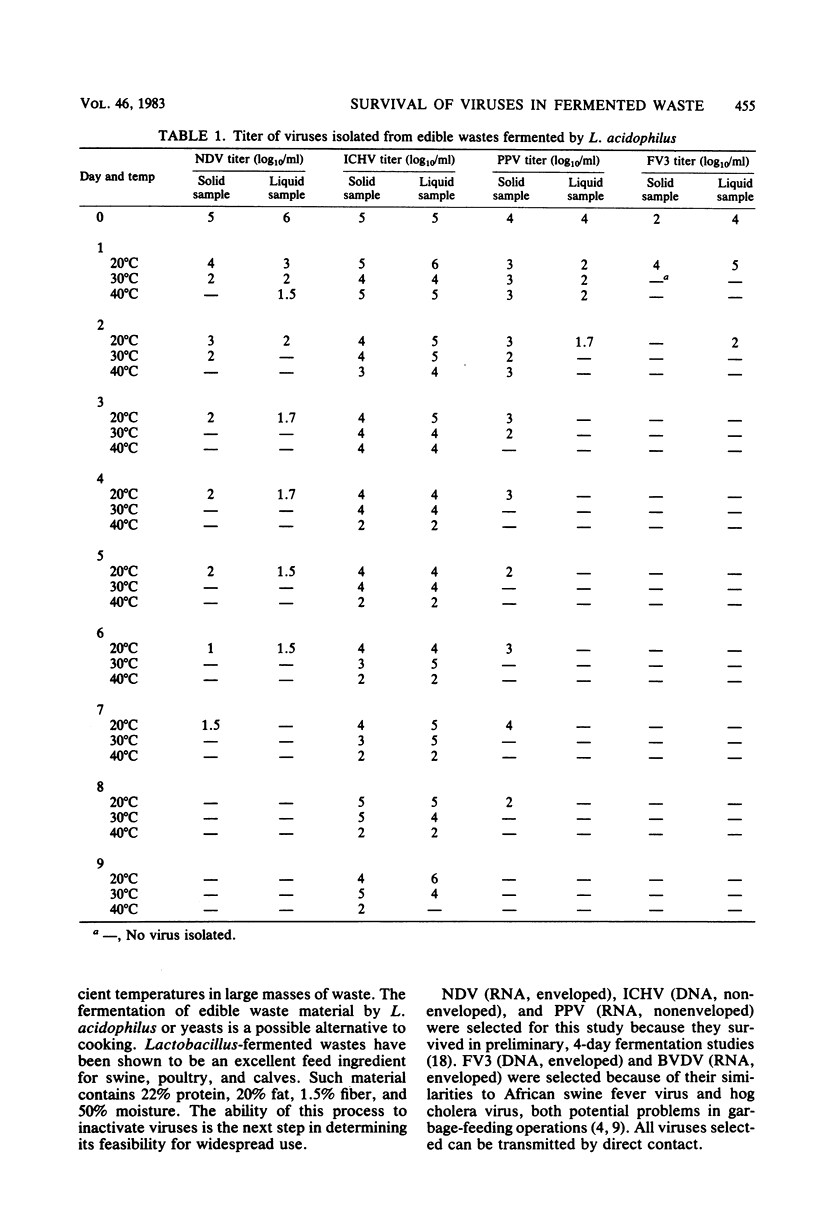
Abstract
The survival of selected viruses in Lactobacillus- and yeast-fermented edible waste material was studied to determine the feasibility of using this material as a livestock feed ingredient. Five viruses, including Newcastle disease virus, infectious canine hepatitis virus, a porcine picornavirus, frog virus 3, and bovine virus diarrhea, were inoculated into a mixture of ground food waste (collected from a school lunch program) containing Lactobacillus acidophilus. Mixtures were incubated at 20, 30, and 40°C for 216 h. In a second trial, four viruses, including Newcastle disease virus, infectious canine hepatitis virus, frog virus 3, and a porcine picornavirus, were inoculated into similar edible waste material containing Saccharomyces cerevisiae. Mixtures were incubated at 20 and 30°C for 216 h. Samples were obtained daily for quantitative (trial 1) and qualitative (trial 2) virus isolation. Temperature, pH, and redox potential were monitored. Controlled pH and temperature studies were also done and compared with the inactivation rates in the fermentation processes. In trial 1 (Lactobacillus fermentation), infectious canine hepatitis virus survived the entire test period in the fermentation process but was inactivated below pH 4.5 in the controlled studies. Newcastle disease virus was inactivated by day 8 in the fermentation process and appeared to be primarily heat sensitive and secondarily pH sensitive in the controlled studies. The porcine picornavirus survived the fermentation process for 8 days at 20°C but was inactivated more rapidly at 30 and 40°C. The controlled studies verified these findings. Frog virus 3 was inactivated by day 3 in the fermentation process and appeared to be sensitive to low pH in the controlled studies. Bovine virus diarrhea was rapidly inactivated in the fermentation process (less than 2 h) and was pH and temperature sensitive. In trial 2 (yeast fermentation), infectious hepatitis virus survived the entire test period in the fermentation process. Newcastle disease virus was inactivated by day 7 at 20°C and day 6 at 30°C. The porcine picornavirus was inactivated by day 7 at 30°C but survived the entire test period at 20°C. Frog virus 3 was inactivated by day 3 at 20°C and day 2 at 30°C.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dimmock N. J. Differences between the thermal inactivation of picornaviruses at "high" and "low" temperatures. Virology. 1967 Feb;31(2):338–353. doi: 10.1016/0042-6822(67)90179-1. [DOI] [PubMed] [Google Scholar]
- GARD S. Theoretical considerations in the inactivation of viruses by chemical means. Ann N Y Acad Sci. 1960 Jan 13;83:638–648. doi: 10.1111/j.1749-6632.1960.tb40934.x. [DOI] [PubMed] [Google Scholar]
- Granoff A., Came P. E., Breeze D. C. Viruses and renal carcinoma of Rana pipiens. I. The isolation and properties of virus from normal and tumor tissue. Virology. 1966 May;29(1):133–148. doi: 10.1016/0042-6822(66)90203-0. [DOI] [PubMed] [Google Scholar]
