Abstract
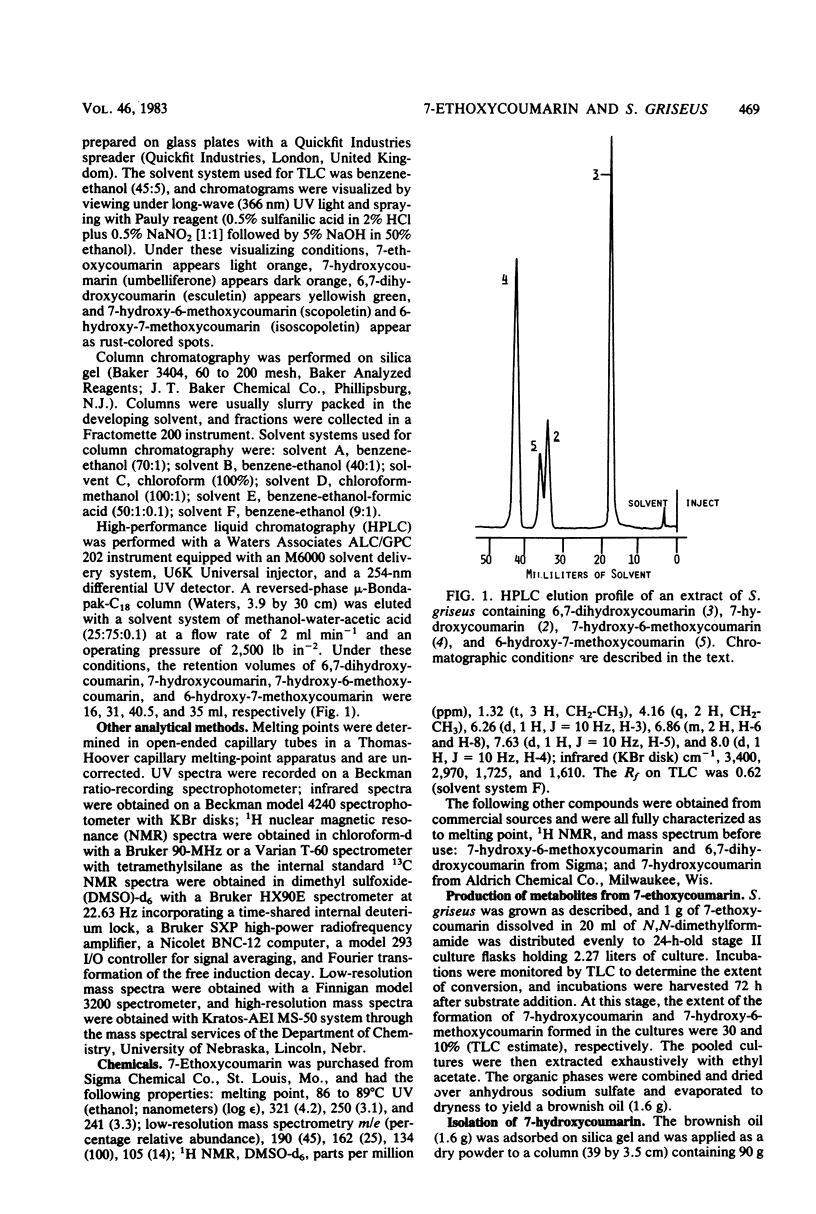
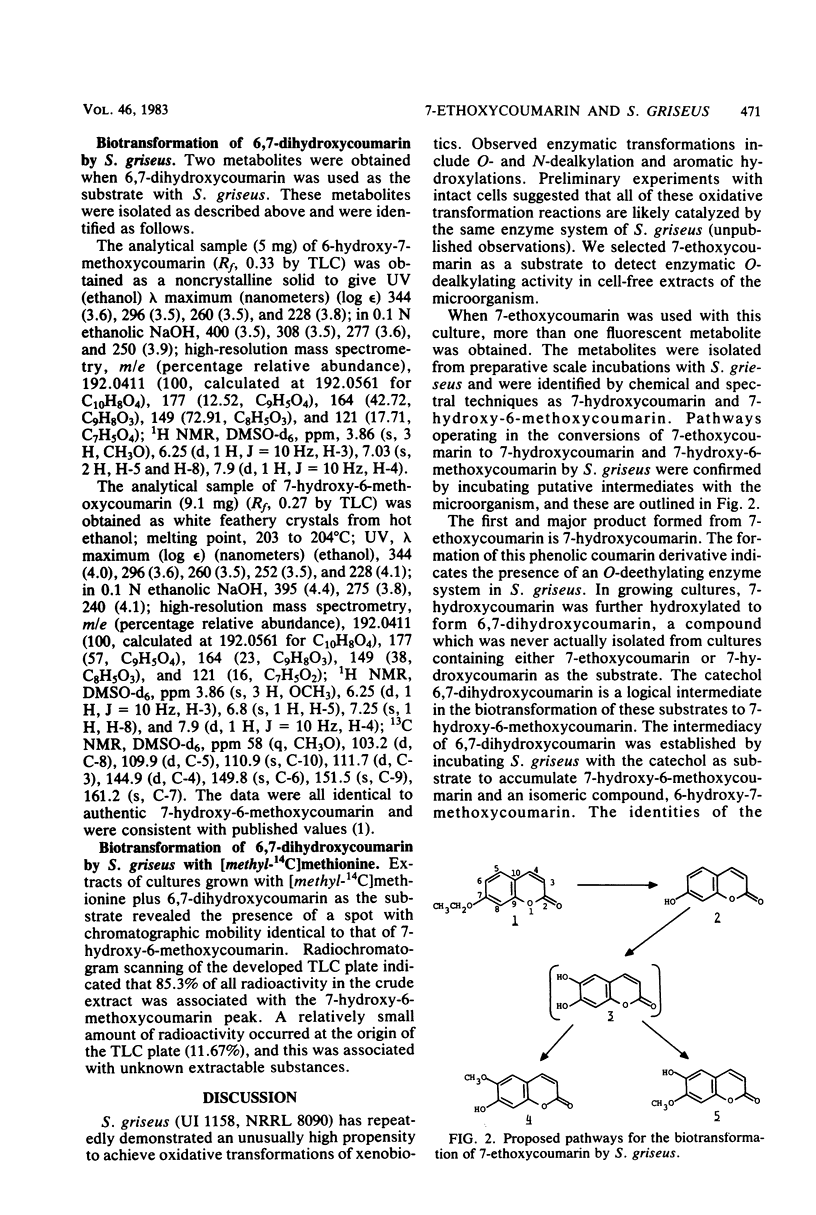
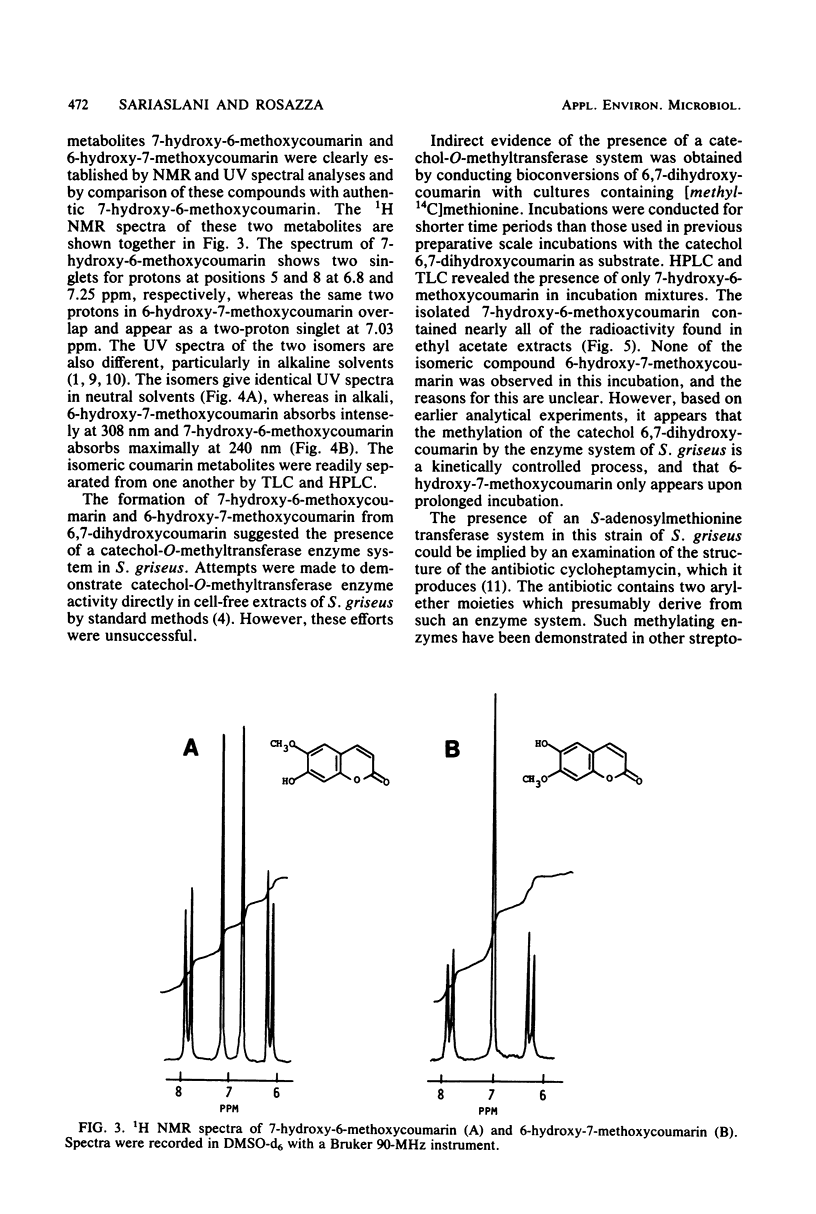
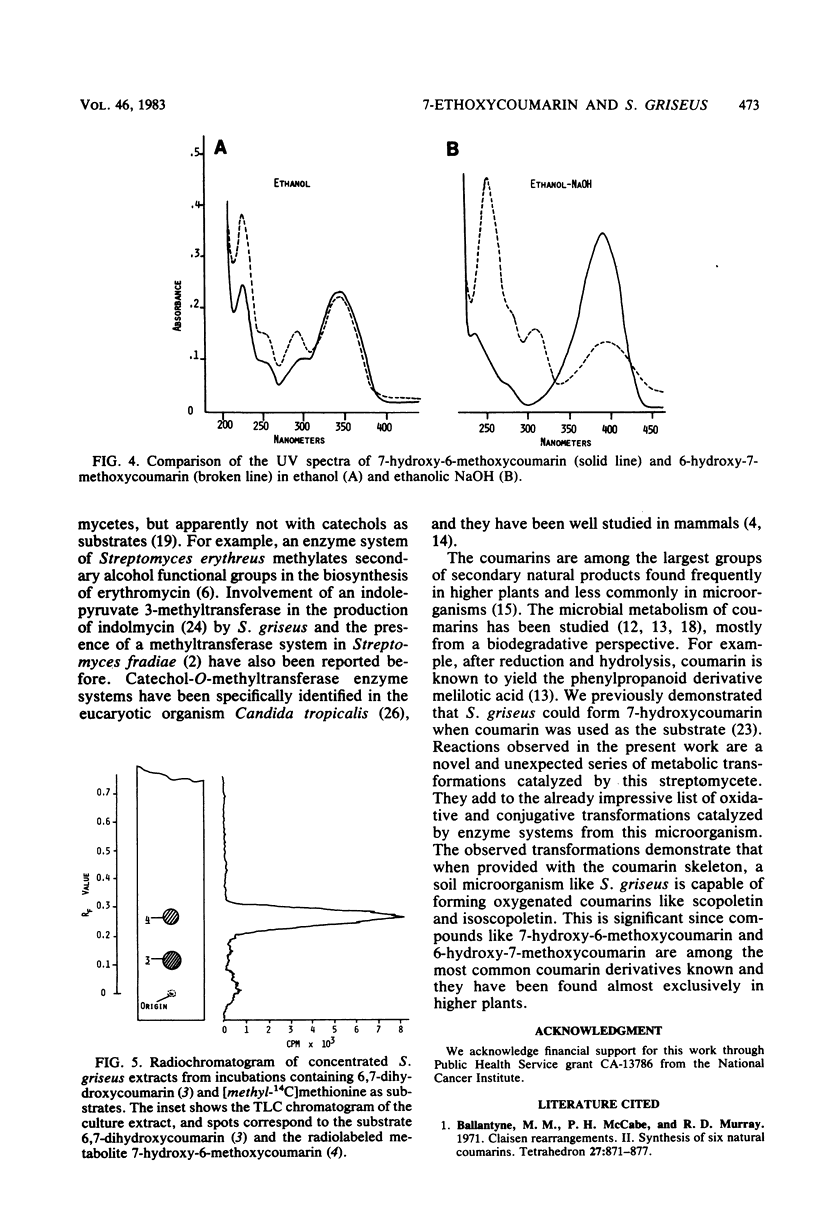
Biotransformation of 7-ethoxycoumarin by Streptomyces griseus resulted in the accumulation of two metabolites which were isolated and identified as 7-hydroxycoumarin and 7-hydroxy-6-methoxycoumarin. A novel series of biotransformation reactions is implicated in the conversion of the ethoxycoumarin substrate to these products, including O-deethylation, 6-hydroxylation to form a 6,7-dihydroxycoumarin catechol, and subsequent O-methylation. Either 7-hydroxycoumarin or 6,7-dihydroxycoumarin was biotransformed to 7-hydroxy-6-methoxycoumarin by S. griseus. Trace amounts of the isomeric 6-hydroxy-7-methoxycoumarin were detected when 6,7-dihydroxycoumarin was used as the substrate. Efforts to obtain a cell-free catechol-O-methyltransferase enzyme system from S. griseus were unsuccessful. However, [methyl-14C]methionine was used with cultures of S. griseus to form 7-hydroxy-6-[14C]methoxycoumarin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltz