Abstract
The role of the anti-apoptotic protein Bcl-2 in lung cancer remains controversial. In order to clarify its impact on survival in small and non-small cell lung cancer (NSCLC), we performed a systematic review of the literature. Trials were selected for further analysis if they provided an independent assessment of Bcl-2 in lung cancer and reported analysis of survival data according to Bcl-2 status. To make it possible to aggregate survival results of the published studies, their methodology was assessed using a quality scale designed by the European Lung Cancer Working Party (including study design, laboratory methods and analysis). Of 28 studies, 11 identified Bcl-2 expression as a favourable prognostic factor and three linked it with poor prognosis; 14 trials were not significant. No differences in scoring measurement were detected between the studies, except that significantly higher scores were found in the trials with the largest sample sizes. Assessments of methodology and of laboratory technique were made independently of the conclusion of the trials. A total of 25 trials, comprising 3370 patients, provided sufficient information for the meta-analysis. The studies were categorised according to histology, disease stage and laboratory technique. The combined hazard ratio (HR) suggested that a positive Bcl-2 status has a favourable impact on survival: 0.70 (95% confidence interval 0.57–0.86) in seven studies on stages I–II NSCLC; 0.50 (0.39–0.65) in eight studies on surgically resected NSCLC; 0.91 (0.76–1.10) in six studies on any stage NSCLC; 0.57 (0.41–0.78) in five studies on squamous cell cancer; 0.75 (0.61–0.93) and 0.71 (0.61–0.83) respectively for five studies detecting Bcl-2 by immunohistochemistry with Ab clone 100 and for 13 studies assessing Bcl-2 with Ab clone 124; 0.92 (0.73–1.16) for four studies on small cell lung cancer; 1.26 (0.58–2.72) for three studies on neuroendocrine tumours. In NSCLC, Bcl-2 expression was associated with a better prognosis. The data on Bcl-2 expression in small cell lung cancer were insufficient to assess its prognostic value.
Keywords: Bcl-2, meta-analysis, survival, non-small cell lung cancer, small cell lung cancer, systematic review
Lung cancer is the most common cause of cancer death in industrialised countries and its incidence is steadily increasing in women. Despite diagnostic and therapeutic improvements, the overall 5-year survival is still less than 15% (Laudanski et al, 1999).
A prognostic factor is a variable measured in individual patients that, alone or in combination with other factors, explains part of the population heterogeneity, and is at the time of diagnosis able to provide information on clinical outcome (Yip and Harper, 2000).
Some independent prognostic factors have been identified in order to predict survival and to help in the management of patients with lung cancer (Paesmans and Sculier, 1998). They include, for small cell lung cancer (SCLC), extent of disease and performance status (Paesmans et al, 2000), for resectable non-small cell lung cancer (NSCLC) performance status, TNM stage and age (Strauss, 1997); for advanced NSCLC, performance status, TNM staging, age, sex and weight loss (Buccheri and Ferrigno, 1994; Paesmans et al, 1995).
It has previously been reported that biological factors, angiogenesis (measurements of number of vessels per mm2), or factors reflecting proliferative state (number of cells in cycle) have a significant impact on survival in NSCLC (Kanters et al, 1995; O'Byrne et al, 2000). Unfortunately, these are relatively crude measures of the biological aggressiveness of the primary cancer because they involve several metabolic pathways.
Analysis and characterisation of proteins and genes involved in cancer development at the molecular level, could add to our knowledge of potential prognostic factors. These factors can be divided into categories according to their biological pathway: tumour suppressor genes, proto-oncogenes, markers of metastatic propensity, and proliferation markers (Strauss et al, 1995). Recent publications have attempted to correlate survival with factors related to angiogenesis (basic fibroblast growth factor, thrombospondin, vascular endothelial growth factor), to apoptosis (Bcl-2, p53), to control cell cycle (cyclins, MDM2, retinoblastoma gene), to growth (epithelial growth factor, erb-B2) and some other factors (serum lactate dehydrogenase (LDH), serum CYFRA21 level, white blood cell count and DNA aneuploidy content). The literature assessing their effects on survival (Strauss et al, 1995; Brambilla et al, 1996; Pujol, 1997; Kim et al, 1998; Kwiatkowski et al, 1998; D'Amico et al, 1999; Choma et al, 2001) remains controversial.
The Bcl-2 gene was originally discovered in a follicular B-cell lymphoma, where a chromosomal translocation t(14:18) moves the Bcl-2 gene into juxtaposition with transcriptional enhancer elements of the immunoglobulin heavy chain locus. (Tsujimoto and Croce, 1986; Aisemberg et al, 1988). In contrast, transregulatory mechanisms appear to be responsible for the high levels of Bcl-2 protein production that occur in many different solid tumours such as prostate cancer (Colombel et al, 2000), breast cancer (Silvestrini et al, 1994) and lung cancer (Pezzella et al, 1993; Fontanini et al, 1995). The Bcl-2 proto-oncogene is encoded by a 230 kb gene. Its product, a 26 kDa protein, is located in the inner mitochondrial membrane, and to a lesser extent in cell membranes (Jong et al, 1994). The major function of Bcl-2 appears to be to inhibit programmed cell death (apoptosis) and to prolong cell survival by arresting cells in the G0/G1 phase of the cell cycle. The ratio of death antagonists (Bcl-2, Bcl-XL, Bcl-W, Mcl-1, A1) to agonists (Bax, Bak, Bcl-Xs, Bad, Bid) determines whether a cell will respond to an apoptotic signal. This death–life rheostat is mediated at least in part, by competitive dimerisations between selective pairs of antagonists and agonists (Kroemer, 1997). It is not clear from the data currently available as to which dimers are true regulators of apoptosis. Moreover, the possibility that at least some dimers form part of a regulatory higher-order, multiprotein complex cannot be excluded. The Bcl-2 protein is expressed in foetal tissues and basal cells of human epithelia, which suggests a role in normal growth regulation and differentiation (Hockenbery et al, 1990; Le Brun et al, 1993).
Although there are now a large number of studies of Bcl-2 expression, their value in predicting the survival of patients with lung cancer remains controversial. We have performed this systematic review of the literature to assess the prognostic value of Bcl-2 overexpression for the survival of lung cancer patients.
MATERIALS AND METHODS
Publication selection
To be eligible for inclusion in this systematic review, a study must have been published as a full paper in the English or French language literature and must meet the following criteria: deal with lung cancer only; analyse patients survival according to Bcl-2 status; measure Bcl-2 expression (protein, DNA or RNA) in the primary tumour (not in metastatic tissue or in tissue adjacent to the tumour) and/or antibodies against Bcl-2 in the serum.
An electronic search on Medline, using the keywords ‘lung neoplasms’ and ‘Bcl-2’, complemented by the personal bibliography of the authors, was used to select the articles. In addition, the bibliographies of studies already identified were used to complete trials identification. Studies published after December 1999 were not included.
Where the same author reported results obtained on the same patient population in several publications, only the most recent report, or the most complete one, was included in the analysis, in order to avoid overlap between cohorts.
Methodological assessment
To assess methodology, 13 investigators (10 physicians, one pathologist, one biostatistician and one biologist) read each publication independently, and scored them according to the ELCWP scoring scale. The scoring system used in this literature review was used for a systematic review of the prognostic value of p53 on survival in lung cancer and has been previously reported (Steels et al, 2001).
Each item was assessed using an ordinal scale (possible values 2, 1, 0). The scores were compared and a consensus value for each item was reached in meetings attended by at least two thirds of the investigators. The participation of many readers was intended to facilitate correct interpretation of the articles.
The score evaluates a number of aspects of methodology, grouped into four main categories: scientific design, the description of laboratory methods used to identify the presence of Bcl-2 (protein, DNA/RNA or antibodies against Bcl-2), generalisability of results and the analysis of the study data. Each category had a maximum score of 10 points, giving a theoretical total maximum score of 40 points. The final scores were expressed as percentages, ranging from 0 to 100%, higher values reflecting better quality methodology. This allowed the value of ‘not applicable’ items to be discounted from the theoretical total of the relevant category.
Statistical methods
A study was considered as significant if the P-value for the statistical test, comparing the survival distributions between the groups with and without Bcl-2 expression, was <0.05 in favour of this latter group. A study was classed as ‘positive’ when Bcl-2 expression was identified as an univariate indicator of good prognosis for survival. Other situations, were called ‘negative’, including the situation where a significant survival difference was found and the group of patients who were Bcl-2-positive fared worse.
