Abstract
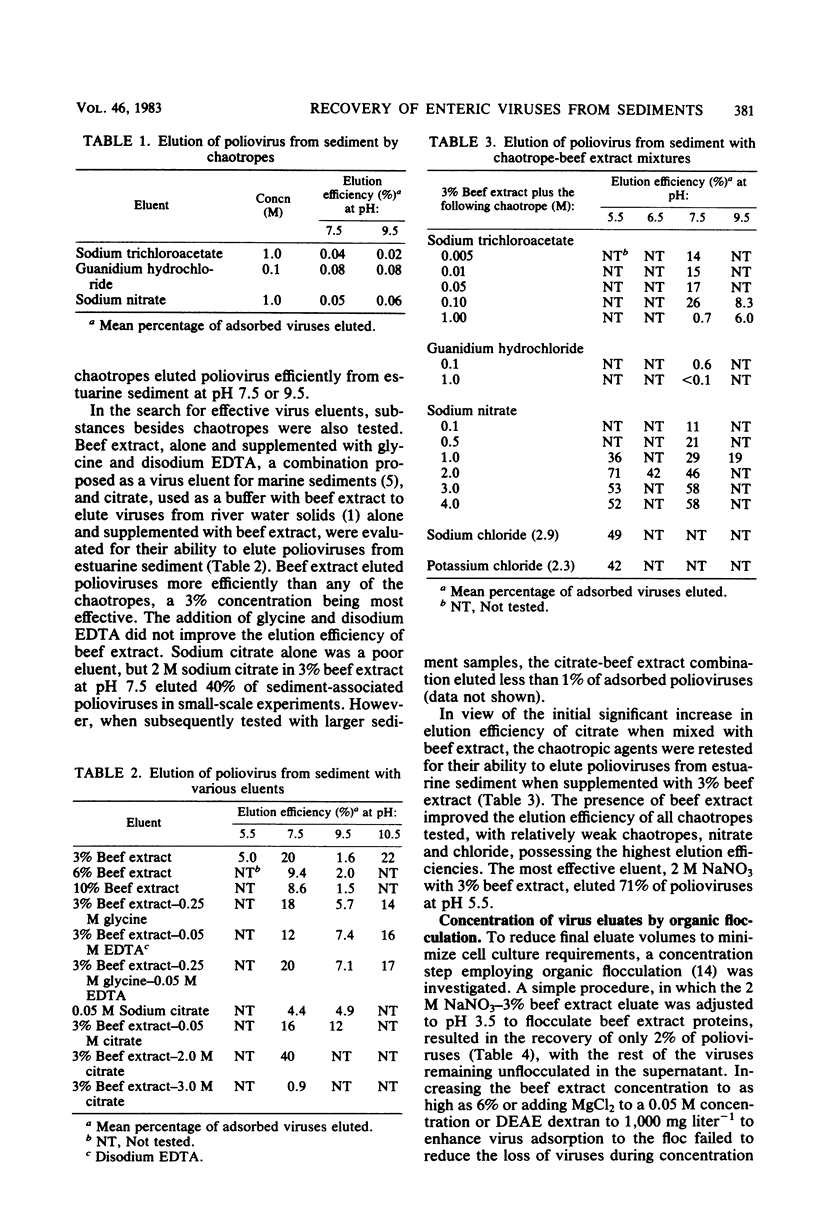
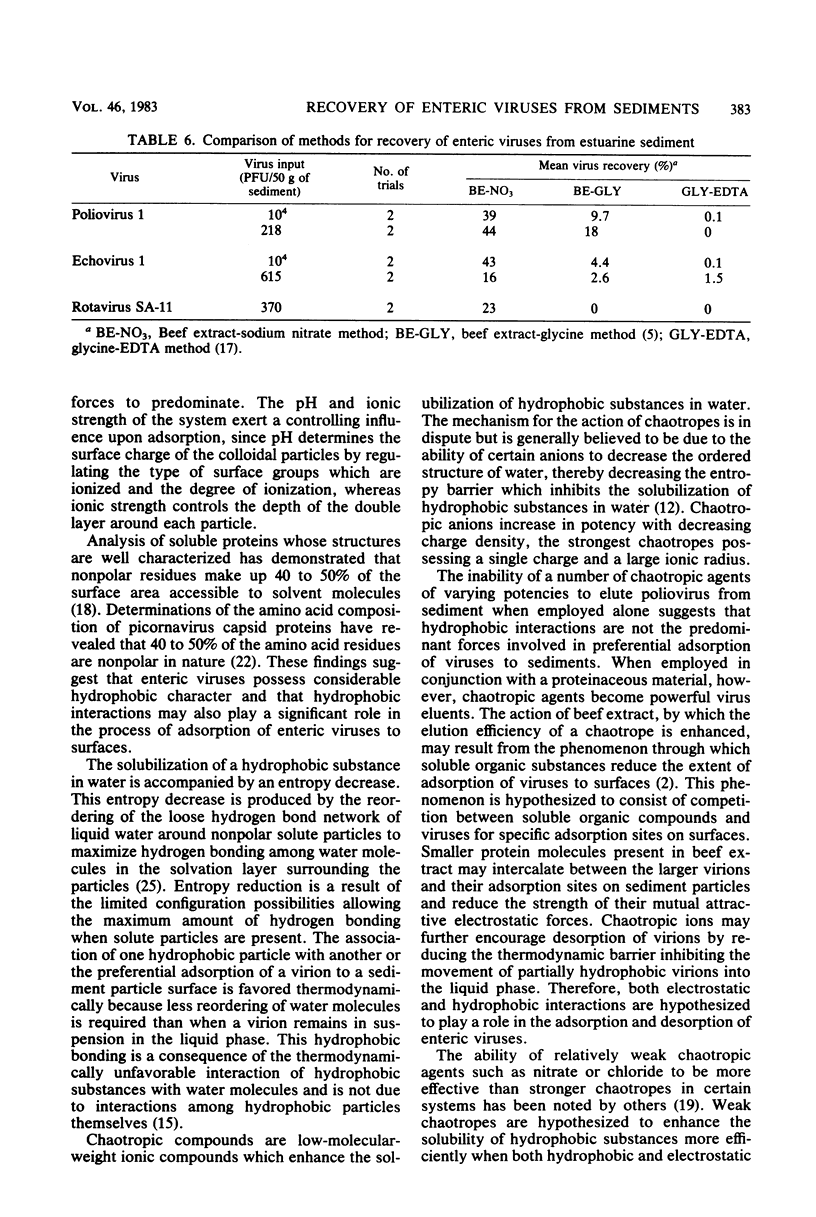
An evaluation was made of the ability of chaotropes, low-molecular-weight ionic compounds which enhance the solubilization of hydrophobic compounds in water, to improve the recovery of enteric viruses from highly organic estuarine sediments. Chaotropic agents alone were poor eluents of polioviruses from sediment but were effective when combined with 3% beef extract. Chaotropes of lower potency, NaNO3, NaCl, and KCl, were more efficient eluents than the stronger chaotropes, guanidium hydrochloride or sodium trichloroacetate. The most effective eluent was 2 M NaNO3 in 3% beef extract at pH 5.5, which eluted 71% of sediment-associated polioviruses. Efficient concentration of the sodium nitrate-beef extract eluate by organic flocculation required the addition of the antichaotrope (NH4)2SO4 to a 2 M concentration and Cat-Floc T (Calgon, Pittsburgh, Pa.) a cationic polyelectrolyte, to a 0.01% concentration. Dialysis of the final concentrate was necessary to reduce salts to nontoxic levels before assay in cell cultures. Trials with highly organic estuarine sediment seeded with high or low numbers of poliovirus 1, echovirus 1, or rotavirus SA-11 demonstrated the superiority of this method over two other methods currently in use.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg G., Dahling D. R. Method for recovering viruses from river water solids. Appl Environ Microbiol. 1980 Apr;39(4):850–853. doi: 10.1128/aem.39.4.850-853.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitton G., Chou Y. J., Farrah S. R. Techniques for virus detection in aquatic sediments. J Virol Methods. 1982 Feb;4(1):1–8. doi: 10.1016/0166-0934(82)90048-9. [DOI] [PubMed] [Google Scholar]
- DOUGHERTY W. J., ALTMAN R. Viral hepatitis in New Jersey 1960-1961. Am J Med. 1962 May;32:704–716. doi: 10.1016/0002-9343(62)90160-2. [DOI] [PubMed] [Google Scholar]
- Farrah S. R., Shah D. O., Ingram L. O. Effects of chaotropic and antichaotropic agents on elution of poliovirus adsorbed on membrane filters. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1229–1232. doi: 10.1073/pnas.78.2.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Destabilization of membranes with chaotropic ions. Methods Enzymol. 1974;31:770–790. doi: 10.1016/0076-6879(74)31080-4. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Solubilization of particulate proteins and nonelectrolytes by chaotropic agents. