Abstract
Hormone–neurotransmitter interactions form an important link through which hormones influence a variety of behavioral processes. Typically, sexual behavior is dimorphic with males mounting receptive females. In the all-female lizard species Cnemidophorus uniparens, individuals display both male-like pseudocopulation and female-like receptivity. These respective behavioral states are correlated with high circulating concentrations of progesterone following ovulation and of estrogen preceding it. In sexual species, serotonin is involved in male-typical mounting, and, as reported here, in male-like pseudosexual behavior in this unisexual species. In the first study, C. uniparens were ovariectomized and treated systemically with exogenous androgen, a hormonal regimen that results in individuals displaying only male-like pseudosexual behavior. An increase in serotonin levels in the preoptic area coupled with the suppression of male-like pseudocopulation was observed in androgen-treated lizards injected with 5-hydroxytryptophan (the precursor of serotonin) and clorgyline (a monoamine oxidase inhibitor) compared to vehicle-treated controls. Our second experiment involved ovariectomizing lizards and either injecting them with estradiol or implanting them with either an empty (Blank) or a progesterone- or testosterone-containing Silastic capsule. Treatment with para-chlorophenylalanine (an inhibitor of tryptophan hydroxylase) facilitated male-like pseudosexual behavior depending on the circulating hormonal milieu and decreased serotonin levels in the preoptic area. Our data suggest that serotonin is inhibitory to male-like pseudosexual behavior in C. uniparens but more importantly that the hormonal environment modulates the serotonin system at the level of the preoptic area, with the serotonergic system then establishing behavioral thresholds that allow for this behavior to be “gated”.
Keywords: Serotonin, Reptile, Sexual behavior, Thresholds, Neurotransmitters, Hormones, Preoptic area
Introduction
Sexual behavior is a composite of male- and female-typical behavioral repertoires including male-typical mounting and female-typical receptivity. The mechanisms underlying both behaviors involve a complex interplay of internal and external factors within a spatial and temporal framework. Circulating sex steroid hormones play a pivotal role in the expression of sexual behavior, and neurotransmitters are thought to be an important link between hormones and behavior in several species (Hull et al., 1999; Summers, 2001). However, details of how steroid hormones and neurotransmitters interact remain poorly understood.
Among the neurotransmitters, serotonin (5-HT) is inhibitory to sexual behavior in a variety of vertebrates (Bitran and Hull, 1987; Gorzalka et al., 1990). Decreasing 5-HT levels via the systemic administration of a tryptophan hydroxylase inhibitor (pCPA) increases mounting, intromission, and ejaculatory behavior in intact male rats (Tagliamonte et al., 1969; Ahlenius et al., 1971; Del Fiacco et al., 1974). Conversely, increasing 5-HT levels by chronic administration of fluoxetine to adult male rats suppresses male-typical sexual behavior (Frank et al., 2000), as does the injection of 5-HT directly into the preoptic area (POA) of male rats (Verma et al., 1989). Administration of pCPA to ovariectomized female rats increases the incidence of mounting behavior when treated females are tested with receptive females, an effect that appears to be independent of the circulating hormonal milieu (Emery and Sachs, 1976; Rodriguez-Sierra et al., 1976; Sodersten et al., 1976; van de Poll et al., 1977).
The unisexual whiptail lizard Cnemidophorus uniparens alternately displays male-like pseudocopulation and female-like receptivity during the course of the ovarian cycle (reviewed in Crews, 2005). High circulating levels of estrogen prior to ovulation are associated with the display of receptive behavior, while the surge in progesterone accompanied by a rapid decline in estrogen levels following ovulation coincides with the display of male-like pseudocopulation. Although this species does not normally secrete androgens, it retains sensitivity to androgen from Cnemidophorus inornatus (its sexual ancestor), and treatment with exogenous androgen results in individuals displaying only male-like pseudocopulatory behavior. As in sexual vertebrate species, the POA is critical to the expression of male-like pseudocopulation (Wade and Crews, 1991; Kingston and Crews, 1994).
Serotonin's role in male-typical sexual behavior in female mammals suggests that the ovarian hormonal environment might interact with the serotonergic system to regulate male-like pseudosexual behavior in C. uniparens. Specifically, we investigated the effect of altering 5-HT levels in hormonally manipulated lizards by assaying for male-like pseudosexual behavior, and measuring levels of serotonin, dopamine, and their metabolites 5-hydroxy indoleacetic acid and 3,4-dihydroxyphenylacetic acid in the POA using high pressure liquid chromatography (HPLC). Our data suggest that 5-HT is inhibitory to male-like pseudosexual behavior in C. uniparens and that the extent of inhibition depends on the circulating hormonal environment. To our knowledge, this is the first study that examines the role of 5-HT in the sexual behavior of a lizard species.
Materials and methods
Animals
Adult C. uniparens were collected from the Sonoran desert in Arizona and New Mexico and transported to the University of Texas at Austin in the summer of 2005. In the laboratory, lizards were group-housed in sand-filled terraria placed in environmentally controlled chambers as described in Woolley and Crews (2004). All animal procedures were carried out in accordance with NIH and IACUC guidelines on the care and use of animals. All the following experiments were carried out in the fall of 2005.
Behavioral testing
In both experiments, a receptive stimulus animal (ovariectomized and estradiol-injected) was introduced into the test animal's tank and the frequency and latency of mounting and pseudocopulatory behavior recorded. Mounting behavior involves climbing on the back of the stimulus animal and being aligned along the longitudinal axis. The riding animal then assumes a characteristic doughnut posture by wrapping itself around the stimulus animal (pseudocopulation) (Fig. 1). In Experiment 2, animals exhibiting mounting behavior on 1 out of the 3 days were assigned a mounting score of 0.33, those mounting on 2 out of 3 days a score of 0.66, and animals mounting on all days received a score of 0.99.
Fig. 1.

Male-like pseudocopulation observed in parthenogenetic Cnemidophorus uniparens. The display of this behavior involves one lizard mounting and riding another, and eventually culminates in the characteristic pseudocopulation posture (Photo credit: Valentino Mauricio).
Tissue collection for HPLC analysis
Lizards were decapitated, brains rapidly dissected, frozen on dry ice, and stored at −80°C until the time of sectioning. Using a cryostat (Microm HM 500 OM), 200-μm coronal sections were thaw mounted onto Superfrost Plus slides (Erie Scientific, USA). Sections were then rapidly frozen using a cooling block set at −20°C (Physitemp Instruments Inc., USA), and the POA was dissected using a 300-μm diameter micropunch, as per Smeets and Steinbusch (1988) and Young et al. (1994). Tissue samples from each animal were assayed independently of each other and not pooled. The punched tissue was stored in ice-cold 70 μl homogenization solution [a mixture of 60 μl homogenization buffer: 0.1 M Perchloric acid (Sigma-Aldrich) containing 347 μM sodium bisulphate (Sigma-Aldrich) and 134 μM EDTA disodium salt (Fluka, USA), and 10 μl of 100 nM Epinine-internal standard; Sigma-Aldrich]. Tissue samples in homogenization solution were frozen at −80°C overnight and thawed after 24 h. The thawed samples were centrifuged at 14,000 rpm at 4°C for 20 min, after which the supernatant was collected and used for HPLC analysis. Protein content in the resulting pellet was determined by resuspending and agitating the pellet in 45 μl ice-cold 0.3N NaOH for 24 h at 4°C and carrying out a modified Bradford assay thereafter (Pierce Biotechnology Inc., USA).
