The human immune system is extraordinarily good at generating antibodies to recognize an almost infinite range of antigens. Antibodies to antigens found on the cells and tissues of the host are repressed by tolerance mechanisms in health, but in autoimmune diseases they can become prominent. Although there is debate in a number of instances about whether autoantibodies are a cause or effect of disease, in many cases it has been established that autoantibodies directly lead to morbidity and even mortality. Although there is no known way to specifically “switch off” the generation of a particular autoreactive antibody response, work by Collin et al. (1) in this issue of PNAS suggests a strategy for reducing the adverse consequences of autoantibodies (1).
Once an antibody (IgG) has engaged a foreign molecule via its variable antigen binding arms (Fab), the subsequent elimination of the target largely depends on the “effector functions” of the constant stem region (Fc). A wide variety of receptor molecules interact with different sites on the Fc, resulting in a range of fates for the bound antigen (2, 3). One of the key effector systems is complement. This central pathway recognizes “nonself” molecular patterns, including arrays of antibody displayed on an antigen surface, and targets them for destruction via a highly efficient array of cell-killing machinery. A specialized Fc adaptor molecule called C1q can bind to a defined binding site on the Fc (4), whereas a separate domain of C1q is associated with enzymes that trigger the rapid activation of the complement pathway. In this way C1q represents a molecular link between the new world of the adaptive immune system and the evolutionarily ancient killing mechanisms of the complement system (5). A second key effector system is provided by IgG Fc receptors (FcγRs) on the surface of many immune cells (3). Immune complexes coated with multiple IgG molecules are able to bind and cross-link FcγRs (2, 6). Upon cross-linking, FcγRs initiate an intracellular signaling cascade via phosphorylation of associated immunoreceptor tyrosine-based activation motifs (7). Despite close similarities in the cytoplasm signaling pathways, the specific consequences of FcγR induced activation depend on the particular cell type. For example, monocytes and macrophages are triggered to engulf and degrade antigens in lysosomal compartments, whereas dendritic cells efficiently capture the IgG-bound antigen and then present antigenic components, via MHC I and MHC II, to T cells (8, 9). Thus the activating FcγRs link the antibody response to cellular immunity.
Although most FcγRs are activating, the FcγRIIB receptor functions as a negative, inhibitory regulator of leukocyte function. Expression of the FcγRIIB receptor is widespread across most classes of leukocytes (9). Its central importance to immune regulation and tolerance in humans is highlighted by the increased susceptibility to autoimmune disease associated with polymorphisms of the FcγRIIB gene and its promoter (10). In mice, Fc binding to the FcγRIIB receptor on plasma cells, in the absence of a compensating costimulatory BCR signal, leads directly to apoptosis (11). The FcγRIIB receptor may also play an inhibitory role in B cell activation, preventing the generation of additional antibodies to a surface that has previously been targeted (12).
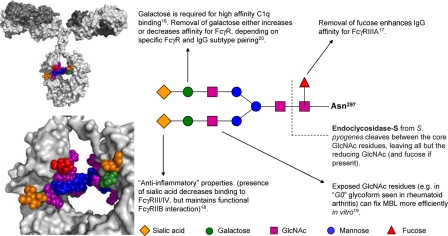
Effector systems have long been known to be sensitive to the glycosylation state of IgG. The twin carbohydrates of the Fc (attached to Asn-297 of each of the two heavy chain Cγ2 domains; see Fig. 1) affect the local structure of the protein (13, 14). The close packing of the glycans appears to determine how closely together the two Cγ2 domains of Fc can interact. Progressive removal of carbohydrates changes the “open” horseshoe shape of the Fc to a more “closed” form (15). Biochemical and crystallographic studies have demonstrated that FcγRs are extraordinarily sensitive to such changes in protein conformation, apparently favoring recognition of the open form of the Fc. The affinity of C1q for IgG Fc is also affected by the type of glycan present on the molecule: for example, the deposition of C1q on agalactosylated IgG is reduced, thus decreasing this glycoform's ability to initiate complement-mediated lysis/phagocytosis (16).
Fig. 1.
