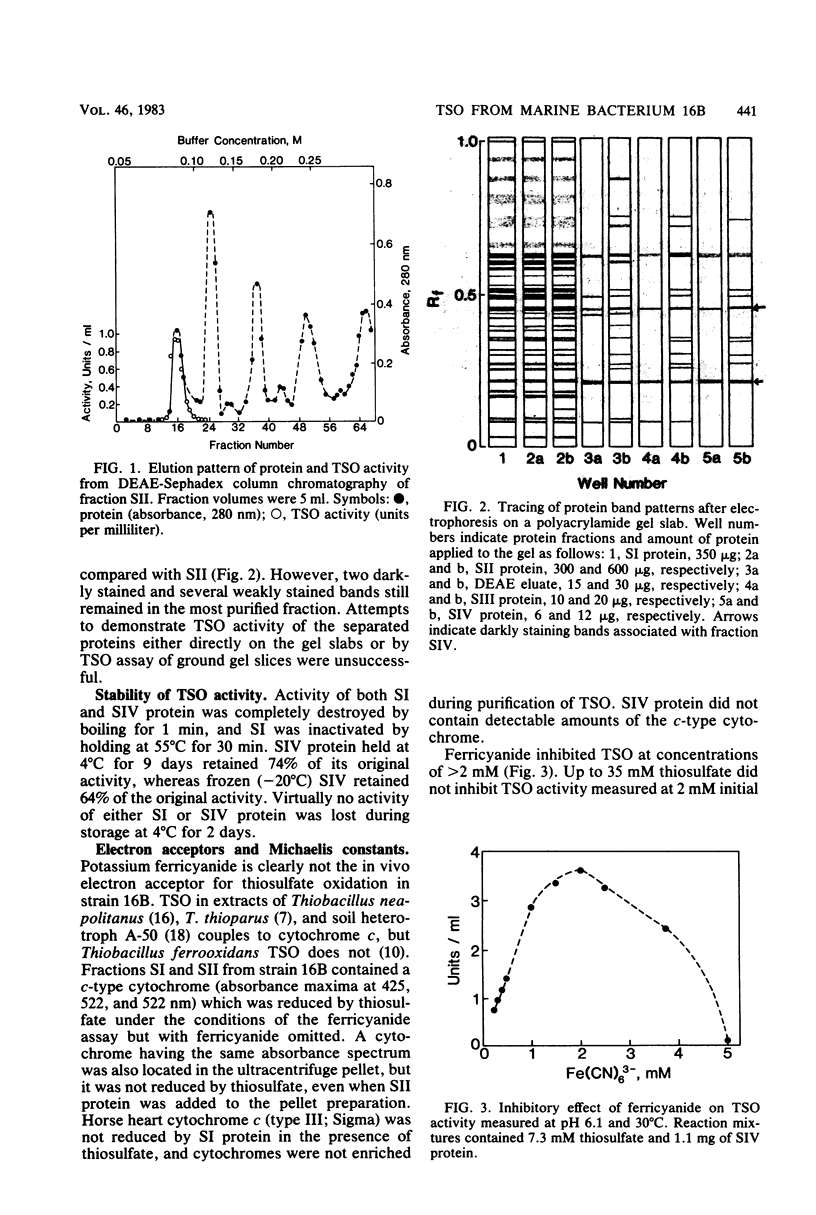
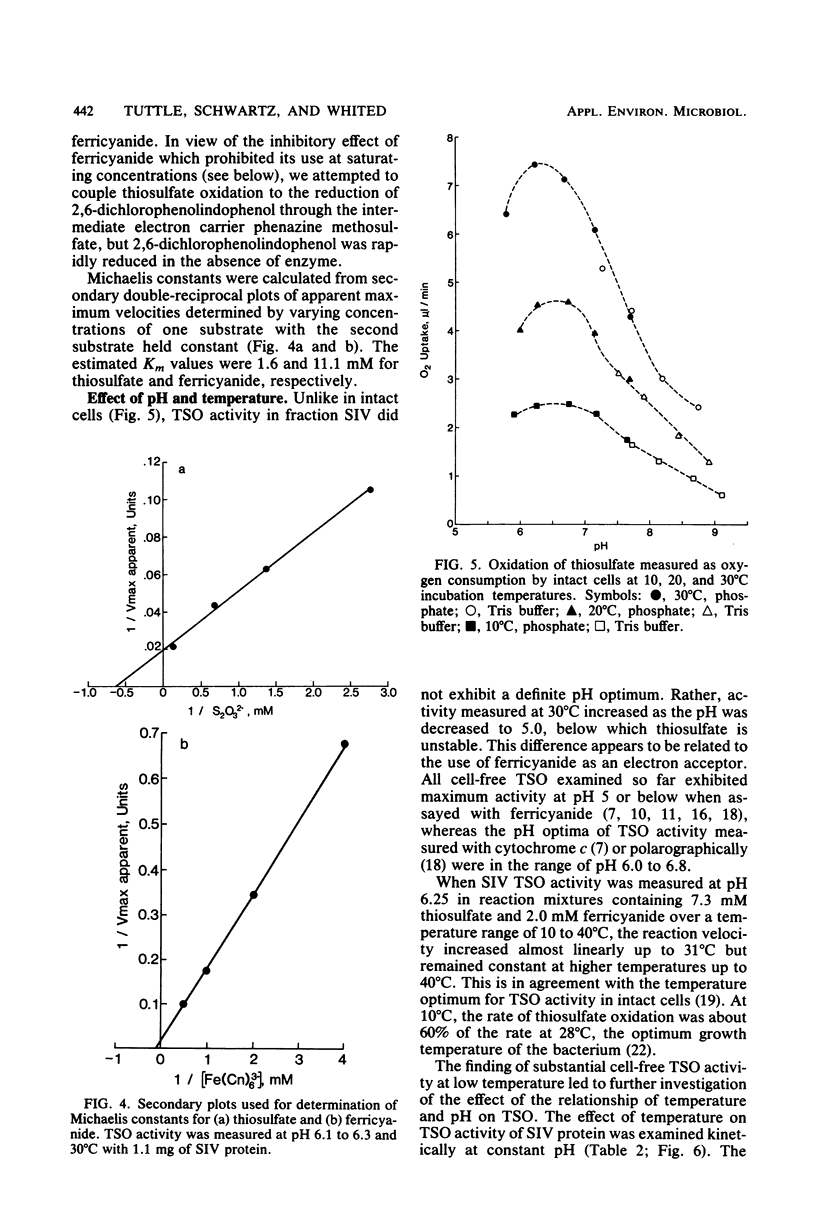
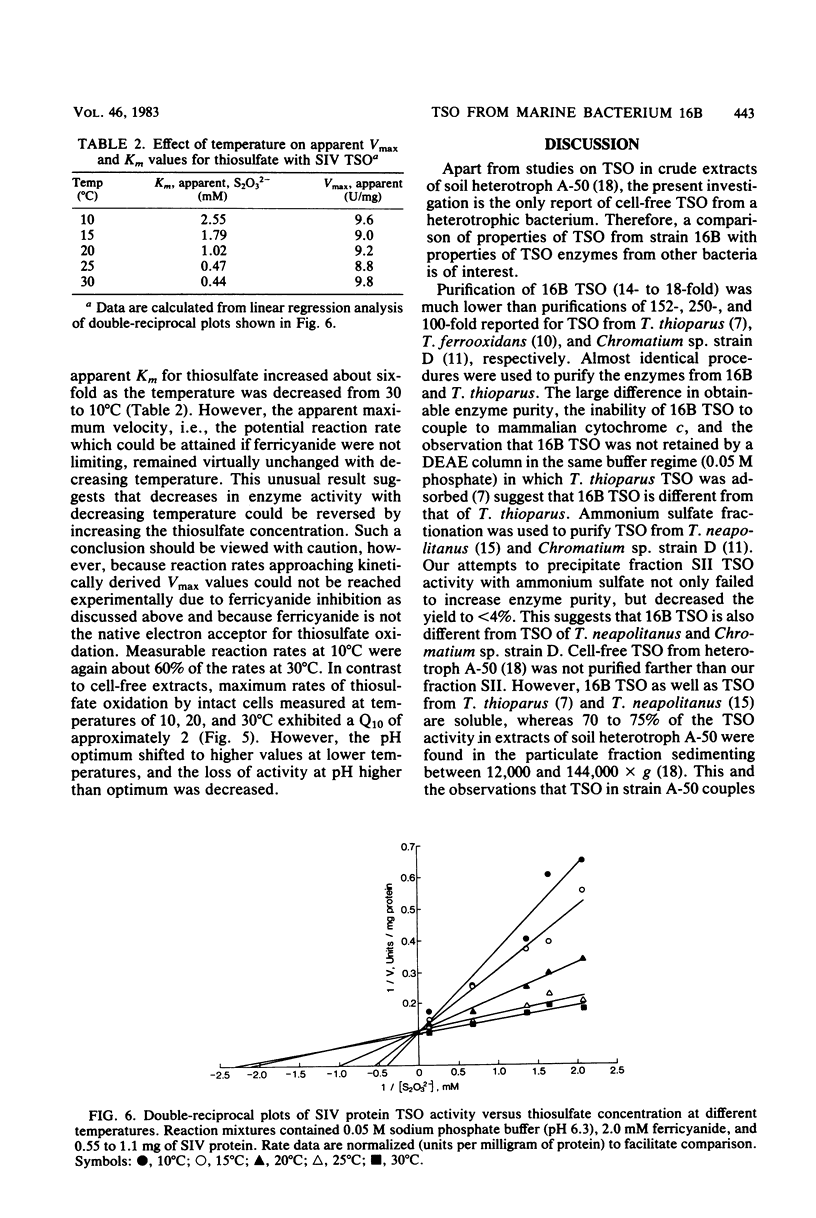
Abstract
Thiosulfate-oxidizing enzyme has been demonstrated in cell-free extracts of the marine, thiosulfate-oxidizing pseudomonad strain 16B. The enzyme, partially purified by ion-exchange chromatography and calcium phosphate gel treatment, catalyzed the oxidation of thiosulfate to tetrathionate with the concomitant reduction of ferricyanide. Native but not mammalian cytochrome c was also reduced by the enzyme in the presence of thiosulfate. The enzyme was located exclusively in the supernatant of ultracentrifuged cell extracts. The most purified enzyme preparation, like intact cells, exhibited a temperature optimum of 30 to 31°C. However, it exhibited no definite pH optimum. At pH 6.1 to 6.3 and 30°C, the Km for thiosulfate was 1.57 mM. At lower temperatures, the apparent Km for thiosulfate increased, but the apparent maximum velocity remained virtually unchanged. Thiosulfate oxidation in intact cells exhibited an increase in the pH optimum at lower temperatures. The thiosulfate-oxidizing enzyme of marine heterotroph 16B is compared with thiosulfate-oxidizing enzymes from other bacteria, and the effect of temperature on the relationship between pH and thiosulfate oxidation is discussed with reference to the natural habitat of the bacterium.