HPLC analysis
Levels of serotonin, dopamine (DA), and their metabolites 5-hydroxy indoleacetic acid (5-HIAA) and 3,4-dihydroxyphenylacetic acid (DOPAC) in the POA were determined by HPLC-EC using modifications of Bai et al. (1999) with the assistance of Dr. Herng-Hsiang Lo in the CRED Analytical Instrumentation Facility Core (UT-Austin). In brief, 50 μl of sample was injected into an HPLC system that comprised a Shimadzu SCL-10A system controller, LC-10AD pump, an SIL-10A auto-sampler (Shimadzu, Columbia, MD), and coupled with a four-channel CoulArray electro-chemical detector (ESA, Chelmsford, MA). The isocratic mobile phase contained 4 mM citric acid, 8 mM ammonium acetate, 120 μM 1-octanesulfonic acid sodium salt, 60 μM EDTA disodium in water and 5% MeOH, pH 3.5. The flow rate of the mobile phase remained at 1 ml/min. Separation was achieved by a 4.6 mm × 80 mm reverse-phase HR-80, 3-μm particle size column (ESA, Chelmsford, MA). The potential of channels 1 through 4 of CoulArray was set at −50, 0, 300, and 400 mV, respectively. Peak area (nC) of 5-HT, 5-HIAA, DA and DOPAC at the corresponding retention time on the chromatogram resulted from 300 mV, and was used to quantify the amount based on the standard curve of each neurotransmitter. Recovery of internal standard was consistently high across all experimental runs (95%–100%) making it unnecessary to correct for recovery of internal standard. Levels of 5-HT, 5-HIAA, DA and DOPAC in the POA were expressed as pg/μg of protein in the microdissected tissue extract.
Neurotransmitter activity can be estimated using a ratio of the neurotransmitter to catabolite (e.g., 5-HIAA/5-HT) taking into account the synthesis and catabolism of the system. However, in some cases (as in the data reported), such estimation might not be accurate since administration of a drug may cause decreased synthesis as well as catabolism, leading to decreases in both neurotransmitter as well as catabolite levels. Such a scenario results in no difference in neurotransmitter to catabolite ratio even though profound changes in neurotransmitter dynamics have occurred. We have thus represented our data as levels of neurotransmitter and catabolite independently and not as a ratio of these two entities.
Statistical analysis
SPSS v12.0 for Windows was used for all statistical analysis with significance set at P < 0.05, and Tukey post hoc analysis conducted where appropriate. For Experiment 1, multivariate ANOVAs were used to test the effect of treatment (VEH or DRUG) on serotonin and dopamine levels within the POA, with separate analyses run on the SAME DAY and NEXT DAY groups. Since the latencies to mount did not follow normal distributions, the non-parametric Mann-Whitney test was used to analyze the effect of vehicle and drug treatment on the latency to mount in the SAME DAY and NEXT DAY groups of animals. In Experiment 2, within each hormone group, the effect of treatment (saline, pCPA, recovery) on mounting score, 5-HT, 5-HIAA, DA and DOPAC levels in the POA were statistically analyzed using a multivariate ANOVA. To assess for an interaction effect between treatment and hormone on mounting score, 5-HT, 5-HIAA, DA and DOPAC levels in the POA, a combined multivariate ANOVA (time point×hormone) with all the hormones was used. The Grubbs test (http://www.graphpad.com/calculators/GrubbsHowTo.cfm) was used to identify outliers; only one value for 5-HT in the OVX + E recovery time point (Experiment 2) qualified and was excluded from analysis.
Experiment 1: Effect of increasing serotonin levels in the POA of androgenized C. uniparens using clorgyline and 5-HTP
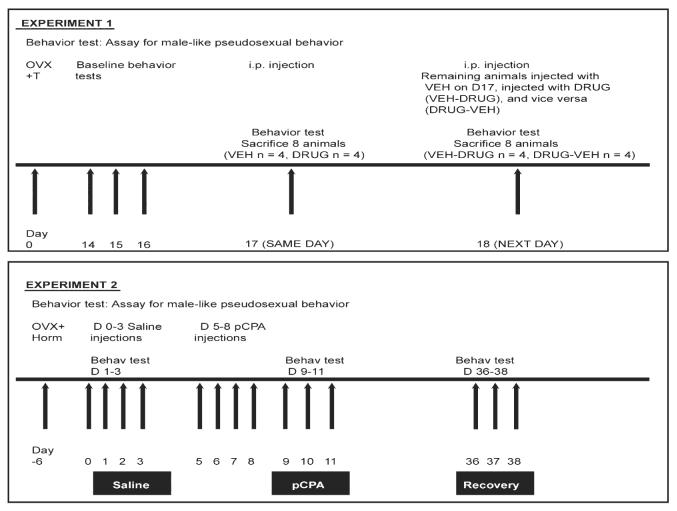
Adult C. uniparens were ovariectomized and implanted with a 12-mm Silastic capsule (Helix Medical Inc., USA) containing testosterone (n = 16). Fourteen days later, animals were tested for male-like pseudocopulation on 3 consecutive days (Baseline tests) (Fig. 2, upper panel). All animals rapidly mounted and pseudocopulated on all test days upon introduction of the receptive stimulus animal, a finding supported by extensive previous research (Crews, 2005). One day after the baseline tests, animals were injected i.p. with either Vehicle (VEH) (DMSO + 0.9% saline) (n = 8), or a combination of the monoamine oxidase inhibitor, Clorgyline (0.3 mg/kg; Sigma-Aldrich, USA), and the precursor of serotonin, 5-hydroxytryptophan (5-HTP) (20 mg/kg; Sigma-Aldrich, USA) (DRUG; n = 8) following a paradigm outlined by Shioda et al. (2004). Ninety minutes after the injection, animals were tested and the latency to mount a receptive stimulus lizard noted. Four animals from each group (VEH, n = 4;DRUG, n = 4) were sacrificed 20 min after the behavioral test (SAME DAY group) and brains set aside for HPLC. The next day, in order to control for order effects and to test the transience of serotonergic modulation of male-like pseudosexual behavior, animals that had been injected with VEH the day before were injected with DRUG (VEH-DRUG, n = 4), and vice versa (DRUG-VEH, n = 4). Once again, the injected animals were tested for male-like pseudocopulation 90 min after the injection and the latency to mount noted. These lizards were then sacrificed for the purpose of HPLC 20 min after their behavioral test (NEXT DAY group).
Fig. 2.
