Abstract
Objective
Protein Z, a vitamin K-dependent plasma protein, has an important role in the regulation of the coagulation cascade. Protein Z deficiency has been associated with unexplained pregnancy loss and adverse pregnancy outcome in patients with thrombophilia. This study was conducted to determine if preeclampsia (PE), small-for-gestational age (SGA) and fetal demise are associated with changes in maternal plasma concentrations of protein Z.
Study Design
This cross-sectional study included normal pregnant women (N=71), patients with PE (N=130), patients who delivered a SGA neonate (N=58), and patients with fetal demise (N=58). Maternal plasma protein Z concentrations were measured by a sensitive and specific immunoassay. Protein Z deficiency was defined as maternal plasma concentrations ≤5th percentile of the normal pregnancy group (≤1.59μg/mL). Non-parametric statistics were used for analysis.
Results
1) Patients with preeclampsia had a lower median plasma concentration of protein Z than normal pregnant women (PE: median: 1.6 μg/mL, range: 0.2-3.3 μg/mLl vs. normal pregnancy: median: 2.4 μg/mL, range: 1.1-3.4 μg/mLl; p<0.01); 2) Patients with SGA (median: 2.3 μg/mL, range: 0.2-3.8 μg/mL) and fetal demise (median: 2.6 μg/mL, range: 0.2-4.3 μg/mL) did not have significant different median protein Z concentrations from normal pregnant women (p>0.05); and 3) Women in the PE and fetal demise groups had significantly higher rate of protein Z deficiency than those with normal pregnancy outcome.
Conclusion
1) PE, but neither SGA nor fetal demise, is associated with significantly lower maternal median plasma concentration of protein Z concentrations than normal pregnancy; and 2) a high rate of protein Z deficiency was observed in patients with PE and fetal demise.
Keywords: SGA, fetal demise, factor X, protein Z dependent protease inhibitor (ZPI), coagulation, pregnancy
Introduction
During pregnancy the changes in the coagulation system are considered to be an adaptive mechanisms for prevention of bleeding at the time of delivery. .[1-5]. Indeed, normal pregnancy is associated with excessive thrombin generation [5,6] and a tendency for platelets to aggregate in response to agonists [7,8]. Thrombosis has been proposed as a mechanism of disease in preeclampsia(PE), [9-13] intrauterine growth restriction (IUGR), [13-17] stillbirth, [18] recurrent pregnancy losses, [19] and preterm delivery [20-22]. Evidence in support of this view includes: 1) an excessive rate of thrombotic lesions in the placental villi [23] and decidual vessels [20,23,24] in patients with these pregnancy complications; and 2) higher maternal plasma concentrations of thrombin-anti- thrombin (TAT) complexes in patients with PE, [25-28] and small-for-gestational age (SGA) neonates [25].
Protein Z is a vitamin-K-dependent plasma glycoprotein that participate in the inhibition of activated factor X (FXa), limiting thrombin generation [29]. This has been attributed to the role of protein Z as a co-factor of the protein Z-dependent protease inhibitor (ZPI .[30-32]. Indeed, in the absence of protein Z, the activity of ZPI is reduced by more than 1,000-fold [32]. Thus, protein Z deficiency has been associated with a procoagulant state [29].
There are conflicting reports concerning the changes in plasma concentrations of protein Z in women with PE. While some authors have reported that there is no significant difference the median plasma concentrations of protein Z between patients with PE and women with normal pregnancy, [33] others have reported that the median plasma protein Z concentrations are significantly lower in women with PE, SGA, and preterm delivery than those with normal pregnancies [34]. However, the authors did not analyzed each complication independently, therefore, the association between PE and changes in protein Z plasma concentration is not clear [34].
The objective of this study was to determine the changes in maternal plasma concentration of protein Z and the rate of protein Z deficiency among patients presenting with PE, SGA, or fetal demise in comparison to women with normal pregnancy outcome.