The association between score measurements or between a score measurement treated as a continuous variable and another continuous variable was measured by the Spearman rank correlation coefficient. Its significance was assessed by testing a null hypothesis of equality to zero for this coefficient. The comparison between score measurement according to the value of a discrete variable was made by nonparametric Mann–Whitney (for dichotomic variables) or Kruskal–Wallis (for nominal variables with multiple classes) tests.
For the quantitative aggregation of the survival results, we measured the impact of Bcl-2 positivity on survival by hazard ratio (HR) between the survival distributions of the two Bcl-2 groups. For each trial, this HR was estimated by a method that depended on the results provided in the publication. The most accurate method was to retrieve the HR estimate and its variance from the reported results, or to calculate them directly using parameters given by the authors for the univariate analysis: the O−E statistic (difference between numbers of observed and expected events), the confidence interval for the HR, the log-rank statistic or its P-value. If these were not available, we looked for the total number of events, the number of patients at risk in each group and the log-rank statistic or its P-value, allowing calculation of an approximation of the HR estimate. Finally, if the only useful data were in the form of graphical representations of the survival distributions, we extracted from them survival rates at specified times in order to reconstruct the HR estimate and its variance, with the assumption that during the study follow-up the number patients counted was constant (Parmar et al, 1998). If authors reported survival of three or more groups (e.g., using several cutoff values for percentage of protein present in the cytoplasm, or regarding the exons of DNA separately), we pooled the results in order to make a comparison between two groups feasible.
Global survival of the entire patient population was analysed, when available. If not, the results of subgroups were treated separately. If survival was reported separately for particular subgroups, these results were treated in the meta-analysis of the corresponding subgroups. The same patients were never considered more than once in each analysis. The individual HR estimates were combined into an overall HR using the method published by Peto (Yusuf et al, 1985). By convention, an HR<1 implied a better survival for the group with positive Bcl-2. This impact of Bcl-2 on survival was considered as statistically significant if the 95% confidence interval (CI) for the overall HR did not overlap 1.
For the subgroups where heterogeneity was detected by χ2 tests for heterogeneity, a calculation of the overall effect using a random-effects model was also included.
The studies eligible for the systematic review were called ‘eligible’ and those providing data for meta-analysis ‘evaluable’.
RESULTS
Studies selection and characteristics
A total of 29 trials, published between 1993 and 1999, were selected (Pezzella et al, 1993; Fontanini et al, 1995, 1996; Walker et al, 1995; Brambilla et al, 1996; Kaiser et al, 1996; Ohsaki et al, 1996; O'Neill et al, 1996; Rao et al, 1996; Takayama et al, 1996; Anton et al, 1997; Apolinario et al, 1997; Higashiyama et al, 1997; Ishida et al, 1997; Koukourakis et al, 1997; Pastorino et al, 1997; Greatens et al, 1998; Kim et al, 1998; Kwiatkowski et al, 1998; Chen et al, 1999; D'Amico et al, 1999; Dingemans et al, 1999; Dosaka-Akita et al, 1999; Eerola et al, 1999; Ghosh et al, 1999; Huang et al, 1999; Laudanski et al, 1999; Maitra et al, 1999; Santinelli et al, 1999). They all report on the prognostic value for survival of Bcl-2 status in lung cancer patients, assessing Bcl-2 protein expression in the primary tumour. One study was excluded because an identical patient cohort was used in another selected publication (references excluded/included: (Fontanini et al, 1996)/(Fontanini et al, 1995)).
The main features of the 28 studies eligible for the systematic review are shown in Table 1 . A total of 21 trials looked at NSCLC, while SCLC and neuroendocrine tumours were studied in four and three trials respectively. Non-small cell lung cancer trials included either all histological subtypes (n=17), or adenocarcinoma (n=2) or squamous cell cancer (n=2). Data related to patients treated by surgery (stages I–IIIB) comprised eight of the 21 NSCLC trials. Six of the 21 NSCLC studies were performed in locoregional disease (stages I–II), while seven were dealt with any stage (stages I–IV).
Table 1. Main characteristics and results of the eligible studies.
|
NSCLC |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
All studies |
Any stage |
Locoregional (I–II) |
Surgical treatment (I–III) |
SCLC |
Neuroendocrine tumours |
|||||||
| Total | S | Total | S | Total | S | Total | S | Total | S | Total | S | |
| Number of studies | 28 (25) | 11 (11) | 7 (6) | 2 (2) | 6 (5) | 2 (2) | 8 (7) | 6 (6) | 4 (4) | 0 | 3 (3) | 1 (1) |
NSCLC=non-small cell lung cancer; SCLC=small cell lung cancer; S=number of studies identifying Bcl-2 positivity as a statistically significant good prognostic factor; ( )=number of studies evaluable for meta-analysis.
Immunohistochemistry techniques (IHC) were used in all the trials to detect the expression of Bcl-2 protein. Various antibodies were used to assess Bcl-2 expression. The two clones most used were clones 100 and 124, in 25% (seven out of 28) and 71% (20 out of 28) of the studies respectively.
Three of the 28 trials eligible for the systematic review reported insufficient data for the HR to be evaluable for the quantitative aggregation. The reasons for not including studies in the meta-analysis were as follows: no survival curve shown (n=1) (Kwiatkowski et al, 1998); no P-value, HR or CI reported (n=1) (Greatens et al, 1998); no proportion of Bcl-2 positive (n=1) (Greatens et al, 1998; Dosaka-Akita et al, 1999).
Studies results report
As shown in Table 1, 11 of the 28 studies (39.3%) identified Bcl-2 expression as a good prognostic factor for survival (all evaluable for meta-analysis), 14 (50%) concluded that Bcl-2 was not a prognostic factor for survival (11 evaluable) and three (10.7%) linked Bcl-2 expression with poor prognosis (three evaluable,).
Of the 21 published NSCLC trials, 11 (57.1%) were positive. All of these studies were evaluable for meta-analysis. None of the four studies dealing with SCLC reported significant results. One of the three concerning neuroendocrine tumours was significant.
Evaluability status for the meta-analysis was associated with trial positivity: the rate of positive results was 44% for evaluable trials (11 out of 25) compared to 0% (zero out of three) for nonevaluable ones (P=0.26).
Quality assessment
Overall, the global quality assessment score, expressed as a percentage, ranged between 32.9 and 79.1%, with a median of 54.6% (Table 2A where only the median values are shown). The design subscore had the lowest values. The most poorly described items (<30% of the maximum) were the a priori estimate of sample size required to conduct the study, the outcome definition, the double-blinding evaluation of the biological marker, the reproducibility control test between the experimenters and the initial disease work-up description.
Table 2. Methodological assessment by ELCWP score, according to trials characteristics: (A) all trials and (B) evaluable trials for meta-analysis.
| Global score (%) | Design (/10) | Laboratory methodology (/10) | Generalisability (/10) | Results analysis (/10) | ||
|---|---|---|---|---|---|---|
| (A) All trials | ||||||
| Total (n=28) | All studies | 54.6 | 4.0 | 6.1 | 6.7 | 5.0 |
| Patient number P-value | 0.0015 | 0.03 | 0.03 | 0.03 | 0.008 | |
| Evaluable for the MA (n=25) | 54.6 | 4.0 | 6.4 | 6.7 | 5.0 | |
| Not evaluable for the MA (n=3) | 54.2 | 5.0 | 7.8 | 6.7 | 5.0 | |
| P-value | 0.53 | 0.28 | 0.17 | 0.91 | 0.50 | |
| Positive (n=11) | 51.5 | 4.0 | 5.7 | 6.6 | 5.0 | |
| Negative (n=17) | 54.6 | 5.0 | 7.14 | 6.6 | 5.0 | |
| P-value | 0.27 | 0.07 | 0.44 | 0.48 | 0.94 | |
| IHC Ab clone 100 (n=5) | 59.4 | 4.0 | 5.0 | 6.7 | 7.5 | |
| IHC Ab clone 124 (n=21) | 52.1 | 4.0 | 6.1 | 6.7 | 5.0 | |
| P-value | 0.25 | 0.81 | 0.54 | 0.54 | 0.08 | |
| (B) Evaluable trials for meta-analysis | ||||||
| Evaluable for the MA (n=25) | All studies | 54.6 | 4.0 | 6.4 | 6.7 | 5.0 |
| Patient number P-value | 0.004 | 0.09 | 0.03 | 0.045 | 0.01 | |
| Positive (n=11) | 51.5 | 4.0 | 5.7 | 6.7 | 5.0 | |
| Negative (n=14) | 57.0 | 5.0 | 6.8 | 7.1 | 5.6 | |
| P-value | 0.35 | 0.01 | 0.64 | 0.53 | 0.80 | |
| IHC Ab clone 100 (n=5) | 59.4 | 4.0 | 5.0 | 6.7 | 7.5 | |
| IHC Ab clone 124 (n=18) | 50.3 | 4.0 | 5.7 | 6.7 | 5.0 | |
| P-value | 0.10 | 0.07 | 0.37 | 0.07 | 1.0 | |
Score distributions are summarised by median values. Positive=studies identifying Bcl-2 positivity as significant good prognostic factor for survival; negative=studies reporting nonsignificant results, or associating Bcl-2 positivity with poor survival; MA=meta-analysis; IHC=immunohistochemistry. The values in bold were significant.