Schematic representation of Experiments 1 and 2. In Experiment 1, Cnemidophorus uniparens were ovariectomized and given a Silastic implant i.p. containing testosterone. After a series of baseline tests to establish robust male-like pseudocopulation, animals were injected i.p. with either saline + DMSO (VEH) or clorgyline + 5-HTP (DRUG) and tested 90 min later; latency to mount was recorded (maximum 600 s). Four VEH and four DRUG animals were sacrificed for HPLC analysis (SAME DAY group). The next day, animals injected with VEH on the previous day were injected i.p. with DRUG (VEH-DRUG animals) and vice versa (DRUG-VEH animals) and tested 90 min later for mounting. These lizards were sacrificed for monoamine analysis using HPLC (NEXT DAY group). In Experiment 2 ovariectomized lizards received a Silastic implant that was either blank (OVX + Bl) or contained progesterone (OVX + P) or testosterone (OVX + T). Another set of animals was ovariectomized and injected i.p. with estradiol to generate an OVX + E group. One week later, animals were injected with saline i.p. and tested for male-like mounting. Five animals from each hormonal group were sacrificed and brains used for HPLC analysis (saline time point). pCPA was administered to the remaining animals for 4 days, and behavioral testing was conducted for 3 consecutive days after the last pCPA injection, after which five animals belonging to each hormonal cohort were sacrificed (pCPA time point). The remaining OVX + Bl, OVX + P and OVX + E animals (n = 5/group) were then tested 27 days after the last pCPA injection and sacrificed (recovery time point).
Experiment 2: Effect of decreasing serotonin levels in the POA via injection of pCPA
Animals were ovariectomized and implanted with either a 12-mm blank Silastic capsule (n = 15, OVX + Bl), one packed with progesterone (n = 15, OVX + P) or packed with testosterone (n = 15, OVX + T); all hormones were purchased from Sigma-Aldrich, USA. The extremely low survival rate of animals implanted with estradiol benzoate (EB) precludes an implanted OVX + E group from being generated. Instead, another 15 adults were ovariectomized and not implanted but administered i.p. injections of 0.5 μg EB twice a week (n = 15, OVX +E). This paradigm has yielded receptive animals in the past and is used as a read-out of estrogen being the predominant hormone in circulation (Young and Crews, 1995). All hormonal manipulations yield physiological concentrations of circulating hormones (Lindzey and Crews, 1986). Animals were housed in isolation and allowed a 6-day recovery period. Each hormonal group was run through the experimental design at the same time (Fig. 2, lower panel) with all behavioral observations recorded and video clips analyzed later. All animals were administered 0.9% saline (i.p.) for 4 days and assayed for male-like mounting behavior on the last 3 days of saline injection. Five animals from each group were then sacrificed for neurochemical analysis (saline time point). The remaining ten lizards were injected i.p. with a tryptophan hydroxylase inhibitor para-chlorophenylalanine (pCPA) (100 mg/kg) for 4 days and scored for male-like mounting behavior for 3 consecutive days starting the day after the last pCPA injection. Five animals representing the OVX + Bl, OVX + P and OVX + E groups, and ten animals from the OVX + T group were sacrificed to conduct HPLC analysis (pCPA time point). Twenty-seven days after the last pCPA injection, the five remaining lizards from the OVX + Bl, OVX + P and OVX + E groups were tested for male-like mounting behavior on 3 consecutive days (recovery time point) and then sacrificed for HPLC analysis.
The OVX + Bl group served as a negative control, as ovariectomy abolishes pseudosexual behavior, while OVX + T animals served as positive controls. A third control group consisted of OVX + T animals (n = 10) sacrificed at the pCPA time point to distinguish between the serotonergic mediation of the behavior and the role of previous experience. Also, it might be argued that the OVX + T animals at the pCPA time point had more T in circulation since they were implanted for 7 days longer than the saline group and that this increase in T level might be related to any behavior observed at the pCPA time point. Although we cannot rule out this possibility, circulating hormone levels in animals receiving Silastic capsules have been shown to plateau after a few days, and no relationship exists between differing testosterone levels brought about by implantation of various lengths of Silastic capsules and sexual behavior in the rat (Johnston and Davidson, 1979; Damassa et al., 1977).
All animals were sacrificed 2 h after the last testing period. This injection and testing paradigm was based on rat studies that showed significant depletion of serotonin levels after four daily injections of pCPA and a return to baseline levels after approximately 3 weeks (Koe and Weissman, 1966).
In both experiments, all animals ate a live cricket following behavior tests, an indication that motivational and motor systems were unaffected by any pharmacological manipulation.
Results
Increased 5-HT level within the POA correlates with suppression of male-like pseudocopulation (Experiment 1)
The combination drug regimen consisting of 5-HTP and clorgyline increased the latency of drug-injected animals to mount a receptive stimulus animal. This increase was accompanied by elevated 5-HT and 5-HIAA levels, while DA and DOPAC levels were unchanged (Table 1, Fig. 3).
Table 1.
Monoamine level (pg/μg of protein) expressed as mean ± SEM
| Day of treatment | Treatment | 5-HT | 5-HIAA | DA | DOPAC |
|---|---|---|---|---|---|
| SAME DAY | VEH | 15.18 ± 3.51 | 17.19 ± 1.60 | 4.98 ± 0.33 | 2.81 ± 0.25 |
| DRUG | 46.17 ± 7.7 | 56.59 ± 6.69 | 5.50 ± 0.72 | 2.13 ± 0.50 | |
| P value | 0.01 | < 0.01 | NS | NS | |
| NEXT DAY | VEH-DRUG | 45.86 ± 6.06 | 26.45 ± 3.89 | 5.66 ± 1.35 | 1.43 ± 0.26 |
| DRUG-VEH | 28.20 ± 5.65 | 11.34 ± 1.39 | 6.16 ± 1.22 | 1.03 ± 0.37 | |
| P value | NS | 0.01 | NS | NS | |
| Combined (SAME DAY and NEXT DAY) | |||||
| 5-HT | 5-HIAA | DA | DOPAC | ||
| VEHICLE | 21.69 ± 3.95 | 14.26 ± 1.48 | 5.57 ± 1.76 | 1.92 ± 1.12 | |
| DRUG | 46.01 ± 4.55 | 41.52 ± 6.81 | 5.58 ± 0.71 | 1.78 ± 0.29 | |
| P value | 0.001 | 0.002 | NS | NS |
Monoamine levels of 5-HT, 5-HIAA, DA and DOPAC in the POA of the parthenogenetic lizard, Cnemidophorus uniparens obtained in Experiment 1. Data expressed as pg/μg of protein (mean ± SEM). Top: SAME DAY animals were injected with either VEH or DRUG and sacrificed the same day (n = 4/group). NEXT DAY animals were injected with VEH on the day of sacrifice after having been injected with DRUG the day before (Drug-VEH) and vice versa (n = 4/group). Bottom: Data combined over both days that consist of animals receiving either VEHICLE or DRUG on the day of sacrifice.
Fig. 3.