Material and Methods
Study population
This cross-sectional study included the following study groups: 1) patients with PE (N=130); 2) patients who delivered a SGA neonate (N=58); 3) patients with fetal demise (N=58); and 4) patients with a normal pregnancy outcome served as a control group (N=71). Patients with multiple pregnancies or fetuses with congenital defects and/ or chromosomal anomalies were excluded.
Clinical definitions
Preeclampsia was defined as hypertension (systolic blood pressure of ≥140 mmHg or diastolic blood pressure of ≥90 mmHg on at least two occasions, four hours to one week apart) associated with proteinuria (≥300 mg in a 24-hour urine collection or one dipstick measurement of ≥2+). Severe PE was defined as diastolic blood pressure ≥110 mmHg or systolic blood pressure ≥160 mmHg and/or proteinuria ≥3+ by dipstick. Fetal demise was defined as a fetal death occurring after 19 weeks of gestation. SGA was defined as a birth weight below the 10th percentile [35]. Birth weight percentiles for gestational age were classified in four groups as follows: 1) ≤5th percentile; 2) 5th-10th percentile; 3) 10th – 90th percentile; and 4) >90th percentile.[35] Protein Z deficiency was defined as maternal plasma concentrations ≤5th percentile[36] of the normal pregnancy group (≤ 1.59μg/mL).
Samples collection
All blood samples were collected with a vacutainer into 0.109M trisodium citrate anticoagulant solution (BD; San Jose, CA, USA). The samples were centrifuged at 1300g for ten minutes at 4°C and stored at -70°C until assay.
All women provided an informed consent prior to the collection of maternal blood. The collection and utilization of samples for research purposes was approved by the institutional review boards of Wayne State University, and the National Institute of Child Health and Human Development (NICHD/NIH/DHHS).
Human protein Z immunoassays
Concentrations of protein Z in maternal plasma were determined by sensitive and specific immunoassays obtained from Diagnostica Stago (Asnieres-sur-Seine, France). The protein Z immunoassay utilizes the quantitative sandwich enzyme immunoassay technique. Briefly, the standards and samples were incubated in duplicate wells of a 96-well microtiter plate pre-coated with a monoclonal antibody specific for protein Z. During this incubation, the protein Z present in the standards and samples were bound to the immobilized protein Z antibodies in the microtiter plate. Following incubation, repeated washings were performed to remove unbound materials. The next step involved incubation with a second monoclonal antibody coupled with peroxidase and directed against another epitope of protein Z that binds to the captured antigen in the microtiter plate forming a sandwich. This step was followed by additional washes to remove unbound materials and an equal amount of stabilized chromogen, ortho-phenylenediamine (OPD) and urea peroxide substrate were added to each well. This initiated the development of color, which was halted at a set time by the addition of sulphuric acid (3M). The optical density of each well was determined using a programmable microplate reader (Bio-Tek Instruments, Winooski, Vermont USA) set at a wavelength of 492 nm. Protein Z concentrations in the samples were determined by interpolation from the standard curve. The calculated inter-and intra-assay coefficients of variation (CVs) were 3.1% and 2.4%, respectively. The sensitivity of the protein Z immunoassay in our laboratory was 0.05μg/mL.
Statistical analysis
Protein Z plasma concentrations were not normally distributed; thus, Kruskal–Wallis and Mann–Whitney U tests were used for comparisons among groups. The Chi-square was used to compare categorical variables. A p value < 0.05 was considered statistically significant. The statistical package used was SPSS, version 12 (SPSS Inc., Chicago, IL USA).
Results
The demographic and clinical characteristics of the study groups are displayed in Table I. There was no significant correlation between gestational age and protein Z concentrations in women with normal pregnancy (R=0.003; p=0.6).
Table I.