The two conserved N-linked carbohydrate chains of IgG are attached to Asn-297 of the Cγ2 domains of each IgG heavy chain. A sialylated biantennary glycan is shown [based on published crystallographic data (3, 15), with nonreducing sialic acid residues modeled according to likely linkage constraints]. The close packing of the carbohydrates between the two chains is evident. A range of other glycoforms also exists on serum IgG (13). These other glycans are based mostly on the biantennary structure but without some or all of the sialic acid or galactose residues from the nonreducing terminus (or fucose residues from the core). The presence of a particular carbohydrate maintains a defined structure of the Fc region, with specific consequences for Fc effector function. Some of the key glycan-mediated interactions are indicated.
Glycan modification of IgG can alter the relative binding to activating and inhibitory FcRs and thus alter the nature of antibody responses. The affinity of the activating FcγRIIIA for all classes of IgG is increased by an order of magnitude, after removal of the single core fucose residue (3, 17) (see Fig. 1). Importantly, however, not all FcR interactions are affected equally by changes in Fc glycosylation. Thus, the presence of sialic acid on some Fcs markedly decreases affinity for activating FcγR (notably FcγRIII/IV) but does not prevent interaction with the inhibitory, antiinflammatory FcγRIIB receptor (3, 18). Thus sialylated IgG has antiinflammatory properties not shared by desialylated IgG glycoforms, such as the IgG0 form prevalent in rheumatoid arthritis. Glycosylation emerges as a potential regulator of activating and inhibitory effector functions.
In addition to indirect modulation of protein–protein interactions, another arm of the immune system can directly recognize the carbohydrates of IgG. Mannose binding lectin (MBL), an initial component of the lectin-activated complement pathways), recognizes mannose and N-acetyl glucosamine (GlcNAc) but not galactose or sialic acid on Fc glycans. Thus the reduction or removal of galactose (observed in several autoimmune conditions, notably rheumatoid arthritis) can expose GlcNAc residues and lead to an increase in MBL binding (19). [However, the inhibition of FcγR binding by Fc sialic acid, rather than MBL binding to nonreducing galactose residues, probably determines the proinflammatory properties of IgG0 (20) in vivo.]
From the above, the concept has emerged of carbohydrates modulating or “fine-tuning” the affinity of antibody for effector molecules and therefore the immune response. However, the sheer variety of interactions involved might suggest that it would be difficult to directly target the Fc carbohydrates for therapeutic modulation of the antibody effector response.
Collin et al. (1) adopt a novel experimental approach to this challenge: enzymatic removal of carbohydrates from IgG Fc in vivo. The enzyme chosen, EndoS, is isolated from a common human pathogen Streptococcus pyogenes and specifically hydrolyzes the Fc glycans between the two core GlcNAc residues. This modification of Fc abolishes the binding of IgG to Fc receptors and reduces complement activation. In their study, Collin et al. first showed that EndoS, at low concentration, efficiently hydrolyzes the glycan on IgG in the complex environment of human blood in vitro. They then went on to show that the hydrolysis could be achieved efficiently in the circulation of a live animal by injecting rabbits with EndoS and monitoring total IgG. Low concentrations of EndoS were effective without perturbing the total IgG concentration or disrupting other serum glycoproteins. Furthermore, the IgG glycan hydrolysis effect was observed even in the face of a host antienzyme response. To investigate whether the glycan modification might have utility in modifying IgG activity, they chose a mouse model of a human autoimmune disease, immune thrombocytopenic purpura (ITP), in which platelets are targeted by autoantibodies. In the model, polyclonal antiplatelet IgG is injected in to the mice to cause disease. Collin et al. showed that pretreatment of the antiplatelet IgG with EndoS or treatment of the mice with EndoS after initiation of disease prevented lethal thrombocytopenia in the majority of animals. Most impressively, even if disease was allowed to proceed to symptoms, as might be the case for humans presenting with ITP, EndoS treatment was able to provide considerable benefit.
How does EndoS exercise a protective function? One plausible mechanism is that “activating” signals from C1q, FcγRs, and MBL binding are all reduced or abolished, whereas the interaction with the “inhibitory” FcγRIIB receptor is preserved. This mechanism may reflect the natural immuno-suppressive role of this enzyme. S. pyogenes has evolved several mechanisms to evade and diminish the adaptive immune response, one of these appears to effectively switch off the effector functions of IgG.