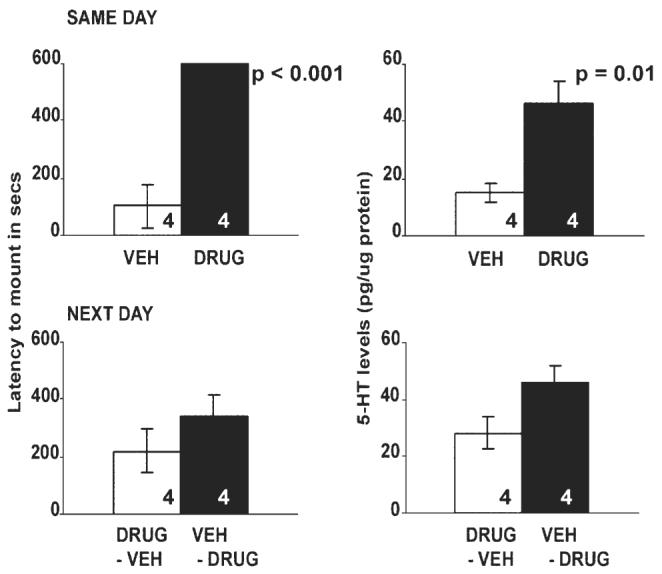
Latency to mount (seconds) and 5-HT levels (expressed as pg/μg of protein) in the preoptic area of ovariectomized, testosterone-implanted Cnemidophorus uniparens receiving either vehicle (DMSO + saline; VEH) or drug (5-HTP + clorgyline; DRUG) in Experiment 1. All data are expressed as mean ± SEM. SAME DAY animals were injected with either VEH or DRUG and sacrificed the same day (n = 4/group). NEXT DAY animals were injected with VEH on the day of sacrifice after having been injected with DRUG the day before (DRUG-VEH) and vice versa (n = 4/group). A significant increase in the latency to mount was observed in DRUG-injected animals compared to VEH-injected controls (P < 0.001), and was mirrored by a significant increase in 5-HT levels in the POA of DRUG-treated lizards compared to VEH-treated animals (F1,6 = 13.33, P = 0.01). No significant differences were observed for either parameter in the NEXT DAY animals.
SAME DAY group: All lizards injected with drug took significantly longer to mount when compared to vehicle-injected controls (P < 0.001) and also had significantly higher levels of 5-HT and 5-HIAA in the POA.
NEXT DAY group: The latency to mount was not significantly different between the DRUG-VEH and VEH-DRUG-injected group (P > 0.05), nor was there a significant difference in 5-HT levels within the POA. 5-HIAA levels were significantly higher in the VEH-DRUG group compared to DRUG-VEH animals.
There was a positive relationship between 5-HT level in the POA and latency to mount (Fig. 4).
Fig. 4.
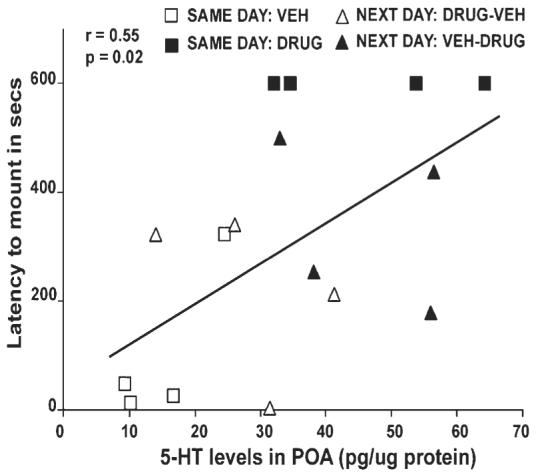
Correlation between 5-HT levels in the preoptic area of Cnemidophorus uniparens that were ovariectomized and received a Silastic capsule containing testosterone. Presented is the latency to mount (seconds) across all animals (SAME DAY and NEXT DAY groups; n = 16) in Experiment 1. A positive relationship exists with high 5-HT levels correlated with a longer latency to mount (F1,14 = 6.06, P = 0.02, r = 0.55). SAME DAY animals are expressed as open squares (Vehicle-injected) or closed squares (Drug-injected). NEXT DAY animals are represented as open triangles (Drug-Vehicle injected) or closed triangles (Vehicle-Drug injected).
DA and DOPAC levels in the POA were not significantly different between drug- and vehicle-injected animals in either group (P > 0.05).
Facilitation of male-like mounting in ovariectomized, hormonally implanted C. uniparens after the administration of pCPA (Experiment 2)
Male-like mounting behavior was increased significantly after pCPA administration to OVX + Bl and OVX + T lizards (Fig. 5). In OVX + Bl animals, male-like mounting behavior was significantly enhanced in pCPA-treated animals. The OVX + T animals injected with pCPA showed significantly more mounting behavior than the saline-treated lizards. No significant differences in mounting behavior between animals at any of the time points were noted in the OVX + P and OVX + E groups.
Fig. 5.
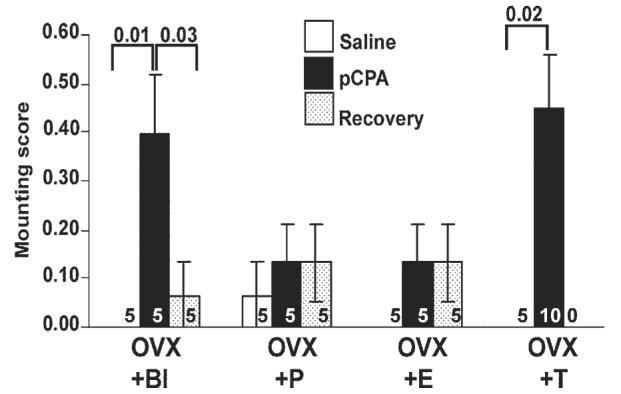
Relationship between steroid hormones, serotonin manipulation and mounting behavior in the unisexual whiptail lizard, Cnemidophorus uniparens using pCPA in Experiment 2. Mounting score is expressed as mean ± SEM at three time points: saline, pCPA and recovery. Ovariectomized lizards received a Silastic implant that was either blank (OVX + Bl) or contained progesterone (OVX + P) or testosterone (OVX + T). Another set of animals was ovariectomized and injected i.p. with estradiol to generate an OVX + E group. Numbers within bars indicate sample size of group. A significant induction in mounting behavior is observed between saline and pCPA-treated OVX + Bl (F2,12 = 6.89, P = 0.01: saline vs. pCPA, P = 0.01) and OVX + T (F1,13 = 7.05, P = 0.02) lizards. In the OVX + Bl group, mounting scores were also significantly different between animals at the pCPA time point and the recovery time point (P = 0.03). No significant differences were detected in OVX + P and OVX + E animals (P > 0.05).
Differences in baseline 5-HT and DA levels in the POA of hormonally manipulated C. uniparens (Experiment 2)
There was a significant effect of the different hormonal regimens on 5-HT levels within the POA at a baseline state (saline time point) (F3,16 = 5.40, P < 0.01). Post hoc analysis of these data indicates that 5-HT levels in the POA of OVX + Bl animals were significantly greater than levels in the OVX + T group (P = 0.006) (Fig. 6). 5-HT levels in the POA of OVX + P and OVX + E were intermediate compared to OVX + Bl and OVX + T animals (P > 0.05). There was no difference in DA levels in the POA between any of the hormonally manipulated lizards at the saline time point (P > 0.05) (Fig. 7).