Demographic and clinical characteristics of the study population
| Normal Pregnancy
N= 71 |
Preeclampsia
(N= 130) |
SGA
(N= 58) |
FD
(N= 58) |
|
|---|---|---|---|---|
| Maternal age (years) | 24.2±4.5 | 25.9± 6.9 | 26.1± 6.7 | 25.9±6.5 |
| Gravidity* | ||||
| 1 | 14(20.3%) | 44 (34.1%) | 12 (21.4%) | 17 (30.4%) |
| 2-5 | 45(65.2%) | 69 (53.5%) | 37 (66.1%) | 28 (50%) |
| ≥6 | 10(14.5%) | 16 (12.4%) | 7 (12.5%) | 11(19.6%) |
| Parity** | ||||
| 1 | 39 (55.7%) | 93(72.1%)* | 38 (66.7%) | 37 (64.9%) |
| 2-5 | 30(42.9%) | 32(24.8%) | 18(31.6%) | 17 (29.8%) |
| ≥6 | 1 (1.4%) | 4 (3.1%) | 1 (1.7%) | 3 (5.3%) |
| Ethnic origin † | ||||
| African-Americans | 53 (77.9%) | 108 (84.37%) | 48 (87.3%) | 51 (91.07%) |
| Caucasian | 11(16.1%) | 13 (10.15%) | 4 (7.3%) | 3 (5.35%) |
| Hispanic | 2 (3%) | 5 (3.9%) | 1 (1.8%) | 1 (1.75%) |
| Asian | 2(3%) | 1 (0.78%) | 1 (1.8%) | 1 (1.75%) |
| Other | 0 | 1 (0.78%) | 1 (1.8%) | 0 |
| Gestational age at blood collection (weeks) | 30.8 ± 5.1 | 33.9± 4.7* | 33.8±4.8* | 29.9 ±6.02 |
| Gestational age at delivery (weeks) | 39.4 ± 1.5 | 34.1± 4.6* | 35.3± 4.1* | 30.1± 5.9* |
| Induction of labor*** | 10 (17.8%) | 59 (45.7%)* | 32 (55.2%)* | 48 (85.7%)* |
| Cesarean delivery**** | 22 (33.3%) | 68 (53.1%)* | 17 (29.3%) | 1 (1.8%)* |
Data are presented as mean ± standard deviation or numbers (%)
= Normal pregnancy (N=69); Preeclampsia (N=129); SGA (N=56); FD (N=56)
= Normal pregnancy (N=70); Preeclampsia (N=129); SGA (N=57); FD (N=57)
= Normal pregnancy (N=68); Preeclampsia (N=128); SGA (N=55); FD (N=56)
SGA= small for gestational age
FD= fetal demise
The groups were compared with the normal pregnancy group: *P<0.05
Normal pregnancy (N=56), Preeclampsia (N=129), FD (N=56).
Normal pregnancy (N=66), Preeclampsia (N=128), FD (N=56).
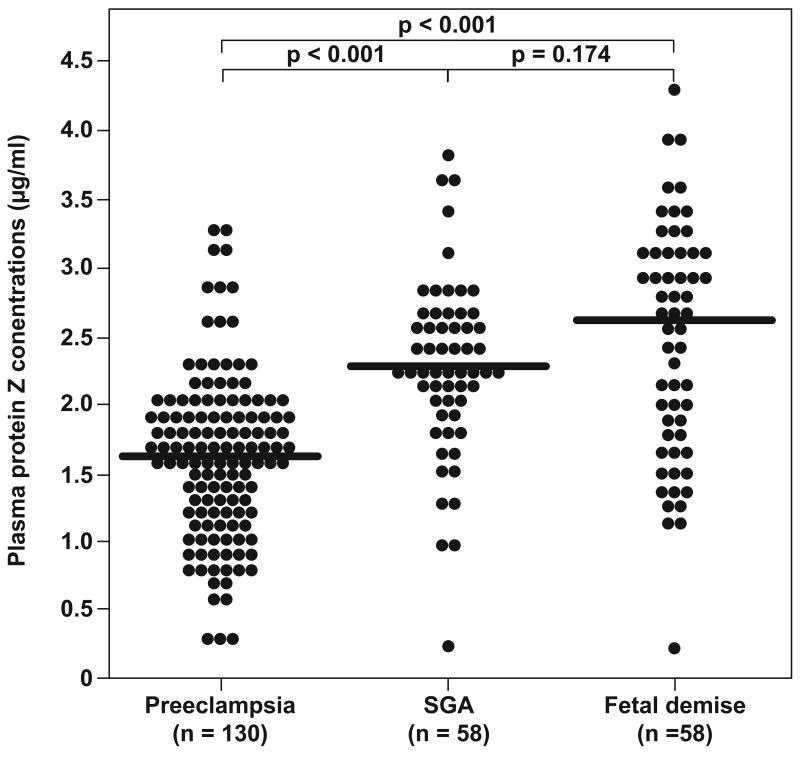
The median maternal plasma protein Z concentration in preeclamptic patients (median: 1.6 μg/mL, range: 0.2-3.3) was significantly lower than that of the other study groups (controls: median: 2.4 μg/mL, range: 1.1-3.4, p<0.0001; SGA: median: 2.3 μg/mL, range: 0.2-3.8, p<0.0001; and fetal demise: median: 2.6 μg/mLl, range: 0.2-4.3, p<0.0001). No differences were observed in the median maternal plasma concentration of protein Z between normal patients and those with SGA and fetal demise (see Figures 1 and 2).
Figure 1.
Comparison of the median maternal plasma protein Z concentrations between women with normal pregnancy and those with pregnancy complications.
Figure 2.
Comparison of the median maternal plasma protein Z concentrations between women with pregnancy complications.