R. H., Seno E. T. Properties of Streptomyces fradiae mutants blocked in biosynthesis of the macrolide antibiotic tylosin. Antimicrob Agents Chemother. 1981 Aug;20(2):214–225. doi: 10.1128/aac.20.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts R. E., Walters D. E., Rosazza J. P. Microbial transformations of antitumor compounds. 1. Conversion of acronycine to 9-hydroxyacronycine by Cunninghamella echinulata. J Med Chem. 1974 Jun;17(6):599–602. doi: 10.1021/jm00252a006. [DOI] [PubMed] [Google Scholar]
- Borchardt R. T. A rapid spectrophotometric assay for catechol-O-methyltransferase. Anal Biochem. 1974 Apr;58(2):382–389. doi: 10.1016/0003-2697(74)90206-1. [DOI] [PubMed] [Google Scholar]
- Corcoran J. W. S-adenosylmethionine:erythromycin C O-methyltransferase. Methods Enzymol. 1975;43:487–498. doi: 10.1016/0076-6879(75)43109-3. [DOI] [PubMed] [Google Scholar]
- Davis P. J., Rosazza J. P. Microbial transformations of natural antitumor agents. 2. Studies with d-tetrandrine and laudanosine. J Org Chem. 1976 Jul 23;41(15):2548–2551. doi: 10.1021/jo00877a009. [DOI] [PubMed] [Google Scholar]