Fig. 6.
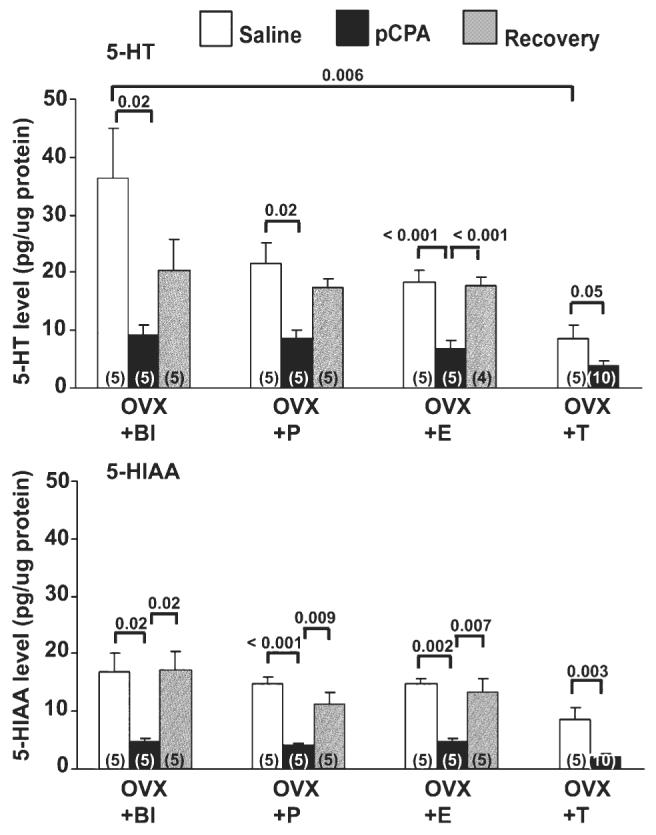
Relationship between steroid hormones and serotonin manipulation on serotonin and its metabolite in the preoptic area (POA) of the unisexual whiptail lizard, Cnemidophorus uniparens in Experiment 2. Presented are levels of 5-HT (top) and 5-HIAA (bottom) (pg/μg of protein). Numbers in parentheses indicate sample size per group. See Fig. 5 for groups. A significant decrease in 5-HT levels is observed at the pCPA time point of OVX + Bl (P = 0.02), OVX + P (P = 0.02), OVX + E (P < 0.001) and OVX + T (P = 0.05) lizards in comparison with the saline time point. 5-HT levels in the OVX + E group at the pCPA time point were also significantly different from those at the recovery time point (P < 0.001). Mean 5-HT levels at the saline time point in the POA of OVX + Bl and OVX + T differ significantly from each other (P < 0.01). 5-HIAA levels in the POA at the pCPA time point are significantly different from levels at the saline and recovery time points (OVX + Bl: saline vs. pCPA, P = 0.02; recovery vs. pCPA, P = 0.02; OVX + P: saline vs. pCPA, P < 0.001; recovery vs. pCPA, P < 0.01; OVX + E: saline vs. pCPA, P < 0.01; recovery vs. pCPA, P < 0.01; OVX + T: saline vs. pCPA, P < 0.01).
Fig. 7.
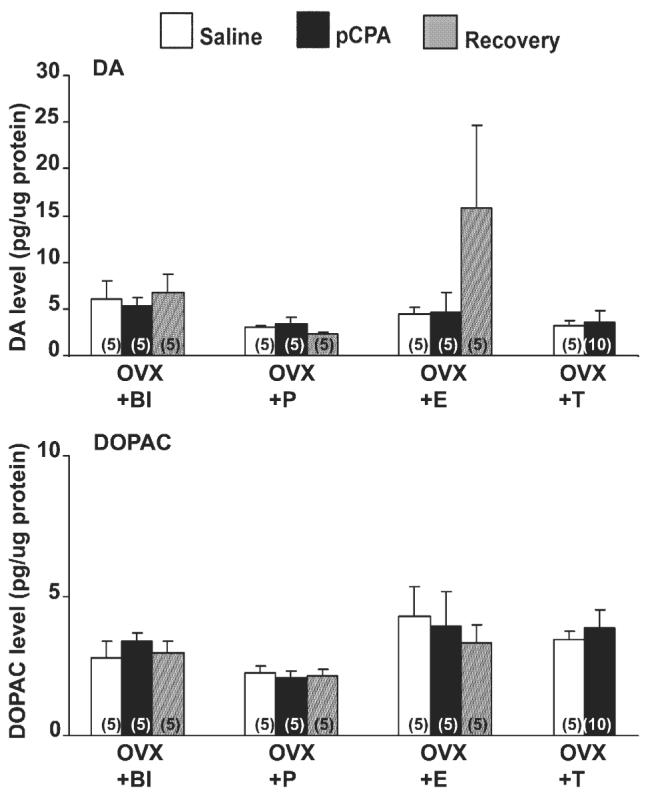
Relationship between steroid hormones and serotonin manipulation on dopamine (DA) and its metabolite (DOPAC) in the preoptic area (POA) of the unisexual whiptail lizard, Cnemidophorus uniparens in Experiment 2. Data expressed as pg/μg of protein in the POA of hormonally manipulated C. uniparens. See Fig. 5 for groups. Numbers in parentheses indicate sample size per group. No significant differences were observed across time points in any of the hormonal groups (P > 0.05).
Alterations in 5-HT, 5-HIAA, DA and DOPAC levels across time points of the pCPA regimen (Experiment 2)
Administration of pCPA significantly decreased 5-HT levels within the POA in OVX + Bl, OVX + P, OVX + E and OVX + T groups when compared to saline-treated animals (Fig. 6). 5-HT levels in the OVX + E group at the pCPA time point were also significantly different from those at the recovery time point.
5-HIAA levels were significantly decreased at the pCPA time point compared to the saline and recovery time points in OVX + Bl, OVX + P, OVX + E, and OVX + T animals (Fig. 6).
DA and DOPAC levels at all time points across all hormonal regimens were not significantly different from each other in the POA (P > 0.05) (Fig. 7).
Discussion
We have demonstrated that increasing 5-HT levels inhibited male-like pseudosexual behavior in the parthenogenetic whiptail lizard C. uniparens (Experiment 1). Decreasing 5-HT levels using pCPA facilitated male-like mounting, an effect influenced by hormone treatment (Experiment 2). Significant alterations in 5-HT and 5-HIAA levels in the POA accompanied the expression or lack thereof of male-like pseudosexual behavior, without any concomitant changes in DA and DOPAC levels.