There was no significant difference in the median maternal plasma protein Z concentration between patients with severe PE and those with mild PE (severe PE: median: 1.7 μg/mL, range: 0.2-3.3 vs. mild PE: median: 1.5 μg/mL, range: 0.3-1.9; p=0.08, respectively). In the PE group there were no significant differences in median maternal plasma protein Z concentrations between the different neonatal birth weight percentiles [≤5th percentile- median: 1.56 μg/mL (0.54-3.16); 2) 5th-10th percentile median: 1.45 μg/mL (0.3-2.7); 3) 10th – 90th percentile median: 1.73 μg/mL (0.2-3.28); and 4) >90th percentile median: 1.77 μg/mL (1.13-2.34), (p=0.24 Kruskal- Wallis test)].
A higher proportion of patients with PE had protein Z deficiency than those with normal pregnancy [50% (65/130) vs. 4.2% (3/71); OR 22.61, 95% CI 6.79-116.82; p<0.00001]. Similarly protein Z deficiency was significantly more frequent in patients with fetal demise than in women with normal pregnancies [25.9% (15/58) vs. 4.2% (3/71), p=0.012]. In contrast, patients who delivered an SGA neonate did not have a higher frequency of protein Z deficiency than women with normal pregnancies, [20.7 % (12/58) vs. 4.2% (3/71); p=0.09).
Discussion
Principal findings: 1) women with PE, but not those with SGA or fetal demise, had significantly lower median plasma concentrations of protein Z than women with normal pregnancies; and 2) ghe rate of protein Z deficiency was significantly higher in patients with PE and fetal demise than in those with normal pregnancies.
Why do women with preeclampsia have lower plasma concentrations of protein Z than those with normal pregnancies?
Normal pregnancy is characterized by an increased plasma concentration of protein Z,[37] which has been proposed to be part of a compensatory mechanism for the increased concentration of factor X [37] and perhaps for the increased thrombin generation. Preeclampsia is associated with an exaggerated hypercoagulable state and excessive thrombin generation,[1,25,38] as determined by higher maternal plasma concentrations of TAT complexes [25-28,39,40] and lower antithrombin III concentrations [41-45] than patients with a normal pregnancies. Moreover, patients with PE who delivered preterm have a higher rate of thrombotic lesions in the decidua [9,20] and in the placental villi [23] than normotensive patients with indicated or spontaneous preterm delivery [20,23]. Therefore, it is possible that an exaggerated procoagulant state may account for the lower plasma concentration of protein Z among women with preclampsia.