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lyric R. M., Suzuki I. Enzymes involved in the metabolism of thiosulfate by Thiobacillus thioparus. 3. Properties of thiosulfate-oxidizing enzyme and proposed pathway of thiosulfate oxidation. Can J Biochem. 1970 Mar;48(3):355–363. doi: 10.1139/o70-058. [DOI] [PubMed] [Google Scholar]
- Silver M., Lundgren D. G. The thiosulfate-oxidizing enzyme of Ferrobacillus ferrooxidans (Thiobacillus ferrooxidans). Can J Biochem. 1968 Oct;46(10):1215–1220. doi: 10.1139/o68-181. [DOI] [PubMed] [Google Scholar]
- Smith A. J. The role of tetrathionate in the oxidation of thiosulphate by Chromatium sp. strain D. J Gen Microbiol. 1966 Mar;42(3):371–380. doi: 10.1099/00221287-42-3-371. [DOI] [PubMed] [Google Scholar]
- Starkey R. L. The Production of Polythionates from Thiosulfate by Microörganisms. J Bacteriol. 1934 Oct;28(4):387–400. doi: 10.1128/jb.28.4.387-400.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRUDINGER P. A. Thiosulphate oxidation and cytochromes in Thiobacillus X. 1. Fractionation of bacterial extracts and properties of cytochromes. Biochem J. 1961 Apr;78:673–680. doi: 10.1042/bj0780673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRUDINGER P. A. Thiosulphate oxidation and cytochromes in Thiobacillus X. 2. Thiosulphate-oxidizing enzyme. Biochem J. 1961 Apr;78:680–686. doi: 10.1042/bj0780680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudinger P. A. Metabolism of thiosulfate and tetrathionate by heterotrophic bacteria from soil. J Bacteriol. 1967 Feb;93(2):550–559. doi: 10.1128/jb.93.2.550-559.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle J. H., Holmes P. E., Jannasch H. W. Growth rate stimulation of marine pseudomonads by thiosulfate. Arch Microbiol. 1974;99(1):1–14. doi: 10.1007/BF00696218. [DOI] [PubMed] [Google Scholar]
- Tuttle J. H., Jannasch H. W. Dissimilatory reduction of inorganic sulfur by facultatively anaerobic marine bacteria. J Bacteriol. 1973 Sep;115(3):732–737. doi: 10.1128/jb.115.3.732-737.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle J. H. Organic carbon utilization by resting cells of thiosulfate-oxidizing marine heterotrophs. Appl Environ Microbiol. 1980 Sep;40(3):516–521. doi: 10.1128/aem.40.3.516-521.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle J. H. Thiosulfate Oxidation and Tetrathionate Reduction by Intact Cells of Marine Pseudomonad Strain 16B. Appl Environ Microbiol. 1980 Jun;39(6):1159–1166. doi: 10.1128/aem.39.6.1159-1166.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VISHNIAC W., SANTER M. The thiobacilli. Bacteriol Rev. 1957 Sep;21(3):195–213. doi: 10.1128/br.21.3.195-213.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]