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1129–1136. doi: 10.1073/pnas.62.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- Katzenelson E., Fattal B., Hostovesky T. Organic flocculation: an efficient second-step concentration method for the detection of viruses in tap water. Appl Environ Microbiol. 1976 Oct;32(4):638–639. doi: 10.1128/aem.32.4.638-639.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBelle R. L., Gerba C. P., Goyal S. M., Melnick J. L., Cech I., Bogdan G. F. Relationships between environmental factors, bacterial indicators, and the occurrence of enteric viruses in estuarine sediments. Appl Environ Microbiol. 1980 Mar;39(3):588–596. doi: 10.1128/aem.39.3.588-596.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBelle R. L., Gerba C. P. Influence of estuarine sediment on virus survival under field conditions. Appl Environ Microbiol. 1980 Apr;39(4):749–755. doi: 10.1128/aem.39.4.749-755.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Wilson C. B., Dixon F. J. Human glomerular basement membrane. Selective solubilization with chaotropes and chemical and immunologic characterization of its components. Biochemistry. 1973 Aug 14;12(17):3260–3266. doi: 10.1021/bi00741a018. [DOI] [PubMed] [Google Scholar]
- Metcalf T. G., Moulton E., Eckerson D. Improved method and test strategy for recovery of enteric viruses from shellfish. Appl Environ Microbiol. 1980 Jan;39(1):141–152. doi: 10.1128/aem.39.1.141-152.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy B. L., Mackowiak P. A., Caraway C. T., Walker J. A., McKinley T. W., Klein C. A., Jr Oyster-associated hepatitis. Failure of shellfish certification programs to prevent outbreaks. JAMA. 1975 Sep 8;233(10):1065–1068. doi: 10.1001/jama.233.10.1065. [DOI] [PubMed] [Google Scholar]
- Smith E. M., Gerba C. P., Melnick J. L. Role of sediment in the persistence of enteroviruses in the estuarine environment. Appl Environ Microbiol. 1978 Apr;35(4):685–689. doi: 10.1128/aem.35.4.685-689.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Carrick R. J., Jensen H. R. Improved methods for detecting enteric viruses in oysters. Appl Environ Microbiol. 1978 Jul;36(1):121–128. doi: 10.1128/aem.36.1.121-128.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillinger F. H. Water revisited. Science. 1980 Jul 25;209(4455):451–457. doi: 10.1126/science.209.4455.451. [DOI] [PubMed] [Google Scholar]