An addition novel finding is the significantly higher rate of protein Z deficiency in women with PE than in women with normal pregnancy outcome (OR 22.65, 95% CI 6.79-116.82). In a previous report, [33] the rate of protein Z deficiency (defined as the 10th percentile of the normal population [33]) in women with PE was not significantly different from the rate observed in women with normal pregnancy. In contrast, we found that 50% of the patients with PE had protein Z deficiency; this difference may be attributed to the larger sample size of patients with PE included in our study, different definition of protein Z deficiency (<5th percentile of the normal pregnant population) and differences in the study population. Protein Z deficiency has been reported in non-pregnant women [36] as well, suggesting that in some of the patients protein Z deficiency may precede the clinical presentation of PE, and a low maternal plasma concentration of protein Z can be a risk factor for the subsequent development of PE in a subset of patients.
There were no significant differences between the median maternal protein Z plasma concentrations of patients who delivered an SGA neonate or had fetal demise in comparison to women with normal pregnancy; these results are consistent with a previous report [33]. In contrast, a recent study [34] reported that patients with adverse pregnancy outcome, including PE, SGA, recurrent unexplained vaginal bleeding, and preterm parturition, had lower mean plasma concentrations of protein Z than patients with normal pregnancy outcome in all three trimesters [34]. However, the authors analyzed all the patients with pregnancy complications together and cases of fetal demise were not included [34]. Thus, it is unclear what was the individual contribution of each of the pregnancy complications to the lower protein Z plasma concentrations observed in that study [34].
Protein Z deficiency and fetal demise
The finding that women with fetal demise have a higher rate of protein Z deficiency than women with normal pregnancy is in accord with previous report [33]. Of interest, the rates of protein Z deficiency that were observed in women with normal pregnancy (4.2%) and those with fetal demise (25%) in the current study, are similar to those reported in non-pregnant women [36] with normal obstetric history (4%) and with a history of previous fetal loss between 10-15 weeks gestation (22%). The similarity in the rate of protein Z deficiency between pregnant and non-pregnant women in both groups (those who had a normal pregnancy and those with fetal demise) suggests that a subset of women in the latter group might have a predisposing protein Z deficiency. Moreover, we have proposed that pregnancy could be considered as a stress test to the hemostatic system [46]. Thus, the physiologic hypercoagulable state that accompanies pregnancy may facilitate the occurrence of thrombotic events of the placenta and adverse pregnancy outcome (i.e. fetal demise) in potentially thrombophilic patients that were clinically “silent” in the non-pregnant state [46]. In addition, Gris et al [36] reported that six out of eight patients with protein Z deficiency had one parent who is also protein Z deficient; [36] thus, the possibility that in some cases protein Z deficiency may be inherited can not be ruled out.
Conclusions
The results of this study indicate that PE and fetal demise are associated with maternal protein Z deficiency; however, only patients with PE have a lower median maternal plasma concentration of protein Z that may be secondary to a higher activation of the coagulation system in patients with this pregnancy complication.