A weak but significant correlation between the global score and the number of patients included in the study was observed (Spearman's correlation coefficient r=0.56, P=0.0015).
No statistically significant difference was found between the 25 evaluable and the three nonevaluable studies either for the global score (median 54.6% in comparison to 54.2%, P=0.53 by the Mann–Whitney test), or for the four subgroups scores.
There was also no statistically significant difference between the global scores of 11 positive trials and the 17 negative trials (median 51.5% in comparison to 54.6%, P=0.27 by Mann–Whitney test), nor for their four subscores.
The score difference between the studies classified according to the types of monoclonal antibody used was not significant. The overall median score was respectively 59.4 and 52.1% when clone 100 or clone 124 antibodies were used (P=0.25 by Mann–Whitney test).
Table 2B describes the scores for the 25 trials classified as evaluable for meta-analysis. Their overall quality score ranged between 32.9 and 79.1%, with a median of 53.9%. There was a significant correlation between the global score and the number of patients included in the study (Spearman's correlation coefficient r=0.55, P=0.004). The scores of the four subgroups matched those of the 28 studies, with the design subscore again being the worse reported. The most poorly described items (<30% of maximal score) were the a priori estimate of sample size required to conduct the study, the outcome definition, the double-blinding evaluation, the reproducibility control test between the experimenters, the initial disease work-up description and the number of unassessable samples, with the reason for their exclusion. There was no significant difference between positive and negative trials in their global score with a median of 51.5 and 57.0% respectively for the positive and the negative studies (P=0.35).
The type of monoclonal antibody did not affect the overall quality assessment, which had a median global score of 59.4% for clone 100 and of 50.3% for clone 124 (P=0.10).
Meta-analysis
The absence of any significant qualitative difference between positive and negative trials allowed us to perform a quantitative aggregation of the survival data. However, only subgroup analysis could be performed due to the heterogeneity of the trials: the trials authors had reported on patients with different histological subtypes (NSCLC, SCLC or neuroendocrine tumours); stages (localised, locoregional or extensive); or treatments. The subgroups were defined according to histology, extent of the disease, technique used to detect Bcl-2 (IHC with the two most frequently used monoclonal antibodies clone 124 and 100) and the threshold used to determine Bcl-2 positivity.
The hazard ratios were retrieved by one of the three methods reported in the Materials and methods section. Only four studies reported the data necessary to estimate the HR directly. In eight trials, the HR was approximated using the total number of events and the log-rank statistic or its P-value. For the 13 remaining studies, the HR was extrapolated from the graphical representations of the estimated survival distributions.
In all, 28 eligible trials analysed overall survival in relation to Bcl-2 expression in 3829 patients. Three trials were excluded and thus the analysis was restricted to 3370 patients (88%).
Overall, Bcl-2 protein was expressed in 39% of the lung tumours studied: 71% in SCLC, 55% in neuroendocrine tumours and 35% in NSCLC. In the NSCLC group, 32% of the squamous cell cancer and 61% of the adenocarcinoma expressed Bcl-2. Bcl-2 expression was found in 23, 37 and 50% respectively for the subgroups of patients with stage I–II, surgically treated stage I–III, and any stage disease.
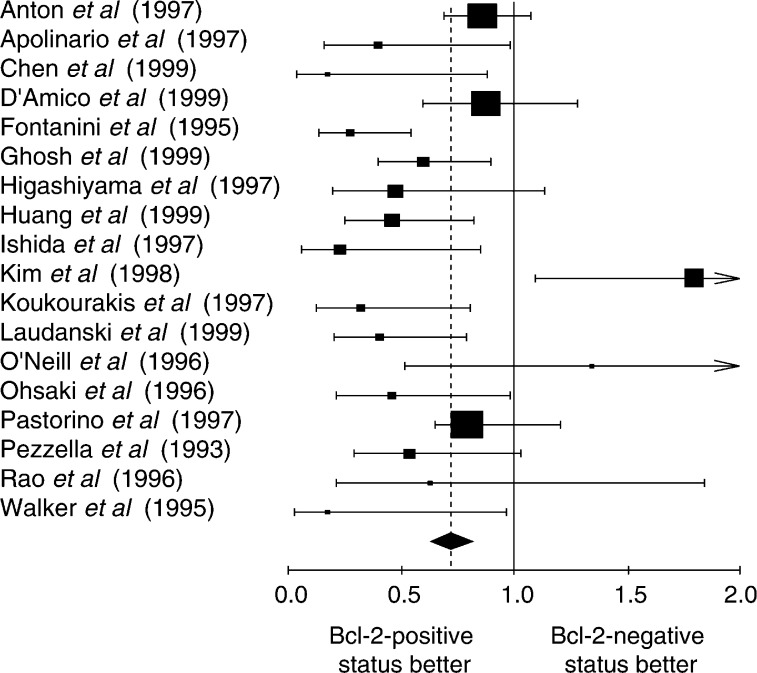
The NSCLC subgroup included 18 trials comprising 2909 patients. The aggregated survival data showed a good survival prognosis where there was Bcl-2 positivity (HR=0.72; 95% CI 0.64–0.82).
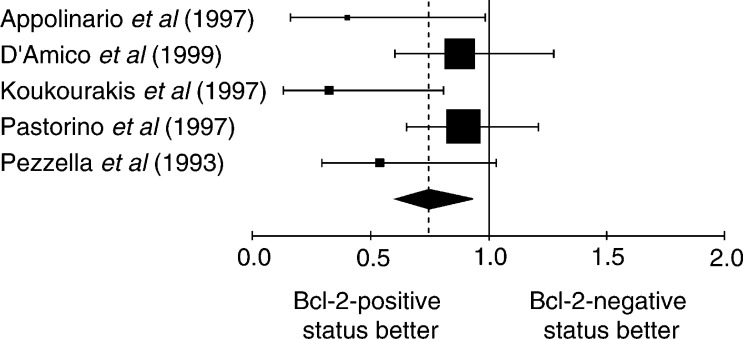
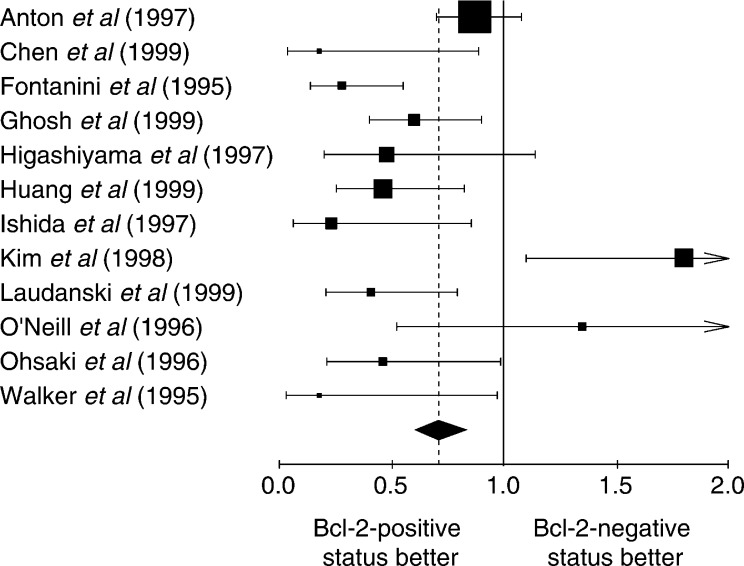
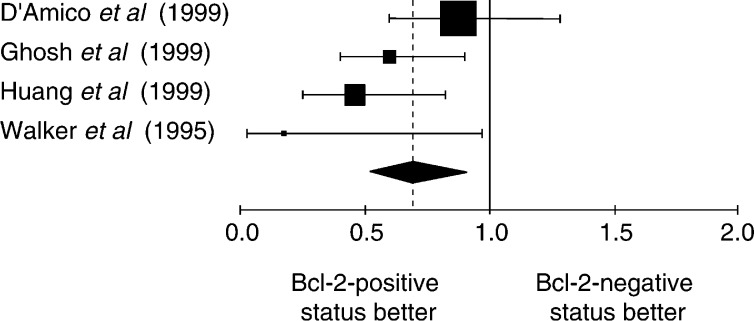
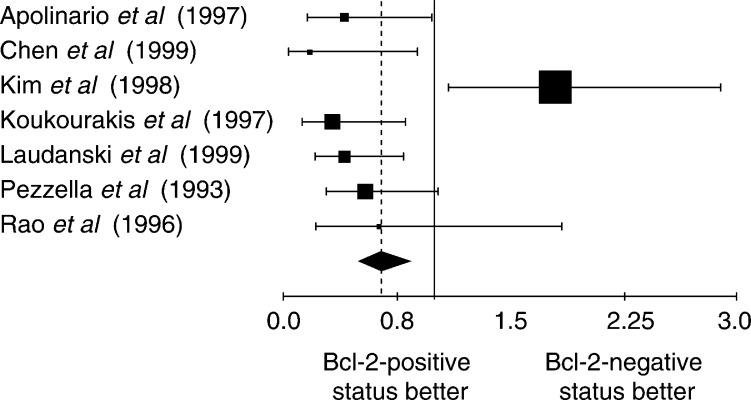
Stages I and II NSCLC subgroup included eight trials comprising 1311 patients. The aggregation produced a statistically significant HR of 0.70 (95% CI 0.57–0.86) (Table 3 ). The result of the test for heterogeneity was not significant (P=0.08), but it was not possible to go further in categorising the trials, and to treat separately papers reporting on stage I patients. The use of a random-effects model did not change the conclusion, with a combined HR of 0.59 (95% CI 0.42–0.83). The surgically treated NSCLC (NSCLC completely removed by surgery for stages I–IIIB), with seven out of eight trials evaluable, showed, a significant HR of 0.50 (95% CI 0.39–0.65) (Table 4 ). Once again, the introduction of a random effect did not change the interpretation of the HR (HR 0.50; 95% CI 0.33–0.77). The subgroup of studies including any stage NSCLC had an HR of 0.91 (95% CI 0.76–1.10) (Table 5 ).
