine transporters: From genes to behavior. Annu Rev Pharmacol Toxicol. 2003;43:261–284. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- 5.Hahn MK, Blakely RD. The functional impact of SLC6 transporter genetic variation. Annu Rev Pharmacol Toxicol. 2007;47:401–441. doi: 10.1146/annurev.pharmtox.47.120505.105242. [DOI] [PubMed] [Google Scholar]
- 6.Torres GE, Amara SG. Glutamate and monoamine transporters: New visions of form and function. Curr Opin Neurobiol. 2007;17:304–312. doi: 10.1016/j.conb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 8.Jones SR, et al. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci USA. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: A review. Prog Neurobiol. 2005;75(6):406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Seeman P, Madras BK. Anti-hyperactivity medication: Methylphenidate and amphetamine. Mol Psychiatry. 1998;5:386–396. doi: 10.1038/sj.mp.4000421. [DOI] [PubMed] [Google Scholar]
- 11.Madras BK, Miller GM, Fischman AJ. The dopamine transporter and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1397–1409. doi: 10.1016/j.biopsych.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Dougherty DD, et al. Dopamine transporter density in patients with attention deficit hyperactivity disorder. Lancet. 1999;354:2132–2133. doi: 10.1016/S0140-6736(99)04030-1. [DOI] [PubMed] [Google Scholar]
- 13.Volkow ND, et al. Brain dopamine transporter levels in treatment and drug naïve adults with ADHD. NeuroImage. 2007;34:1182–1190. doi: 10.1016/j.neuroimage.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Sacchetti P, Brownschidle LA, Granneman JG, Bannon MJ. Characterization of the 5′-flanking region of the human dopamine transporter gene. Brain Res Mol Brain Res. 1999;74:167–174. doi: 10.1016/s0169-328x(99)00275-2. [DOI] [PubMed] [Google Scholar]
- 15.Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J Neurosci. 1996;15:436–447. doi: 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hersch SM, Yi H, Heilman CJ, Edwards RH, Levey AI. Subcellular localization and molecular topology of the dopamine transporter in the striatum and substantia nigra. J Comp Neurol. 1997;388:211–227. [PubMed] [Google Scholar]
- 17.Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heintz N. BAC to the future: The use of bac transgenic mice for neuroscience research. Nat Rev Neurosci. 2001;12:861–870. doi: 10.1038/35104049. [DOI] [PubMed] [Google Scholar]
- 19.Donovan DM, et al. Cocaine reward and MPTP toxicity: Alteration by regional variant dopamine transporter overexpression. Brain Res Mol Brain Res. 1999;73:37–49. doi: 10.1016/s0169-328x(99)00235-1. [DOI] [PubMed] [Google Scholar]
- 20.Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: Structure, regulation and function. Nat Rev Neurosci. 2003;1:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- 21.Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc London B. 2006;361:1149–1158. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gainetdinov RR, et al. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- 23.Spielewoy C, et al. Hypolocomotor effects of acute and daily d-amphetamine in mice lacking the dopamine transporter. Psychopharmacology (Berlin) 2001;159:2–9. doi: 10.1007/s002130100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salahpour A, Medvedev IO, Beaulieu JM, Gainetdinov RR, Caron MG. Local knockdown of genes in the brain using small interfering RNA: A phenotypic comparison with knockout animals. Biol Psychiatry. 2007;61:65–69. doi: 10.1016/j.biopsych.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Zhuang X, et al. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci USA. 2001;98:1982–1987. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gainetdinov RR, et al. Dopaminergic supersensitivity in G protein-coupled receptor kinase 6-deficient mice. Neuron. 2003;38:291–303. doi: 10.1016/s0896-6273(03)00192-2. [DOI] [PubMed] [Google Scholar]
- 27.Michael D, Travis ER, Wightman RM. Color images for fast-scan CV measurements in biological systems. Anal Chem. 1998;70:586A–592A. doi: 10.1021/ac9819640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.