Acknowledgments
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.de B K, ten Cate JW, Sturk A, Borm JJ, Treffers PE. Enhanced thrombin generation in normal and hypertensive pregnancy. Am J Obstet Gynecol. 1989:95–100. doi: 10.1016/0002-9378(89)90096-3. [DOI] [PubMed] [Google Scholar]
- 2.Yuen PM, Yin JA, Lao TT. Fibrinopeptide A levels in maternal and newborn plasma. Eur J Obstet Gynecol Reprod Biol. 1989:239–44. doi: 10.1016/0028-2243(89)90007-5. [DOI] [PubMed] [Google Scholar]
- 3.Sorensen JD, Secher NJ, Jespersen J. Perturbed (procoagulant) endothelium and deviations within the fibrinolytic system during the third trimester of normal pregnancy. A possible link to placental function. Acta Obstet Gynecol Scand. 1995:257–61. doi: 10.3109/00016349509024445. [DOI] [PubMed] [Google Scholar]
- 4.Walker MC, Garner PR, Keely EJ, Rock GA, Reis MD. Changes in activated protein C resistance during normal pregnancy. Am J Obstet Gynecol. 1997:162–9. doi: 10.1016/s0002-9378(97)70456-3. [DOI] [PubMed] [Google Scholar]
- 5.Bellart J, Gilabert R, Miralles RM, Monasterio J, Cabero L. Endothelial cell markers and fibrinopeptide A to D-dimer ratio as a measure of coagulation and fibrinolysis balance in normal pregnancy. Gynecol Obstet Invest. 1998:17–21. doi: 10.1159/000009989. [DOI] [PubMed] [Google Scholar]
- 6.Chaiworapongsa T, Espinoza J, Yoshimatsu J, Kim YM, Bujold E, Edwin S, Yoon BH, Romero R. Activation of coagulation system in preterm labor and preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2002:368–73. doi: 10.1080/jmf.11.6.368.373. [DOI] [PubMed] [Google Scholar]
- 7.Yoneyama Y, Suzuki S, Sawa R, Otsubo Y, Power GG, Araki T. Plasma adenosine levels increase in women with normal pregnancies. Am J Obstet Gynecol. 2000:1200–3. doi: 10.1067/mob.2000.104832. [DOI] [PubMed] [Google Scholar]
- 8.Sheu JR, Hsiao G, Luk HN, Chen YW, Chen TL, Lee LW, Lin CH, Chou DS. Mechanisms involved in the antiplatelet activity of midazolam in human platelets. Anesthesiology. 2002:651–8. doi: 10.1097/00000542-200203000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Brosens I, Renaer M. On the pathogenesis of placental infarcts in pre-eclampsia. J Obstet Gynaecol Br Commonw. 1972:794–9. doi: 10.1111/j.1471-0528.1972.tb12922.x. [DOI] [PubMed] [Google Scholar]
- 10.Robertson WB, Brosens I, Dixon G. Uteroplacental vascular pathology. Eur J Obstet Gynecol Reprod Biol. 1975:47–65. doi: 10.1016/0028-2243(75)90130-6. [DOI] [PubMed] [Google Scholar]
- 11.Pijnenborg R, Anthony J, Davey DA, Rees A, Tiltman A, Vercruysse L, van A A. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol. 1991:648–55. doi: 10.1111/j.1471-0528.1991.tb13450.x. [DOI] [PubMed] [Google Scholar]
- 12.Gersell DJ. Selected vascular lesions of the placenta. Clin Lab Med. 1995:611–29. [PubMed] [Google Scholar]
- 13.Sikkema JM, Franx A, Bruinse HW, van der Wijk NG, de Valk HW, Nikkels PG. Placental pathology in early onset pre-eclampsia and intra-uterine growth restriction in women with and without thrombophilia. Placenta. 2002:337–42. doi: 10.1053/plac.2001.0785. [DOI] [PubMed] [Google Scholar]
- 14.Rolschau J. Infarctions and intervillous thrombosis in placenta, and their association with intrauterine growth retardation. Acta Obstet Gynecol Scand Suppl. 1978:22–7. [PubMed] [Google Scholar]