Table 3. Meta-analysis of the subgroup including studies of stages I and II NSCLC with their characteristics.
|
Group of NSCLC: stages I–II (n=8) | |||||||
|---|---|---|---|---|---|---|---|
| Study | Method | Threshold | QS (%) | N Pts | Bcl-2+ (%) | HR | 95% CI |
| Apolinario et al (1997) | IHC-clone 100 | NM | 59 | 73 | 51 | 0.40 | 0.16–0.98 |
| Chen et al (1999) | IHC-clone 124 | NM | 33 | 40 | 43 | 0.18 | 0.04–0.88 |
| D'Amico et al (1999) | IHC-clone 120 | >50 | 72 | 408 | 23 | 0.88 | 0.60–1.28 |
| Higashiyama et al (1997) | IHC-clone 124 | >10 | 52 | 38 | 39 | 0.30 | 0.06–1.62 |
| Koukourakis et al (1997) | IHC-clone 100 | NM | 58 | 107 | 19 | 0.32 | 0.13–0.86 |
| Ohsaki et al (1996) | IHC-clone 124 | >20 | 50 | 45 | 29 | 0.45 | 0.13–1.56 |
| Pastorino et al (1997) | IHC-clone 120 | >10 | 76 | 485 | 17 | 0.89 | 0.65–1.21 |
| Pezzella et al (1993) | IHC-clone 100 | NM | 65 | 115 | 22 | 0.54 | 0.29–1.03 |
| Overall (fixed-effects model) | 1311 | 23 | 0.70 | 0.57–0.86 | |||
| Overall (random-effects model) | 0.59 | 0.42–0.83 | |||||
| χ2 statistic for heterogeneity=12.80, 7 df, P=0.08 | |||||||
IHC-clone 100=immunohistochemistry with monoclonal antibody 100; IHC-clone 124=immunohistochemistry with monoclonal antibody 124; QS=Median quality score; N pts=number of patients; Bcl-2+=presence of Bcl-2; df=degree of freedom; HR=hazard ratio; CI=confidence interval; NM=not clearly mentioned.
Table 4. Meta-analysis of the subgroup including studies performed in NSCLC treated by surgery, with their characteristics.
|
Group of NSCLC: surgical stages (n=7) | |||||||
|---|---|---|---|---|---|---|---|
| Study | Method | Threshold | QS (%) | N Pts | Bcl-2+ (%) | HR | 95% CI |
| Fontanini et al (1995, 1996) | IHC-clone 124 | >1 | 45 | 89 | 66 | 0.28 | 0.14–0.55 |
| Ghosh et al (1999) | IHC-clone 124 | >50 | 41 | 134 | 31 | 0.60 | 0.40–0.90 |
| Higashiyama et al (1997) | IHC-clone 124 | >10 | 52 | 174 | 21 | 0.47 | 0.20–1.14 |
| Huang et al (1999) | IHC-clone 124 | >50 | 70 | 203 | 39 | 0.46 | 0.26–0.82 |
| Ishida et al (1997) | IHC-clone 124 | >10 | 64 | 114 | 38 | 0.23 | 0.06–0.86 |
| Kim et al (1998) | IHC-clone 124 | NM | 79 | NM | NM | 2.50 | 0.90–7.1 |
| Laudanski et al (1999) | IHC-clone 124 | NM | 68 | 84 | 46 | 0.41 | 0.21–0.79 |
| Overall (fixed-effects model) | 798 | 37 | 0.50 | 0.39–0.65 | |||
| Overall (random-effects model) | 0.50 | 0.33–0.77 | |||||
| χ2 statistic for heterogeneity=14.98, 6 df, P=0.02 | |||||||
The meaning of the symbols is described in Table 3.
Table 5. Meta-analysis of the subgroup including studies performed in any stage of NSCLC, with their characteristics.
|
Group of NSCLC: all stages (n=6) | |||||||
|---|---|---|---|---|---|---|---|
| Study | Method | Threshold | QS (%) | N Pts | Bcl-2+ (%) | HR | 95% CI |
| Anton et al (1997) | IHC-clone 124 | >10 | 49 | 427 | 47 | 0.87 | 0.70–1.07 |
| Kim et al (1998) | IHC-clone 124 | NM | 79 | 238 | 72 | 1.80 | 1.1–2.9 |
| O'Neill et al (1996) | IHC-clone 124 | >1 | 55 | 54 | 35 | 1.34 | 0.53–3.44 |
| Ohsaki et al (1996) | IHC-clone 124 | >20 | 50 | 96 | 17 | 0.46 | 0.22–0.98 |
| Rao et al (1996) | IHC-clone 124 | NM | 45 | 41 | 61 | 0.63 | 0.22–1.84 |
| Walker et al (1995) | IHC-clone 124 | >50 | 46 | 27 | 44 | 0.18 | 0.03–0.97 |
| Overall (fixed-effects model) | 883 | 50 | 0.91 | 0.76–1.10 | |||
| Overall (random-effects model) | 0.85 | 0.53–1.37 | |||||
| χ2 statistic for heterogeneity=15.34, 5 df, P=0.009 | |||||||
The meaning of the symbols is described in Table 3.
Five trials for squamous cell cancer were assessable (Table 6 ). Results were significantly in favour of Bcl-2 positivity with an HR of 0.57 (95% CI 0.41–0.78).
Table 6. Meta-analysis of the studies performed in squamous cell cancer, with their characteristics.
|
Group of squamous cell cancer (n=5) | |||||||
|---|---|---|---|---|---|---|---|
| Study | Method | Threshold | QS (%) | N Pts | Bcl-2+ (%) | HR | 95% CI |
| Chen et al (1999) | IHC-clone 124 | NM | 33 | 40 | 42 | 0.18 | 0.03–0.88 |
| Ghosh et al (1999) | IHC-clone 124 | >50 | 41 | 134 | 31 | 0.6 | 0.40–0.89 |
| Higashiyama et al (1997) | IHC-clone 124 | >10 | 52 | 67 | 31 | 0.32 | 0.08–1.25 |
| O'Neill et al (1996) | IHC-clone 124 | >1 | 55 | 54 | 35 | 1.34 | 0.52–3.44 |
| Pezzella et al (1993) | IHC-clone 100 | NM | 65 | 75 | 27 | 0.42 | 0.20–0.91 |
| Overall (fixed-effects model) | 370 | 32 | 0.57 | 0.41–0.78 | |||
| Overall (random-effects model) | 0.54 | 0.33–0.89 | |||||
| χ2 statistic for heterogeneity=6.67, 4 df; P=0.15 | |||||||
The meaning of the symbols is described in Table 3.