A study in rodents indicating that an increase in male mounting behavior after decreasing 5-HT levels using pCPA requires testosterone (Sodersten and Larsson, 1976) is contradicted by another that shows male hypersexuality in response to pCPA to be independent of circulating testosterone (Bond et al., 1972). The observation of male-like mounting in pCPA-injected OVX + Bl lizards suggests that 5-HT does indeed act independently to regulate the expression of male-like pseudosexual behavior with the hormonal environment modulating the nature of this regulation, as observed in the OVX + T, OVX + P and OVX + E groups.
Specificity of the hormonal dependence of pseudosexual behavior in C. uniparens is illustrated by androgen-treated lizards exhibiting male-like pseudocopulation (Experiment 1) and estradiol-injected animals displaying female-like receptivity (Experiment 2). The absence of male-like pseudocopulatory behavior by OVX + P animals might be due to the need for a decrease in levels of estrogen to accompany an increase in progesterone levels (as observed in naturally cycling animals), a hormonal profile not achieved by implanting animals in the OVX + P group with only progesterone. In addition, in Experiment 2, only a week followed the hormone implantation prior to testing, and this is insufficient time for the activation of male-like pseudosexual behavior.
The suppression of male-like mounting behavior in testosterone-treated C. uniparens by increasing 5-HT levels in the POA suggests that, as in other vertebrates studied, 5-HT is inhibitory to male-like pseudosexual behavior and acts in the POA to exert this inhibitory effect, thereby pointing towards an evolutionarily conserved neurochemical pathway that regulates male sexual behavior. Data from animals injected with 5-HTP/clorgyline suggest that since 5-HT levels in the POA of DRUG-VEH animals were still elevated relative to baseline levels, increased mount latencies comparable to those observed in VEH-DRUG animals were noted.
Although 5-HT levels in the POA decreased after pCPA treatment in all treated animals, mounting frequency significantly increased only in the OVX + Bl and OVX + T animals. This observation indicates differential control of male-like pseudosexual behavior in C. uniparens by the circulating hormonal environment. One possible explanation is that factors other than 5-HT are involved in the inhibition of male-like pseudocopulation in progesterone- or estrogen-treated animals and hence relieving only the serotonergic inhibition is insufficient to elicit the behavioral pattern in these cohorts. Interestingly, administration of the D1 receptor agonist SKF 81927 facilitates male-like mounting behavior in estrogen-injected C. uniparens (Woolley et al., 2001). In the present study, no differences in dopaminergic activity as measured by DA and DOPAC levels were observed in any of the hormonal groups. Thus, it may be that 5-HT plays a central role in the male-like pseudosexual behavior expressed by OVX + T lizards, while the dopaminergic system needs to be recruited for the expression of behavior in OVX + E and OVX + P animals.
The POA serves as an integrative area for exogenous and endogenous stimuli regulating the expression of male-typical copulatory behavior across vertebrates (Beach, 1967; Friedman and Crews, 1985; Hull et al., 2002). In C. uniparens, male-like pseudocopulation is mediated by the POA (Wade and Crews, 1991; Kingston and Crews, 1994). A correlation between neuronal activity within the POA and male-like pseudocopulation (Rand and Crews, 1994; Sakata et al., 2002) makes it plausible to suggest that alterations in the neurochemical milieu within the POA may have profound effects on male-like pseudocopulation.
Interactions between neurotransmitter systems are reported to influence the expression of stereotypical sexual behavior across several species (Fernandez-Guasti et al., 1986; Cornil et al., 2005). Testosterone acting at the level of the POA is thought to “gate” male-typical copulatory behavior by increasing extracellular dopamine in this region (Dominguez and Hull, 2005). Successful copulatory behavior is also correlated with an increase in extracellular dopamine in the nucleus accumbens, which in turn is regulated by serotonin levels in the hypothalamus (Lorrain et al., 1999). In the present study, the hormonal environment did not influence baseline DA levels in the POA. In addition, no alterations in DA and DOPAC levels were observed in the POA of lizards expressing male-like mounting behavior, and it seems that the 5-HT system does not affect stored DA levels to elicit behavioral changes. Noradrenergic neurotransmission in the POA is known to be involved in male sexual behavior (Mallick et al., 1996), and norepinephrine and serotonin interact to influence sexual behavior in the male rat (Fernandez-Guasti et al., 1986). Recent work with C. uniparens has also shown the POA to have high levels of norepinephrine (Brian Dias and David Crews, unpublished observations), a neurotransmitter that we were unable to measure using the reported HPLC method. Experiments addressing this issue of independence or interaction between the serotonergic, dopaminergic and noradrenergic systems and their respective roles in pseudosexual behavior in C. uniparens are in progress.
Although little is known about 5-HT levels in the POA under the influence of androgen, 5-HT synthesizing raphe neurons projecting to hypothalamic nuclei are known to express receptors for ovarian steroid hormones (reviewed in Bethea et al., 2002). This provides a mechanism by which ovarian hormonal environments might regulate 5-HT levels. For example, hypothalamic serotonin levels have been shown to be lower in estrous female rats than in intact male rats (Gundlah et al., 1998). Microdialysis studies indicate that decreases in 5-HT levels in the VMN after sequential treatment with estrogen and progesterone coincide with periods of maximal lordosis (Farmer et al., 1996; Glaser et al., 1983). Thus, we propose that different steroid hormones might regulate the serotonergic system that in turn establishes thresholds for the expression of male-like pseudosexual behavior, thereby “gating” the expression of behavioral repertoires. This interpretation is supported by our finding that expression of male-like pseudosexual behavior is the result of disinhibition by 5-HT at the POA. At baseline (saline time point; Fig. 6), OVX + Bl animals have significantly higher 5-HT levels in the POA than do OVX + T animals. Thus, the administration of T disinhibits the effect of serotonin on male-like pseudosexual behavior to a greater extent relative to that seen in blank-implanted animals. This suggests that the degree of disinhibition necessary to elicit a behavioral response will be different for different hormones.
According to this model, the degree of inhibition exerted at the level of the POA by 5-HT prior to administration of pCPA (saline time point), and the extent of disinhibition following pCPA administration, depends upon the nature and amount of steroid hormones in circulation. 5-HT levels in the POA of pCPA-injected blank and hormone-treated ovariectomized lizards were reduced compared to baseline levels in the following order: Bl > E = P > T. Such differences in baseline 5-HT levels might explain why a smaller reduction in 5-HT levels is sufficient to produce an increase in mounting scores in OVX + T lizards, while a greater decrease is required to produce a similar mounting score in OVX + Bl animals. The reductions in 5-HT levels from baseline in the OVX + E and OVX + P groups may not result in disinhibition to the extent achieved in the OVX + Bl group as evidenced by their lack of mounting. Modulation of 5-HT levels might be only one of many ways that the 5-HT system establishes behavioral thresholds. For example, several serotonin receptor subtypes are expressed in the brain of the green anole, Anolis carolinensis (Baxter, 2001; Baxter et al., 2001; Clark and Baxter, 2000).