- 15.Arias F, Romero R, Joist H, Kraus FT. Thrombophilia: a mechanism of disease in women with adverse pregnancy outcome and thrombotic lesions in the placenta. J Matern Fetal Med. 1998:277–86. doi: 10.1002/(SICI)1520-6661(199811/12)7:6<277::AID-MFM5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Mitra SC, Seshan SV, Riachi LE. Placental vessel morphometry in growth retardation and increased resistance of the umbilical artery Doppler flow. J Matern Fetal Med. 2000:282–6. doi: 10.1002/1520-6661(200009/10)9:5<282::AID-MFM5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 17.Sugimura M, Ohashi R, Kobayashi T, Kanayama N. Intraplacental coagulation in intrauterine growth restriction: cause or result? Semin Thromb Hemost. 2001:107–13. doi: 10.1055/s-2001-14068. [DOI] [PubMed] [Google Scholar]
- 18.Ornoy A, Crone K, Altshuler G. Pathological features of the placenta in fetal death. Arch Pathol Lab Med. 1976:367–71. [PubMed] [Google Scholar]
- 19.Preston FE, Rosendaal FR, Walker ID, Briet E, Berntorp E, Conard J, Fontcuberta J, Makris M, Mariani G, Noteboom W, et al. Increased fetal loss in women with heritable thrombophilia. Lancet. 1996:913–6. doi: 10.1016/s0140-6736(96)04125-6. [DOI] [PubMed] [Google Scholar]
- 20.Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, Rotmensch S, Romero R. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003:1063–9. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- 21.Elovitz MA, Baron J, Phillippe M. The role of thrombin in preterm parturition. Am J Obstet Gynecol. 2001:1059–63. doi: 10.1067/mob.2001.117638. [DOI] [PubMed] [Google Scholar]
- 22.Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993:585–91. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 23.Moldenhauer JS, Stanek J, Warshak C, Khoury J, Sibai B. The frequency and severity of placental findings in women with preeclampsia are gestational age dependent. Am J Obstet Gynecol. 2003:1173–7. doi: 10.1067/s0002-9378(03)00576-3. [DOI] [PubMed] [Google Scholar]
- 24.Redline RW, Boyd T, Campbell V, Hyde S, Kaplan C, Khong TY, Prashner HR, Waters BL. Maternal vascular underperfusion: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2004:237–49. doi: 10.1007/s10024-003-8083-2. [DOI] [PubMed] [Google Scholar]
- 25.Chaiworapongsa T, Yoshimatsu J, Espinoza J, Kim YM, Berman S, Edwin S, Yoon BH, Romero R. Evidence of in vivo generation of thrombin in patients with small-for-gestational-age fetuses and pre-eclampsia. J Matern Fetal Neonatal Med. 2002:362–7. doi: 10.1080/jmf.11.6.362.367. [DOI] [PubMed] [Google Scholar]
- 26.Terao T, Maki M, Ikenoue T, Gotoh K, Murata M, Iwasaki H, Shibata J, Nakabayashi M, Muraoka M, Takeda Y, et al. The relationship between clinical signs and hypercoagulable state in toxemia of pregnancy. Gynecol Obstet Invest. 1991:74–85. doi: 10.1159/000293106. [DOI] [PubMed] [Google Scholar]
- 27.Cadroy Y, Grandjean H, Pichon J, Desprats R, Berrebi A, Fournie A, Boneu B. Evaluation of six markers of haemostatic system in normal pregnancy and pregnancy complicated by hypertension or pre-eclampsia. Br J Obstet Gynaecol. 1993:416–20. doi: 10.1111/j.1471-0528.1993.tb15264.x. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi T, Sumimoto K, Tokunaga N, Sugimura M, Nishiguchi T, Kanayama N, Terao T. Coagulation index to distinguish severe preeclampsia from normal pregnancy. Semin Thromb Hemost. 2002:495–500. doi: 10.1055/s-2002-36689. [DOI] [PubMed] [Google Scholar]
- 29.Yin ZF, Huang ZF, Cui J, Fiehler R, Lasky N, Ginsburg D, Broze GJ., Jr Prothrombotic phenotype of protein Z deficiency. Proc Natl Acad Sci U S A. 2000:6734–8. doi: 10.1073/pnas.120081897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han X, Fiehler R, Broze GJ., Jr Isolation of a protein Z-dependent plasma protease inhibitor. Proc Natl Acad Sci U S A. 1998:9250–5. doi: 10.1073/pnas.95.16.9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han X, Huang ZF, Fiehler R, Broze GJ., Jr The protein Z-dependent protease inhibitor is a serpin. Biochemistry. 1999:11073–8. doi: 10.1021/bi990641a. [DOI] [PubMed] [Google Scholar]