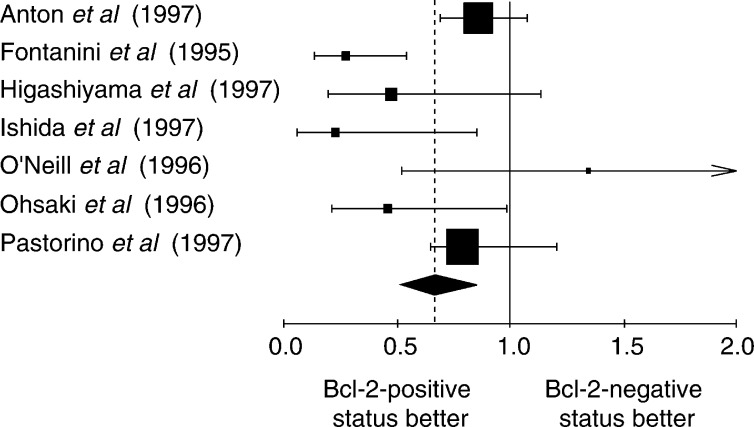
The NSCLC group was meta-analysed according two further criteria: the method used to detect Bcl-2 overexpression and the Bcl-2 positivity threshold. The aggregated results are shown in Figures 1, 2, 3, 4, 5 and 6. The individual data from these studies have been reported in Tables 3, 4, 5 and 6. Firstly, the monoclonal antibodies clones used according to HR for the studies assessing Bcl-2 with antibody clone 100 and clone 124 were respectively 0.75 (95% CI 0.61–0.93) and 0.71 (95% CI 0.61–0.83). Secondly, the studies were divided into four groups according to the definition of the threshold for Bcl-2 positivity: global group, threshold from 1 to 20%, threshold up to 50% and threshold not clearly described. HR, calculated by a fixed-effect model, were respectively 0.73 (95% CI 0.64–0.82), 0.77 (95% CI 0.66–0.91), 0.65 (95% CI 0.51–0.83) and 0.62 (95% CI 0.52–0.91).
Figure 1.
Hazard ratio (HR) and 95% CI of mortality in studies evaluating Bcl-2 status by IHC with Ab 100. χ2 statistic for heterogeneity=8.14, 4 df, P=0.09. NB: HR<1 implies a survival benefit for the group with positive Bcl-2. The square size is proportional to the number of patients included in the study. The centre of the lozenge gives the combined HR of the meta-analysis and its extremities the 95% CI.
Figure 2.
Hazard ratio and 95% CI of mortality in studies evaluating Bcl-2 status by IHC with Ab 124. The meaning of the symbols is described in Figure 1.
Figure 3.
Hazard ratio and 95% CI of mortality in studies incorporating NSCLC whatever the threshold of positivity chosen by the authors. The meaning of the symbols is described in Figure 1.
Figure 4.
Results of the meta-analysis for the subgroup of studies where a tumour was considered as expressing Bcl-2 if 1–20% of the cells were positive for Bcl-2. The meaning of the symbols is described in Figure 1.
Figure 5.
Results of the meta-analysis for the subgroup of studies where a tumour was considered as expressing Bcl-2 if 21–50% of the cells were positive for Bcl-2. The meaning of the symbols is described in Figure 1.
Figure 6.
Results of the meta-analysis for the subgroup of studies where a tumour was considered as expressing Bcl-2 if the percentage of the cells was not clearly mentioned positive for Bcl-2. The meaning of the symbols is described in Figure 1.
In the SCLC subgroup, four studies (all reported as negative) comprised together 317 patients. The aggregation produced an HR of 0.92 (95% CI 0.73–1.16) (Table 7 ).
Table 7. Meta-analysis of the studies performed in small cell lung cancer, with their characteristics.
|
Group of SCLC (n=4) | |||||||
|---|---|---|---|---|---|---|---|
| Study | Method | Threshold | QS (%) | N Pts | Bcl-2+ (%) | HR | 95% CI |
| Dingemans et al (1999) | IHC-clone 100 | >10 | 67 | 91 | 78 | 1.55 | 0.93–2.58 |
| Kaiser et al (1996) | IHC | >50 | 63 | 146 | 75 | 0.76 | 0.55–1.04 |
| Maitra et al (1999) | IHC-clone 100 | >10 | 46 | 42 | 57 | 0.68 | 0.36–1.27 |
| Takayama et al (1996) | IHC-clone 124 | >10 | 55 | 38 | 55 | 1.31 | 0.64–2.70 |
| Overall (fixed-effects model) | 317 | 71 | 0.92 | 0.73–1.16 | |||
| Overall (random-effects model) | 0.99 | 0.66–1.48 | |||||
| χ2 statistic for heterogeneity=7.35, 3 df, P=0.06 | |||||||
The meaning of the symbols is described in Table 3. IHC=Immunohistochemistry with other antibodies than clone 100 and 124.
For the three studies dealing with neuroendocrine tumours (86 patients), the aggregation produced an HR of 1.26 (95% CI 0.58–2.72) (Table 8 ).
Table 8. Meta-analysis of the studies performed in neuroendocrine tumoral lung cancer, with their characteristics.
|
Group of neuroendocrine tumoral lung cancer (n=3) | |||||||
|---|---|---|---|---|---|---|---|
| Study | Method | Threshold | QS (%) | N Pts | Bcl-2+ (%) | HR | 95% CI |
| Brambilla et al (1996) | IHC-clone 124 | >50 | 41 | 43 | 56 | 11.48 | 1.34–98.01 |
| Eerola et al (1999) | IHC-clone 124 | NM | 45 | 20 | 18 | 0.44 | 0.13–1.43 |
| Santinelli et al (1999) | IHC-clone 124 | NM | 49 | 23 | 61 | 1.81 | 0.57–5.77 |
| Overall (fixed-effects model) | 86 | 55 | 1.26 | 0.58–2.72 | |||
| Overall (random-effects model) | 1.71 | 0.35–8.41 | |||||
| χ2 statistic for heterogeneity=6.79, 2 df, P=0.03 | |||||||
The meaning of the symbols is described in Table 3.
DISCUSSION
Our systematic review of the literature shows that overexpression of the Bcl-2 protein is a good prognostic factor for survival in patients with NSCLC. The analysis reveals similar features in different subgroups of localised NSCLC and clarifies the message of individual studies that are somewhat inconsistent.
The decision to perform the meta-analysis was based on a prior methodological assessment of the publications. We have used a methodology similar to previous systematic reviews reported by our group on the treatment of lung cancer (Luce et al, 1998; Sculier et al, 1998; Meert et al, 1999; Mascaux et al, 2000) after an adaptation to biological prognostic factors such as p53 (Steels et al, 2001). By comparing the scores of the studies where Bcl-2 was a significant prognostic factor and those where it was not, we could identify differences, suggesting biases induced by trial methodology. Nevertheless, our approach does not eliminate all potential biases.
First, we have to consider publication bias. Our review took into account only fully published studies. We did not look for unpublished trials and abstracts because the methodology we used required data that are usually only available in full publications. Meta-analysis based on data on individuals is considered by some authors as the gold standard (Stewart and Parmar, 1993). Systematic reviews of the literature and meta-analyses of individual patient data should not be confused. The first approach is based only on fully published studies and provides an exhaustive and critical analysis of the topic with an adequate methodology based on the criteria of Mulrow (1987) and with data aggregation (meta-analysis) when possible. The second approach is, in fact, a new study taking in all trials performed on the topic, whether published or not. It requires that the investigators update individual data. In the latter case, publications are used mainly for identification purposes. In prophylactic cranial irradiation, our meta-analysis (Meert et al, 2001), based on the published data, yielded the same results for patients in complete remission as Aupérin et al (1999) showed in their individual data meta-analysis. This supports the validity of our approach. Our review deals with studies of prognostic factors and, as they are most often retrospective, it is much more difficult to identify unpublished data than it is with clinical trial data. Furthermore, we were not able to include all the papers identified in the meta-analysis due to under-reported results, which occurred more often in papers where an effect of Bcl-2 on survival was not shown.