The complementarity of serotonergic manipulation coupled with the contrasting patterns of behavior (suppression with increases, and facilitation with decreases in 5-HT levels) indicates that serotonergic activity in the POA is a major factor in androgen-induced male-like pseudocopulatory behavior exhibited by C. uniparens. This suggests that the mechanism of androgen action on male-typical mounting behavior might be evolutionarily conserved across a wide array of vertebrate taxa. We have discussed the inhibition of male-like pseudosexual behavior by 5-HT within the POA in C. uniparens, in addition to proposing that the specific endocrine context modulates the serotonergic system in the POA, which in turn establishes thresholds to enable the “gating” of male-like pseudosexual behavior. Future studies of the interaction between the serotonergic system and gonadal hormones, as well as the interactions between different neurotransmitter systems in brain nuclei of C. uniparens, might allow us to integrate neuronal circuits and complementary behavioral patterns, providing insight into how the neurochemical milieu acts at different nuclei to mediate behavioral repertoires.
Acknowledgments
The authors would like to thank Dr. Herng-Hsiang Lo in the CRED Analytical Instrumentation Facility Core at UT-Austin (supported by NIEHS center grant ES07784) for his assistance with the HPLC analysis and Dr. Cliff Summers for his valuable input on the HPLC method. We are also grateful to Sunayana Banerjee, Nicholas Sanderson and Lynn Almli for the comments and suggestions regarding the manuscript. Animals caught in the Sonoran desert were temporarily housed at the Southwestern Research Station (Portal, AZ) before being transported to the University of Texas at Austin. Funding provided by NIMH 41770 to DC.
References
- Ahlenius S, Eriksson H, Larsson K, Modigh K, Sodersten P. Mating behavior in the male rat treated with p-chlorophenylalanine methyl ester alone and in combination with pargyline. Psychopharmacologia. 1971;20:383–388. doi: 10.1007/BF00403569. [DOI] [PubMed] [Google Scholar]
- Bai F, Lau SS, Monks TJ. Glutathione and N-acetylcysteine conjugates of alpha-methyldopamine produce serotonergic neurotoxicity: possible role in methylenedioxyamphetamine-mediated neurotoxicity. Chem. Res. Toxicol. 1999;12:1150–1157. doi: 10.1021/tx990084t. [DOI] [PubMed] [Google Scholar]
- Baxter LR., Jr. Brain mediation of Anolis social dominance displays: III. Differential forebrain 3H-sumatriptan binding in dominant vs. submissive males. Brain Behav. Evol. 2001;57:202–213. doi: 10.1159/000047237. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Jr., Clark EC, Ackermann RF, Lacan G, Melega WP. Brain mediation of Anolis social dominance displays: II. Differential forebrain serotonin turnover, and effects of specific 5-HT receptor agonists. Brain Behav. Evol. 2001;57:184–201. doi: 10.1159/000047236. [DOI] [PubMed] [Google Scholar]
- Beach FA. Cerebral and hormonal control of reflexive mechanisms involved in copulatory behavior. Physiol. Rev. 1967;47:289–316. doi: 10.1152/physrev.1967.47.2.289. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol. 2002;23:41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- Bitran D, Hull EM. Pharmacological analysis of male rat sexual behavior. Neurosci. Biobehav. Rev. 1987;11:365–389. doi: 10.1016/s0149-7634(87)80008-8. [DOI] [PubMed] [Google Scholar]
- Bond VJ, Shillito EE, Vogt M. Influence of age and of testosterone on the response of male rats to parachlorophenylalanine. Br. J. Pharmacol. 1972;46:46–55. doi: 10.1111/j.1476-5381.1972.tb06847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EC, Baxter LR., Jr. Mammal-like striatal functions in Anolis: I. Distribution of serotonin receptor subtypes, and absence of striosome and matrix organization. Brain Behav. Evol. 2000;56:235–248. doi: 10.1159/000047207. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Dejace C, Ball GF, Balthazart J. Dopamine modulates male sexual behavior in Japanese quail in part via actions on noradrenergic receptors. Behav Brain Res. 2005;163:42–57. doi: 10.1016/j.bbr.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Crews D. Evolution of neuroendocrine mechanisms that regulate sexual behavior. Trends Endocrinol. Metab. 2005;16:354–361. doi: 10.1016/j.tem.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Damassa DA, Smith ER, Tennent B, Davidson JM. The relationship between circulating testosterone levels and male sexual behavior in rats. Horm. Behav. 1977;8:275–286. doi: 10.1016/0018-506x(77)90002-2. [DOI] [PubMed] [Google Scholar]
- Del Fiacco M, Fratta W, Gessa GL, Tagliamonte A. Lack of copulatory behaviour in male castrated rats after p-chlorophenylalanine. Br. J. Pharmacol. 1974;51:249–251. doi: 10.1111/j.1476-5381.1974.tb09654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez JM, Hull EM. Dopamine, the medial preoptic area, and male sexual behavior. Physiol. Behav. 2005;86:356–368. doi: 10.1016/j.physbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Emery DE, Sachs BD. Hormonal and monoaminergic influences on masculine copulatory behavior in the female rat. Horm. Behav. 1976;7:341–352. doi: 10.1016/0018-506x(76)90039-8. [DOI] [PubMed] [Google Scholar]
- Farmer CJ, Isakson TR, Coy DJ, Renner KJ. In vivo evidence for progesterone dependent decreases in serotonin release in the hypothalamus and midbrain central grey: relation to the induction of lordosis. Brain Res. 1996;711:84–92. doi: 10.1016/0006-8993(95)01403-9. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Hansen S, Archer T, Jonsson G. Noradrenaline-serotonin interactions in the control of sexual behavior in the male rat: DSP4-induced noradrenaline depletion antagonizes the facilitatory effect of serotonin receptor agonists, 5-MeODMT and lisuride. Brain Res. 1986;377:112–118. doi: 10.1016/0006-8993(86)91196-0. [DOI] [PubMed] [Google Scholar]
- Frank JL, Hendricks SE, Olson CH. Multiple ejaculations and chronic fluoxetine: effects on male rat copulatory behavior. Pharmacol. Biochem. Behav. 2000;66:337–342. doi: 10.1016/s0091-3057(00)00191-x. [DOI] [PubMed] [Google Scholar]
- Friedman D, Crews D. Role of the anterior hypothalamus-preoptic area in the regulation of courtship behavior in the male Canadian red-sided garter snake (Thamnophis sirtalis parietalis): intracranial implantation experiments. Horm. Behav. 1985;19:122–136. doi: 10.1016/0018-506x(85)90013-3. [DOI] [PubMed] [Google Scholar]