- 32.Han X, Fiehler R, Broze GJ., Jr Characterization of the protein Z-dependent protease inhibitor. Blood. 2000:3049–55. [PubMed] [Google Scholar]
- 33.Bretelle F, Arnoux D, Shojai R, D'Ercole C, Sampol J, Dignat F, Camoin-Jau L. Protein Z in patients with pregnancy complications. Am J Obstet Gynecol. 2005:1698–702. doi: 10.1016/j.ajog.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Paidas MJ, Ku DH, Lee MJ, Manish S, Thurston A, Lockwood CJ, Arkel YS. Protein Z, protein S levels are lower in patients with thrombophilia and subsequent pregnancy complications. J Thromb Haemost. 2005:497–501. doi: 10.1111/j.1538-7836.2005.01158.x. [DOI] [PubMed] [Google Scholar]
- 35.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996:163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 36.Gris JC, Quere I, Dechaud H, Mercier E, Pincon C, Hoffet M, Vasse M, Mares P. High frequency of protein Z deficiency in patients with unexplained early fetal loss. Blood. 2002:2606–8. doi: 10.1182/blood.v99.7.2606. [DOI] [PubMed] [Google Scholar]
- 37.Quack Loetscher KC, Stiller R, Roos M, Zimmermann R. Protein Z in normal pregnancy. Thromb Haemost. 2005:706–9. doi: 10.1160/TH04-08-0532. [DOI] [PubMed] [Google Scholar]
- 38.Grisaru D, Zwang E, Peyser MR, Lessing JB, Eldor A. The procoagulant activity of red blood cells from patients with severe preeclampsia. Am J Obstet Gynecol. 1997:1513–6. doi: 10.1016/s0002-9378(97)70100-5. [DOI] [PubMed] [Google Scholar]
- 39.Schjetlein R, Haugen G, Wisloff F. Markers of intravascular coagulation and fibrinolysis in preeclampsia: association with intrauterine growth retardation. Acta Obstet Gynecol Scand. 1997:541–6. doi: 10.3109/00016349709024580. [DOI] [PubMed] [Google Scholar]
- 40.VanWijk MJ, Boer K, Berckmans RJ, Meijers JC, van der Post JA, Sturk A, VanBavel E, Nieuwland R. Enhanced coagulation activation in preeclampsia: the role of APC resistance, microparticles and other plasma constituents. Thromb Haemost. 2002:415–20. [PubMed] [Google Scholar]
- 41.Graninger W, Tatra G, Pirich K, Nasr F. Low antithrombin III and high plasma fibronectin in pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 1985:223–9. doi: 10.1016/0028-2243(85)90033-4. [DOI] [PubMed] [Google Scholar]
- 42.Weenink GH, ten Cate JW, Treffers PE, Smorenberg-Schoorl ME. Blood coagulation in pregnancy induced hypertension. Scand J Clin Lab Invest Suppl. 1985:99–105. [PubMed] [Google Scholar]
- 43.Saleh AA, Bottoms SF, Welch RA, Ali AM, Mariona FG, Mammen EF. Preeclampsia, delivery, and the hemostatic system. Am J Obstet Gynecol. 1987:331–6. doi: 10.1016/s0002-9378(87)80163-1. [DOI] [PubMed] [Google Scholar]
- 44.Weiner CP. The mechanism of reduced antithrombin III activity in women with preeclampsia. Obstet Gynecol. 1988:847–9. doi: 10.1097/00006250-198812000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Osmanagaoglu MA, Topcuoglu K, Ozeren M, Bozkaya H. Coagulation inhibitors in preeclamptic pregnant women. Arch Gynecol Obstet. 2005:227–30. doi: 10.1007/s00404-003-0596-4. [DOI] [PubMed] [Google Scholar]
- 46.Romero R, Dekker G, Kupferminc M, Saade G, Livingston J, Peaceman A, Mazor M, Yoon BH, Espinoza J, Chaiworapongsa T, et al. Can heparin prevent adverse pregnancy outcome? J Matern Fetal Neonatal Med. 2002:1–8. doi: 10.1080/jmf.12.1.1.8. [DOI] [PubMed] [Google Scholar]