- Hafez S. M., Liess B. Studies on bovine viral diarrhea-mucosal disease virus. II. Stability and some physico-chemical properties. Acta Virol. 1972 Sep;16(5):399–408. [PubMed] [Google Scholar]
- Kohn A., Gitelman J., Inbar M. Interaction of polyunsaturated fatty acids with animal cells and enveloped viruses. Antimicrob Agents Chemother. 1980 Dec;18(6):962–968. doi: 10.1128/aac.18.6.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera L. S. Effects of temperature on frog polyhedral cytoplasmic deoxyribovirus multiplication: thermosensitivity of initiation, replication, and encapsidation of viral DNA. Virology. 1970 Nov;42(3):576–589. doi: 10.1016/0042-6822(70)90304-1. [DOI] [PubMed] [Google Scholar]
- Lodetti E., De Simone F., Nardelli L. Neutralization tests for foot-and-mouth disease, equine rhino-, porcine entero-, Aujeszky- and rhinopneumonitis viruses. Comparison of results obtained by a simple microculture plaque reduction test and various traditional tests. Zentralbl Veterinarmed B. 1972 Dec;19(10):848–857. doi: 10.1111/j.1439-0450.1972.tb00378.x. [DOI] [PubMed] [Google Scholar]
- Mengeling W. L., Gutekunst D. E., Fernelius A. L., Pirtle E. C. Demonstration of an Antigenic Relationship Between Hog Cholera and Bovine Viral Diarrhea Viruses by Immunofluorescence. Can J Comp Med Vet Sci. 1963 Jul;27(7):162–164. [PMC free article] [PubMed] [Google Scholar]
- Moore N. F., Patzer E. J., Shaw J. M., Thompson T. E., Wagner R. R. Interaction of vesicular stomatitis virus with lipid vesicles: depletion of cholesterol and effect on virion membrane fluidity and infectivity. J Virol. 1978 Aug;27(2):320–329. doi: 10.1128/jvi.27.2.320-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao N. U., Labzoffsky N. A. A simple method for the detection of low concentration of viruses in large volumes of water by the membrane filter technique. Can J Microbiol. 1969 May;15(5):399–403. doi: 10.1139/m69-071. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Gaffney B. J. Effect of the viral proteins on the fluidity of the membrane lipids in Sindbis virus. J Mol Biol. 1974 Dec 5;90(2):343–358. doi: 10.1016/0022-2836(74)90378-7. [DOI] [PubMed] [Google Scholar]
- WOESE C. Thermal inactivation of animal viruses. Ann N Y Acad Sci. 1960 Jan 13;83:741–751. doi: 10.1111/j.1749-6632.1960.tb40943.x. [DOI] [PubMed] [Google Scholar]
- Wooley R. E., Brown J., Gratzek J. B., Kleven S. H., Scott T. A. Microculture system for detection of Newcastle disease virus antibodies. Appl Microbiol. 1974 May;27(5):890–895. doi: 10.1128/am.27.5.890-895.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooley R. E., Brown J., Scott T. A., Lukert P. D., Crowell W. A. Effect of polyinosinic-polycytidylic acid in dogs experimentally infected with infectious canine hepatitis virus. Am J Vet Res. 1974 Sep;35(9):1217–1219. [PubMed] [Google Scholar]
- Wooley R. E., Gilbert J. P., Whitehead W. K., Shotts E. B., Jr, Dobbins C. N. Survival of viruses in fermented edible waste material. Am J Vet Res. 1981 Jan;42(1):87–90. [PubMed] [Google Scholar]