The comparison of the score of the two groups (positive and negative trials) showed no statistically significant difference, allowing a meaningful data aggregation. The three studies excluded from the meta-analysis due to a lack of reported data were all negative. There is, thus, a potential bias in favour of positive trials. It should, however, be stressed that results were significantly better reported in the positive studies than in the negative ones. Indeed, studies with no statistically significant results are less often published or, if they are published, it is with more concise reports of results, meaning that they are more often unassessable. Moreover, there is a language bias. We have restricted our review to articles published in English and French, because all our readers did not know other languages such as Japanese or German. This bias could favour the positive studies that are more often published in English, while the negative ones are more often reported in native languages (Egger et al, 1997).
Another potential source of bias is related to the method for extrapolating the HR. If they were not reported by the authors, HR were calculated from the data available in the article and, if that was not possible, they were extrapolated from the survival curves, which involves making assumptions. Moreover, there is no consensus over the choice of time intervals for reading survival rates on the curves. Finally, we would emphasise that a global meta-analysis did not appear meaningful because of the heterogeneity of the patient populations. The patient population of the studies available is very heterogeneous, often they were restricted to patients with a specific histological subtype or a selected tumour stage. For this reason, we did not perform a global analysis and instead focused our analysis on more homogeneous subgroups of patients by aggregating data from studies conducted in similar patient populations or on similar tumours. When using a random-effects models, we came to the same conclusions as we did with fixed-effects models. However, such models do not identify the source of the heterogeneity, itself an important clinical point. It was not possible, on the basis of published data, to adjust our results in a multivariate analysis.
Our results are based on an aggregation of data obtained by univariate survival analysis in retrospective trials. The results need to be confirmed by an adequately designed prospective study and the exact value at which Bcl-2 should be considered ‘overexpressed’ determined by an appropriate multivariate analysis taking into account the classical well-defined prognostic factors for lung cancer. A meta-analysis based on the individual data of the patients included in studies (Stewart and Parmar, 1993) would help to define by multivariate methods the prognostic role of Bcl-2, but it would require the collection of a huge amount of retrospective data, with the potential problem of dealing with a lot of missing data. But such a study could never have the equivalent value of a well-designed prospective study (Cappelleri et al, 1996).
Another possible source of confusion is the use of same cohort of patients for different publications (Fontanini et al, 1996). If the same patients are included twice or more in a meta-analysis, it may give a higher weighting to these studies. In the systematic review, we have excluded the studies for which it was possible to identify with certainty that similar patients cohorts had been used in different publications (Fontanini et al, 1995). On the other hand, when the data in the publication did not allow us to decide if the same cohort of patients was being investigated (Pezzella et al, 1993; Koukourakis et al, 1997), we have assumed that the authors have been sufficiently honest not to re-report the results from the same cohort of patients without making this clear in the paper.
Finally, for practical purposes, and because of their small number, we have included in the negative group the three trials that showed that of the presence of Bcl-2 had a significant negative effect on survival.
The techniques used to identify overexpression of Bcl-2 status can also be a potential source of bias. The IHC used to reveal the Bcl-2 protein is not always performed with the same antibody. Sometimes the protocol was performed without prior reaction of epitope unmasking on fixed issue (Cattoretti et al, 1993). To try to exclude technical biases, we performed subgroup analysis according to the most frequently used methods: IHC with antibody clone 100 and clone 124 (Figure 1 and Figure 2). In both cases, the results were consistent with a favourable survival in the case of Bcl-2 overexpression, making it improbable that the techniques were a source of bias. Moreover, the cutoff in the number of positive cells defining a tumour with Bcl-2 overexpression is often arbitrary and varies according to the investigators, from a few percent to 50%. The use of different cutoff points for IHC is of critical importance, as was shown by Lee et al (1995). Some investigators selected the cutoff point based on the minimum P-value approach, which can lead to seriously biased conclusions (Altman et al, 1994). If a chosen cutoff is often arbitrary, selection according to the median value of expression levels provides a more standardised approach to prognostic factors, although it may lead to some loss of information (Altman et al, 1994). An optimal threshold still needs to be defined for Bcl-2.
It should be noted that the four eligible studies reporting on SCLC and two of the three studies concerning neuroendocrine tumours were negative. In fact, it is very difficult to draw a definite conclusion because of the small number of patients included in these trials. Consequently, further studies are necessary to determine the value of Bcl-2 as a prognostic factor for survival in SCLC and in neuroendocrine tumours.
In our systematic review with meta-analysis, patients with Bcl-2-positive tumours had significantly better survival than those with Bcl-2-negative tumours. The mechanism underlying the effect of Bcl-2 oncoprotein expression on tumour progression and prognosis remains essentially uncertain. Originally, the Bcl-2 gene product was implicated in oncogenesis because of its ability to prolong cell survival through the inhibition of apoptosis (Adams and Cory, 1998; Antonsson and Martinou, 2000). The process of apoptosis involves many proteins such as the antiapoptotic proteins (Bcl-2, Bcl-X, Bfl-1) and the proapoptotic proteins (Bax, Bak, Bad) (Kroemer, 1997). These proteins can interact in order to regulate cellular apoptosis by balancing pro- and antiapoptotic mechanisms. Thus, the study of only one apoptotic protein produces an incomplete appraisal of apoptosis and it would be interesting to conduct a survival analysis of a combination of these proteins. Moreover, the distribution of Bcl-2 protein observed in normal tissues and embryonic tissues indicates that it has a function in morphogenesis linked to cell proliferation via escape from cell death (Le Brun et al, 1993; Adams and Cory, 1998; Antonsson and Martinou, 2000). In NSCLC, Fontanini et al (1995) stated that Bcl-2 oncoprotein expression status was not correlated with proliferative potential indicators including PCNA and Ki-67. On the other hand, considering how rarely extrathoracic metastasis in NSCLC express Bcl-2, it could be proposed that this oncoprotein plays an inhibitory role in the haematogenous metastatic process through tumour progression. The question of whether Bcl-2 oncoprotein biologically participates in the haematogenous metastatic process and reduces the incidence of distant metastasis has still to be elucidated.
In conclusion, our systematic review of the lung cancer literature suggests that overexpression of Bcl-2, in patients with NSCLC has good prognostic value for survival, whatever the biological test used. This observation is potentially important. Identification of independent prognostic factors allows us to define high-risk patients for whom specific therapy may be designed or to introduce stratification in randomised trials. In lung cancer, the prognostic factors currently used are clinical variables such as performance status or disease extent. The results of our meta-analysis, which suggest a relation between Bcl-2 and survival, should encourage properly designed prospective studies, with an appropriate statistical methodology including multivariate analysis, in order to demonstrate the usefulness of molecular biological markers like Bcl-2, assessed by IHC.
Acknowledgments
B Martin received a fellowship of an FNRS-Télévie Grant (7.4512.98), Belgium.