- Davis P. J., Wiese D., Rosazza J. P. Microbial transformations of glaucine. J Chem Soc Perkin 1. 1977;1:1–6. doi: 10.1039/p19770000001. [DOI] [PubMed] [Google Scholar]
- Dean F. M., Parton B., Somvichien N., Taylor D. A. The coumarins of Ptaeroxylon obliquum. Tetrahedron Lett. 1967 Jun;23:2147–2151. doi: 10.1016/s0040-4039(00)90785-8. [DOI] [PubMed] [Google Scholar]
- Godtfredsen W. O., Vangedal S., Thomas D. W. Cycloheptamycin, a new peptide antibiotic. Structure determination by mass spectrometry. Tetrahedron. 1970 Nov;26(21):4931–4946. doi: 10.1016/s0040-4020(01)93145-x. [DOI] [PubMed] [Google Scholar]
- Kunc F. Microbial decomposition of coumarin in soil. Folia Microbiol (Praha) 1974;19(3):209–217. doi: 10.1007/BF02895020. [DOI] [PubMed] [Google Scholar]
- Levy C. C., Frost P. The metabolism of coumarin by a microorganism. V. Melilotate hydroxylase. J Biol Chem. 1966 Feb 25;241(4):997–1003. [PubMed] [Google Scholar]