The idea that antibody-mediated autoimmunity could be therapeutically controlled by a single enzyme is certainly an attractive one, but will Fc deglycosylation become a therapeutic reality? Several questions remain. EndoS is a bacterial protein and is readily targeted for removal by antibodies, wihch would normally limit the half-life of the protein in the blood. However, Collin et al. (1) report that, although antibodies to EndoS were observed in their study, the enzyme nonetheless exhibited reasonable pharmacokinetics. A possible explanation for this observation is that the normal Fc-mediated mechanisms for clearing foreign proteins are reduced, because the IgGs bound to the enzyme are themselves deglycosylated by the enzyme. Another potential obstacle might be that EndoS would cleave glycans other than those on IgG with unknown consequences. Encouragingly, however, EndoS appears unusually specific for the glycans on IgG, probably a consequence of its intrinsic affinity for Fc.
A final concern is that the deglycosylation of autoantibodies is inevitably concurrent with the removal of effector functions from protective, nonself restricted antibodies. The therapeutic benefit of a reduction in autoimmunity is thus bought at the price of decreased antibody protection. The reversibility and magnitude of the IgG-mediated component of autoimmune pathology will determine whether such a tradeoff is medically justified.
Footnotes
The authors declare no conflict of interest.
See companion article on page 4265.
References
- 1.Collin M, Shannon O, Björck L. Proc Natl Acad Sci USA. 2008;105:4265–4270. doi: 10.1073/pnas.0711271105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dijstelbloem HM, van de Winkel JG, Kallenberg CG. Trends Immunol. 2001;22:510–516. doi: 10.1016/s1471-4906(01)02014-2. [DOI] [PubMed] [Google Scholar]
- 3.Nimmerjahn F, Ravetch JV. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 4.Zlatarova AS, Rouseva M, Roumenina LT, Gadjeva M, Kolev M, Dobrev I, Olova N, Ghai R, Jensenius JC, Reid KB, et al. Biochemistry. 2006;45:9979–9988. doi: 10.1021/bi060539v. [DOI] [PubMed] [Google Scholar]
- 5.Ghai R, Waters P, Roumenina LT, Gadjeva M, Kojouharova MS, Reid KB, Sim RB, Kishore U. Immunobiology. 2007;212:253–266. doi: 10.1016/j.imbio.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 7.Humphrey MB, Lanier LL, Nakamura MC. Immunol Rev. 2005;208:50–65. doi: 10.1111/j.0105-2896.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 8.Regnault A, Lankar D, Lacabanne V, Rodriguez A, Thery C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, Amigorena S. J Exp Med. 1999;189:371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nimmerjahn F, Ravetch JV. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Tarasenko T, Dean JA, Bolland S. Autoimmunity. 2007;40:409–417. doi: 10.1080/08916930701464665. [DOI] [PubMed] [Google Scholar]
- 11.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, Hiepe F. Nat Rev Immunol. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 12.Hjelm F, Carlsson F, Getahun A, Heyman B. Scand J Immunol. 2006;64:177–184. doi: 10.1111/j.1365-3083.2006.01818.x. [DOI] [PubMed] [Google Scholar]
- 13.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 14.Lund J, Takahashi N, Pound JD, Goodall M, Jefferis R. J Immunol. 1996;157:4963–4969. [PubMed] [Google Scholar]
- 15.Krapp S, Mimura Y, Jefferis R, Huber R, Sondermann P. J Mol Biol. 2003;325:979–989. doi: 10.1016/s0022-2836(02)01250-0. [DOI] [PubMed] [Google Scholar]
- 16.Tsuchiya N, Endo T, Matsuta K, Yoshinoya S, Aikawa T, Kosuge E, Takeuchi F, Miyamoto T, Kobata A. J Rheumatol. 1989;16:285–290. [PubMed] [Google Scholar]
- 17.Shields RL, Lai J, Keck R, O'Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. J Biol Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 18.Kaneko Y, Nimmerjahn F, Ravetch JV. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 19.Malhotra R, Wormald MR, Rudd PM, Fischer PB, Dwek RA, Sim RB. Nat Med. 1995;1:237–243. doi: 10.1038/nm0395-237. [DOI] [PubMed] [Google Scholar]
- 20.Nimmerjahn F, Anthony RM, Ravetch JV. Proc Natl Acad Sci USA. 2007;104:8433–8437. doi: 10.1073/pnas.0702936104. [DOI] [PMC free article] [PubMed] [Google Scholar]