References
- Adams JM, Cory S (1998) The Bcl-2 protein family: arbiters of cell survival. Science 281: 1322–1326 [DOI] [PubMed] [Google Scholar]
- Aisemberg AC, Wilkes DM, Jacobson JO (1988) The bcl-2 gene is rearranged in many diffuse B-cell lymphomas. Blood 71: 969–972 [PubMed] [Google Scholar]
- Altman DG, Lausen B, Sauerbrei W, Schumacher M (1994) Dangers of using ‘optimal’ cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst 86: 829–835 [DOI] [PubMed] [Google Scholar]
- Anton RC, Brown RW, Younes M, Gondo MM, Stephenson MA, Cagle PT (1997) Absence of prognostic significance of bcl-2 immunopositivity in non-small cell lung cancer: analysis of 427 cases. Hum Pathol 28: 1079–1082 [DOI] [PubMed] [Google Scholar]
- Antonsson B, Martinou J-C (2000) The Bcl-2 protein family. Exp Cell Res 256: 50–57 [DOI] [PubMed] [Google Scholar]
- Apolinario RM, Van Der Valk P, de Jong JS, Deville W, Ark-Otte J, Dingemans AM, Van Mourik JC, Postmus PE, Pinedo HM, Giaccone G (1997) Prognostic value of the expression of p53, bcl-2, and bax oncoproteins, and neovascularization in patients with radically resected non-small-cell lung cancer. J Clin Oncol 15: 2456–2466 [DOI] [PubMed] [Google Scholar]
- Aupérin A, Arriagada R, Pignon J-P, LePechoux C, Grégor A, Stephens RJ, Kristjansen PEG, Johnson BE, Ueoka H, Wagner H, Aisner J (1999) Prophylactic cranial irradiation for patient with small-cell lung cancer in complete remission. N Engl J Med 341: 476–483 [DOI] [PubMed] [Google Scholar]
- Brambilla E, Negoescu A, Gazzeri S, Lantuejoul S, Moro D, Brambilla C, Coll JL (1996) Apoptosis-related factors p53, bcl-2, and bax in neuroendocrine tumors. Am J Pathol 149: 1941–1952 [PMC free article] [PubMed] [Google Scholar]
- Buccheri G, Ferrigno D (1994) Prognostic factors in lung cancer: tables and comments. Eur Respir J 7: 1350–1364 [DOI] [PubMed] [Google Scholar]
- Cappelleri J, Loannidis J, Schmid C, de Ferranti S, Aubert M, Chalmers T, Lau J (1996) Large trials versus meta-analysis of smaller trials. JAMA 276: 1332–1338 [PubMed] [Google Scholar]
- Cattoretti G, Pileri S, Parravicini C, Becker MH, Poggi S, Bifulco C, Key G, D'amato L, Sabattini E, Feudale E (1993) Antigen unmasking on formalin-fixed paraffin-embedded tissue sections. J Pathol 171: 83–98 [DOI] [PubMed] [Google Scholar]
- Chen Y, Sato M, Fujimura S, Endo C, Sakurada A, Aikawa H, Takahashi H, Tanita T, Kondo T, Saito Y, Sagawa M (1999) Expression of Bcl-2, Bax, and p53 proteins in carcinogenesis of squamous cell lung cancer. Anticancer Res 19: 1351–1356 [PubMed] [Google Scholar]
- Choma D, Daures JP, Quantin X, Pujol JL (2001) Aneuploidy and prognosis of non-small cell lung cancer: a meta-analysis of published data. Br J Cancer 85: 14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombel M, Symmans F, Gil S, O'Toole KM, Chopin D, Benson M, Olsson CA, Korsmeyer S, Buttyan R (2000) Detection of the apoptosis-suppressing oncoprotein bcl-2 in hormone-refractory human prostate cancers. Am J Pathol 143: 390–400 [PMC free article] [PubMed] [Google Scholar]
- D'Amico TA, Massey M, Herndon JE, Moore MB, Harpole Jr DH (1999) A biologic risk model for stage I lung cancer: immunohistochemical analysis of 408 patients with the use of ten molecular markers. J Thorac Cardiovasc Surg 117: 736–743 [DOI] [PubMed] [Google Scholar]
- Dingemans AM, Witlox MA, Stallaert RA, van der Valk P, Postmus PE, Giaccone G (1999) Expression of DNA topoisomerase IIalpha and topoisomerase IIbeta genes predicts survival and response to chemotherapy in patients with small cell lung cancer. Clin Cancer Res 5: 2048–2058 [PubMed] [Google Scholar]
- Dosaka-Akita H, Katabami M, Hommura H, Fujioka Y, Katoh H, Kawakami Y (1999) Bcl-2 expression in non-small cell lung cancers: higher frequency of expression in squamous cell carcinomas with earlier pT status. Oncology 56: 259–264 [DOI] [PubMed] [Google Scholar]
- Eerola AK, Ruokolainen H, Soini Y, Raunio H, Paakko P (1999) Accelerated apoptosis and low bcl-2 expression associated with neuroendocrine differentiation predict shortened survival in operated large cell carcinoma of the lung. Pathol Oncol Res 5: 179–186 [DOI] [PubMed] [Google Scholar]
- Egger M, Zellweger-Zahner T, Schneider M, Junker C, Lengeler C, Antes G (1997) Language bias in randomised controlled trials published in English and German. Lancet 350: 326–329 [DOI] [PubMed] [Google Scholar]
- Fontanini G, Vignati S, Bigini D, Mussi A, Lucchi M, Angeletti CA, Basolo F, Bevilacqua G (1995) Bcl-2 protein: a prognostic factor inversely correlated to p53 in non-small-cell lung cancer. Br J Cancer 71: 1003–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanini G, Vignati S, Bigini D, Mussi A, Lucchi M, Chine S, Angeletti CA, Bevilacqua G (1996) Recurrence and death in non-small cell lung carcinomas: a prognostic model using pathological parameters, microvessel count, and gene protein products. Clin Cancer Res 2: 1067–1075 [PubMed] [Google Scholar]
- Ghosh M, Crocker J, Morris AG (1999) CD40 and bcl-2 expression in squamous cell carcinoma of the lung: correlation with apoptosis, survival, and other clinicopathological factors. J Pathol 189: 363–367 [DOI] [PubMed] [Google Scholar]
- Greatens TM, Niehans GA, Rubins JB, Jessurun J, Kratzke RA, Maddaus MA, Niewoehner DE (1998) Do molecular markers predict survival in non-small-cell lung cancer? Am J Respir Crit Care Med 157: 1093–1097 [DOI] [PubMed] [Google Scholar]
- Higashiyama M, Doi O, Kodama K, Yokouchi H, Nakamori S, Tateishi R (1997) bcl-2 Oncoprotein in surgically resected non-small cell lung cancer: possibly favorable prognostic factor in association with low incidence of distant metastasis. J Surg Oncol 64: 48–54 [DOI] [PubMed] [Google Scholar]
- Hockenbery D, Numez G, Milliman C, Schreiber RD, Korsmeyer S (1990) Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 348: 334. [DOI] [PubMed] [Google Scholar]
- Huang C, Kohno N, Inufusa H, Kodama K, Taki T, Miyake M (1999) Overexpression of bax associated with mutations in the loop–sheet–helix motif of p53. Am J Pathol 155: 955–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida H, Irie K, Itoh T, Furukawa T, Tokunaga O (1997) The prognostic significance of p53 and bcl-2 expression in lung adenocarcinoma and its correlation with Ki-67 growth fraction. Cancer 80: 1034–1045 [PubMed] [Google Scholar]
- Jong D, Prins FA, Masson DY, Reed JC, Van Ommen GB, Kluin PM (1994) Subcellular localization of the bcl-2 protein in malignant and normal lymphoid cells. Cancer Res 54: 256–260 [PubMed] [Google Scholar]
- Kaiser U, Schilli M, Haag U, Neumann K, Kreipe H, Kogan E, Havemann K (1996) Expression of bcl-2 protein in small cell lung cancer. Lung Cancer 15: 31–40 [DOI] [PubMed] [Google Scholar]
- Kanters SD, Lammers JW, Voest EE (1995) Molecular and biological factores in the prognosis of non-small cell lung cancer. Eur Respir J 8: 1389–1397 [DOI] [PubMed] [Google Scholar]
- Kim YC, Park KO, Kern JA, Park CS, Lim SC, Jang AS, Yang JB (1998) The interactive effect of Ras, HER2, p53 and Bcl-2 expression in predicting the survival of non-small cell lung cancer patients. Lung Cancer 22: 181–190 [DOI] [PubMed] [Google Scholar]
- Koukourakis MI, Giatromanolaki A, O'Byrne KJ, Whitehouse RM, Talbot DC, Gatter KC, Harris AL (1997) Potential role of bcl-2 as a suppressor of tumour angiogenesis in non-small-cell lung cancer. Int J Cancer 74: 565–570 [DOI] [PubMed] [Google Scholar]
- Kroemer G (1997) The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med 3: 614–620 [DOI] [PubMed] [Google Scholar]
- Kwiatkowski DJ, Harpole Jr DH, Godleski J, Herndon JE, Shieh DB, Richards W, Blanco R, Xu HJ, Strauss GM, Sugarbaker DJ (1998) Molecular pathologic substaging in 244 stage I non-small cell lung cancer patients: clinical implications. J Clin Oncol 16: 2468–2477 [DOI] [PubMed] [Google Scholar]