- Lin R. L., Narasimhachari N. Specific tlc, gc, and gc-ms methods for kinetic studies with COMT. Anal Biochem. 1974 Jan;57(1):46–58. doi: 10.1016/0003-2697(74)90048-7. [DOI] [PubMed] [Google Scholar]
- Nabih T., Davis P. J., Caputo J. F., Rosazza J. P. Microbial transformations of natural antitumor agents. 3. Conversion of thalicarpine to (+)-hernandalinol by Streptomyces punipalus. J Med Chem. 1977 Jul;20(7):914–917. doi: 10.1021/jm00217a010. [DOI] [PubMed] [Google Scholar]
- Nambudiri A. M., Bhat J. V. Conversion of p-coumarate into caffeate by Streptomyces nigrifaciens. Purification and properties of the hydroxylating enzyme. Biochem J. 1972 Nov;130(2):425–433. doi: 10.1042/bj1300425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogell B. M. S-adenosylmethionine:O-demethylpuromycin O-methyltransferase. Methods Enzymol. 1975;43:508–515. doi: 10.1016/0076-6879(75)43112-3. [DOI] [PubMed] [Google Scholar]
- Rosazza J. P., Kammer M., Youel L. Microbial models of mammalian metabolism O-demethylations of papaverine. Xenobiotica. 1977 Mar;7(3):133–143. doi: 10.3109/00498257709036245. [DOI] [PubMed] [Google Scholar]