- Glaser JH, Rubin BS, Barfield RJ. Onset of the receptive and proceptive components of feminine sexual behavior in rats following the intravenous administration of progesterone. Horm. Behav. 1983;17:18–27. doi: 10.1016/0018-506x(83)90012-0. [DOI] [PubMed] [Google Scholar]
- Gorzalka BB, Mendelson SD, Watson NV. Serotonin receptor subtypes and sexual behavior. Ann. N. Y. Acad. Sci. 1990;600:435–444. doi: 10.1111/j.1749-6632.1990.tb16900.x. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Simon LD, Auerbach SB. Differences in hypothalamic serotonin between estrous phases and gender: an in vivo microdialysis study. Brain Res. 1998;785:91–96. doi: 10.1016/s0006-8993(97)01391-7. [DOI] [PubMed] [Google Scholar]
- Hull EM, Lorrain DS, Du J, Matuszewich L, Lumley LA, Putnam SK, Moses J. Hormone–neurotransmitter interactions in the control of sexual behavior. Behav. Brain Res. 1999;105:105–116. doi: 10.1016/s0166-4328(99)00086-8. [DOI] [PubMed] [Google Scholar]
- Hull EM, Meisel R, Sachs B. Male sexual behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Horm. Brain Behav. Vol. 1. Academic Press; New York: 2002. pp. 3–137. [Google Scholar]
- Johnston PG, Davidson JM. Priming action of estrogen: minimum duration of exposure for feedback and behavioral effects. Neuroendocrinology. 1979;28:155–159. doi: 10.1159/000122857. [DOI] [PubMed] [Google Scholar]
- Kingston PA, Crews D. Role of the effects of hypothalamic lesions on courtship and copulatory behavior in sexual and unisexual whiptail lizards. Brain Res. 1994;643:349–351. doi: 10.1016/0006-8993(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Koe BK, Weissman A. p-Chlorophenylalanine: a specific depletor of brain serotonin. J. Pharmacol. Exp. Ther. 1966;154:499–516. [PubMed] [Google Scholar]
- Lindzey J, Crews D. Hormonal control of courtship and copulatory behavior in male Cnemidophorus inornatus, a direct sexual ancestor of a unisexual, parthenogenetic lizard. Gen. Comp. Endocrinol. 1986;64:411–418. doi: 10.1016/0016-6480(86)90077-8. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Riolo JV, Matuszewich L, Hull EM. Lateral hypothalamic serotonin inhibits nucleus accumbens dopamine: implications for sexual satiety. J. Neurosci. 1999;19:7648–7652. doi: 10.1523/JNEUROSCI.19-17-07648.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick H, Manchanda SK, Kumar VM. beta-adrenergic modulation of male sexual behavior elicited from the medial preoptic area in rats. Behav. Brain Res. 1996;74:181–187. doi: 10.1016/0166-4328(95)00168-9. [DOI] [PubMed] [Google Scholar]
- Rand MS, Crews D. The bisexual brain: sex behavior differences and sex differences in parthenogenetic and sexual lizards. Brain Res. 1994;663:163–167. doi: 10.1016/0006-8993(94)90474-x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sierra JF, Naggar AN, Komisaruk BR. Monoaminergic mediation of masculine and feminine copulatory behavior in female rats. Pharmacol. Biochem. Behav. 1976;5:457–463. doi: 10.1016/0091-3057(76)90110-6. [DOI] [PubMed] [Google Scholar]
- Sakata JT, Gupta A, Gonzalez-Lima F, Crews D. Heterosexual housing increases the retention of courtship behavior following castration and elevates metabolic capacity in limbic brain nuclei in male whiptail lizards, Cnemidophorus inornatus. Horm. Behav. 2002;42:263–273. doi: 10.1006/hbeh.2002.1829. [DOI] [PubMed] [Google Scholar]
- Shioda K, Nisijima K, Yoshino T, Kato S. Extracellular serotonin, dopamine and glutamate levels are elevated in the hypothalamus in a serotonin syndrome animal model induced by tranylcypromine and fluoxetine. Prog. Neuro-psychopharmacol. Biol. Psychiatry. 2004;28:633–640. doi: 10.1016/j.pnpbp.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Smeets WJ, Steinbusch HW. Distribution of serotonin immunoreactivity in the forebrain and midbrain of the lizard Gekko gecko. J. Comp. Neurol. 1988;271:419–434. doi: 10.1002/cne.902710309. [DOI] [PubMed] [Google Scholar]
- Sodersten P, Larsson K. Sexual behavior in castrated male rats treated with monoamine synthesis inhibitors and testosterone. Pharmacol. Biochem. Behav. 1976;5:319–327. doi: 10.1016/0091-3057(76)90084-8. [DOI] [PubMed] [Google Scholar]
- Sodersten P, Larsson K, Ahlenius S, Engel J. Stimulation of mounting behavior but not lordosis behavior in ovariectomized female rats by p-chlorophenylalanine. Pharmacol. Biochem. Behav. 1976;5:329–333. doi: 10.1016/0091-3057(76)90085-x. [DOI] [PubMed] [Google Scholar]
- Summers CH. Mechanisms for quick and variable responses. Brain Behav. Evol. 2001;57:283–292. doi: 10.1159/000047246. [DOI] [PubMed] [Google Scholar]
- Tagliamonte A, Tagliamonte P, Gessa GL, Brodie BB. Compulsive sexual activity induced by p-chlorophenylalanine in normal and pine-alectomized male rats. Science. 1969;166:1433–1435. doi: 10.1126/science.166.3911.1433. [DOI] [PubMed] [Google Scholar]
- van de Poll NE, van Dis H, Bermond B. The induction of mounting behavior in female rats by p-chlorophenylalanine. Eur. J. Pharmacol. 1977;41:225–229. doi: 10.1016/0014-2999(77)90214-x. [DOI] [PubMed] [Google Scholar]
- Verma S, Chhina GS, Mohan Kumar V, Singh B. Inhibition of male sexual behavior by serotonin application in the medial preoptic area. Physiol. Behav. 1989;46:327–330. doi: 10.1016/0031-9384(89)90275-8. [DOI] [PubMed] [Google Scholar]
- Wade J, Crews D. The relationship between reproductive state and “sexually” dimorphic brain areas in sexually reproducing and parthenogenetic whiptail lizards. J. Comp. Neurol. 1991;309:507–514. doi: 10.1002/cne.903090407. [DOI] [PubMed] [Google Scholar]
- Woolley SC, Crews D. Species differences in the regulation of tyrosine hydroxylase in Cnemidophorus whiptail lizards. J. Neurobiol. 2004;60:360–368. doi: 10.1002/neu.20044. [DOI] [PubMed] [Google Scholar]
- Woolley SC, Sakata JT, Gupta A, Crews D. Evolutionary changes in dopaminergic modulation of courtship behavior in Cnemidophorus whiptail lizards. Horm. Behav. 2001;40:483–489. doi: 10.1006/hbeh.2001.1713. [DOI] [PubMed] [Google Scholar]
- Young LJ, Crews D. Comparative neuroendocrinology of steroid receptor gene expression and regulation: relationship to physiology and behavior. Trends Endocrinol. Metab. 1995;6:317–323. doi: 10.1016/1043-2760(95)00175-1. [DOI] [PubMed] [Google Scholar]
- Young LJ, Lopreato GF, Horan K, Crews D. Cloning and in situ hybridization analysis of estrogen receptor, progesterone receptor, and androgen receptor expression in the brain of whiptail lizards (Cnemido- and C. inornatus) J. Comp. Neurol. 1994;347:288–300. doi: 10.1002/cne.903470210. [DOI] [PubMed] [Google Scholar]