- Laudanski J, Chyczewski L, Niklinska WE, Kretowska M, Furman M, Sawicki B, Niklinski J (1999) Expression of bcl-2 protein in non-small cell lung cancer: correlation with clinicopathology and patient survival. Neoplasma 46: 25–30 [PubMed] [Google Scholar]
- Le Brun DP, Warnke RA, Cleary ML (1993) Expression of bcl-2 in fetal tissues suggests a role in morphogenesis. Am J Pathol 142: 743. [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Yoon A, Kalapurakal SK, Ro JY, Lee JJ, Tu M, Hittelman WN, Hong WK (1995) Expression of p53 oncoprotein in non-small cell lung cancer: a favorable pronostic factor. J Clin Oncol 13: 1893–1903 [DOI] [PubMed] [Google Scholar]
- Luce S, Paesmans M, Berghmans T, Castaigne C, Sotiriou C, Vermylen P, Sculier J-P (1998) Revue critique des études randomisées évaluant le rôle de la radiothérapie dans le traitement du cancer bronchique à petites cellules au stade limité. Rev Mal Respir 15: 633–641 [PubMed] [Google Scholar]
- Maitra A, Amirkhan RH, Saboorian MH, Frawley WH, Ashfaq R (1999) Survival in small cell lung carcinoma is independent of Bcl-2 expression. Hum Pathol 30: 712–717 [DOI] [PubMed] [Google Scholar]
- Mascaux C, Paesmans M, Berghmans T, Branle F, Lafitte J-J, Lemaitre F, Meert A-P, Vermylen P, Sculier J-P (2000) A systematic review of the role of etoposide and cisplatin in the chemotherapy of small cell lung cancer with methodology assessment and meta-analysis. Lung Cancer 30: 23–36 [DOI] [PubMed] [Google Scholar]
- Meert A-P, Berghmans T, Branle F, Lemaître F, Mascaux C, Rubesova E, Vermylen P, Paesmans M, Sculier J-P (1999) Phase II et III with new drugs for non-small cell lung cancer: a systematic review of the literature with a methodology quality assessment. Anticancer Res 19: 4379–4390 [PubMed] [Google Scholar]
- Meert A-P, Paesmans M, Berghmans T, Martin B, Mascaux C, Vallot F, Verdebout J-M, Lafitte J-J, Sculier J-P (2001) Prophylactic cranial irradiation in small cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer 1: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulrow CD (1987) The medical review article: state of the Science. Ann Intern Med 106: 485–488 [DOI] [PubMed] [Google Scholar]
- O'Byrne KJ, Koukourakis MI, Giatromanolaki A, Cox G, Turley H, Steward WP, Gatter K, Harris AL (2000) Vascular endothelial growth factor, platelet-derived endothelial cell growth factor and angiogenesis in non-small cell lung cancer. Br J Cancer 82: 1427–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill AJ, Staunton MJ, Gaffney EF (1996) Apoptosis occurs independently of bcl-2 and p53 over-expression in non-small cell lung carcinoma. Histopathology 29: 45–50 [DOI] [PubMed] [Google Scholar]
- Ohsaki Y, Toyoshima E, Fujiuchi S, Matsui H, Hirata S, Miyokawa N, Kubo Y, Kikuchi K (1996) Bcl-2 and p53 protein expression in non-small cell lung cancers: correlation with survival time. Clin Cancer Res 2: 915–920 [PubMed] [Google Scholar]
- Paesmans M, Sculier J-P, Lecomtre J, Thiriaux J, Libert P, Sergysels R, Bureau G, Dabouis G, Van Custem O, Mommen P, Ninane V, Klastersky J, for the European Lung Cancer Working Party (2000) Prognostic factors in patients with small cell lung cancer: analysis of a series of 763 patients included in four consecutive prospective trials and with a minimal 5-year follow-up duration. Cancer 89: 523–533 [DOI] [PubMed] [Google Scholar]
- Paesmans M, Sculier J-P, Libert P, Bureau G, Dabouis G, Thiriaux J, Michel J, Van Custem O, Sergysels R, Mommen P (1995) Prognostic factors for survival in advanced non-small cell lung cancer: univariate and multivariate analyses including recursive partitioning and amalgamation algorithms in 1052 patients. J Clin Oncol 13: 1221–1230 [DOI] [PubMed] [Google Scholar]
- Paesmans M, Sculier J-P (1998) Facteurs pronostiques des cancers bronchopulmonaires. In Cancer Broncho-pulmonaires, Milleron B, Depierre A (eds) pp 239–247. Arnette F-78141 Velizy-Villacoublay, France [Google Scholar]
- Parmar, Mahesh KB, Torri V, Stewart L (1998) Extracting summary statistics to perfom meta-analyses of the published literature for survical endpoinds. Statist Med 17: 2815–2834 [DOI] [PubMed] [Google Scholar]
- Pastorino U, Andreola S, Tagliabue E, Pezzella F, Incarbone M, Sozzi G, Buyse M, Menard S, Pierotti M, Rilke F (1997) Immunocytochemical markers in stage I lung cancer: relevance to prgnosis. J Clin Oncol 15: 2858–2865 [DOI] [PubMed] [Google Scholar]
- Pezzella F, Turley H, Kuzu I, Tungekar MF, Dunnill MS, Pierce CB, Harris A, Gatter KC, Mason DY (1993) bcl-2 Protein in non-small-cell lung carcinoma [see comments]. N Engl J Med 329: 690–694 [DOI] [PubMed] [Google Scholar]
- Pujol JL (1997) Cyfra 21-1. Rev Mal Respir 14: 3s31–3s36 [PubMed] [Google Scholar]
- Rao SK, Krishna M, Woda BA, Savas L, Fraire AE (1996) Immunohistochemical detection of bcl-2 protein in adenocarcinoma and non-neoplastic cellular compartments of the lung. Mod Pathol 9: 555–559 [PubMed] [Google Scholar]
- Santinelli A, Ranaldi R, Baccarini M, Mannello B, Bearzi I (1999) Ploidy, proliferative activity, p53 and bcl-2 expression in bronchopulmonary carcinoids: relationship with prognosis. Pathol Res Pract 195: 467–474 [DOI] [PubMed] [Google Scholar]
- Sculier J-P, Berghmans T, Castaigne C, Luce S, Sotiriou C, Vermylen P, Paesmans M (1998) Maintenance chemotherapy for small cell lung cancer: a critical review of the literature. Lung Cancer 19: 141–151 [DOI] [PubMed] [Google Scholar]
- Silvestrini R, Veneroni S, Daidone MG, Benini E, Boracchi P, Mezzetti M, Di Fronzo G, Rilke F, Veronesi U (1994) The bcl-2: a prognostic indicator strongly related to p53 protein in lymph node-negative breast cancer patients. J Natl Cancer Inst 86: 499–504 [DOI] [PubMed] [Google Scholar]
- Steels E, Paesmans M, Berghmans T, Branle F, Burniat A, Ghisdal L, Lafitte J-J, Lemaitre F, Mascaux C, Meert A-P, Vallot F, Sculier J-P (2001) Role of p53 on the survival of patients with lung cancer as assessed by a systematic review of the literature. Eur Respir J 18: 705–719 [DOI] [PubMed] [Google Scholar]
- Stewart A, Parmar K (1993) Meta-analysis of the literature or of individual patient data l is there a difference? Lancet 341: 418–422 [DOI] [PubMed] [Google Scholar]
- Strauss GM (1997) Prognostic markers in resectable non-small cell lung cancer. Hematol Oncol Clin North Am 11: 409–434 [DOI] [PubMed] [Google Scholar]
- Strauss GM, Kwiatkowski DJ, Harpole DH, Lynch TJ, Skarin AT, Sugarbaker DJ (1995) Molecular and pathologic analysis of stade I non-small cell lung carcinoma of the lung. J Clin Oncol 13: 1265–1279 [DOI] [PubMed] [Google Scholar]
- Takayama K, Ogata K, Nakanishi Y, Yatsunami J, Kawasaki M, Hara N (1996) Bcl-2 expression as a predictor of chemosensitivities and survival in small cell lung cancer. Cancer J Sci Am 2: 212–216 [PubMed] [Google Scholar]
- Tsujimoto Y, Croce CM (1986) Analysis of the structure, transcripts and protein products of Bcl-2 the gene involved in human follicular lymphoma. Proc Natl Acad Sci USA 83: 5214–5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C, Robertson L, Myskow M, Dixon G (1995) Expression of the Bcl-2 protein in normal and dysplastic bronchial epithelium and in lung carcinomas. Br J Cancer 72: 164–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip D, Harper PG (2000) Predictive and prognostic factors in small cell lung cancer: current status. Lung Cancer 28: 173–185 [DOI] [PubMed] [Google Scholar]
- Yusuf S, Peto R, Lewis J, Collins R, Sleight P (1985) Blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis 27: 335–371 [DOI] [PubMed] [Google Scholar]