- Rosazza J. P., Stocklinski A. W., Gustafson M. A., Adrian J., Smith R. V. Microbial models of mammalian metabolism. O-Dealkylation of 10,11-dimethoxyaporphine. J Med Chem. 1975 Aug;18(8):791–794. doi: 10.1021/jm00242a006. [DOI] [PubMed] [Google Scholar]
- Smith R. V., Rosazza J. P. Microbial models of mammalian metabolism. Aromatic hydroxylation. Arch Biochem Biophys. 1974 Apr 2;161(2):551–558. doi: 10.1016/0003-9861(74)90338-5. [DOI] [PubMed] [Google Scholar]
- Speedie M. K., Hornemann U., Floss H. G. S-adenoxylmethionine:indolepyruvate 3-methyltransferase. Methods Enzymol. 1975;43:498–502. doi: 10.1016/0076-6879(75)43110-x. [DOI] [PubMed] [Google Scholar]
- Ullrich V., Weber P. The O-dealkylation of 7-ethoxycoumarin by liver microsomes. A direct fluorometric test. Hoppe Seylers Z Physiol Chem. 1972 Jul;353(7):1171–1177. doi: 10.1515/bchm2.1972.353.2.1171. [DOI] [PubMed] [Google Scholar]
- Veser J., Martin R., Thomas H. Immunocytochemical demonstration of catechol methyltransferase in Candida tropicalis. J Gen Microbiol. 1981 Sep;126(1):97–101. doi: 10.1099/00221287-126-1-97. [DOI] [PubMed] [Google Scholar]


