Abstract
The activity of ZD 1839 on brain metastases (BM) from Non-Small-Cell Lung Cancer (NSCLC) is unknown. We report four cases of BM responding to ZD 1839 theraphy.
Keywords: tyrosine kinase inhibitor, ZD 1839, non-small-cell lung cancer
ZD 1839 (gefitinib, Iressa; AstraZeneca Pharmaceuticals, Wilmington, DE, USA) is an orally active, selective epidermal growth factor receptor (EGFR) tyrosine-kinase inhibitor (TKI), which demonstrated antitumour activity in vitro and in vivo (Ciardiello et al, 2000). In pretreated non-small-cell lung cancer (NSCLC) patients, phase I and II studies demonstrated that ZD 1839 is active and well tolerated, with a response rate of about 10–15% (Kris et al, 2000,2002; Fukuoka et al, 2002). The presence of brain metastases (BM) has been considered as an exclusion criterion in all trials conducted so far (Kris et al, 2000,2002; Fukuoka et al, 2002; Giaccone et al, 2002; Johnson et al, 2002). Therefore, the activity of ZD 1839 on BM is unknown. Since January 2001, we participated in the ZD 1839 compassionate use programme, in which pretreated fit NSCLC patients were candidates to receive ZD 1839 at 250 mg daily dose. Compassionate use programme criteria do not exclude patients with BM. Here, we report four cases of BM from NSCLC patients responding to ZD 1839 therapy.
Patients were 53, 54, 55, and 65 years old, two male and two female patients. Histology was adenocarcinoma (two cases), squamous cell carcinoma (one case) and bronchiolo-alveolar carcinoma (one case). All patients developed BM in the presence of extracranial disease. All patients had received a first-line platinum-based chemotherapy, and two patients were treated with whole-brain radiotherapy (WBRT) for BM terminated at least 3 months before starting ZD 1839, with evidence of brain disease progression when therapy with ZD 1839 was started. In the other two patients, ZD 1839 was begun for appearance of asymptomatic BM and progressive disease on the extracranial sites. After 3 months of ZD 1839 therapy, all the patients had a partial response both in the brain and in the extracranial sites (Figure 1). At the time of this analysis, two patients discontinued the treatment after 8 and 15 months for disease progression, while two patients are still on treatment with no evidence of treatment failure after 6+ and 11+ months. ZD 1839 therapy was generally well tolerated, with skin toxicity recorded in three patients (two patients grade 1 and one patient grade 2). All four patients experienced symptomatic improvement while on treatment.
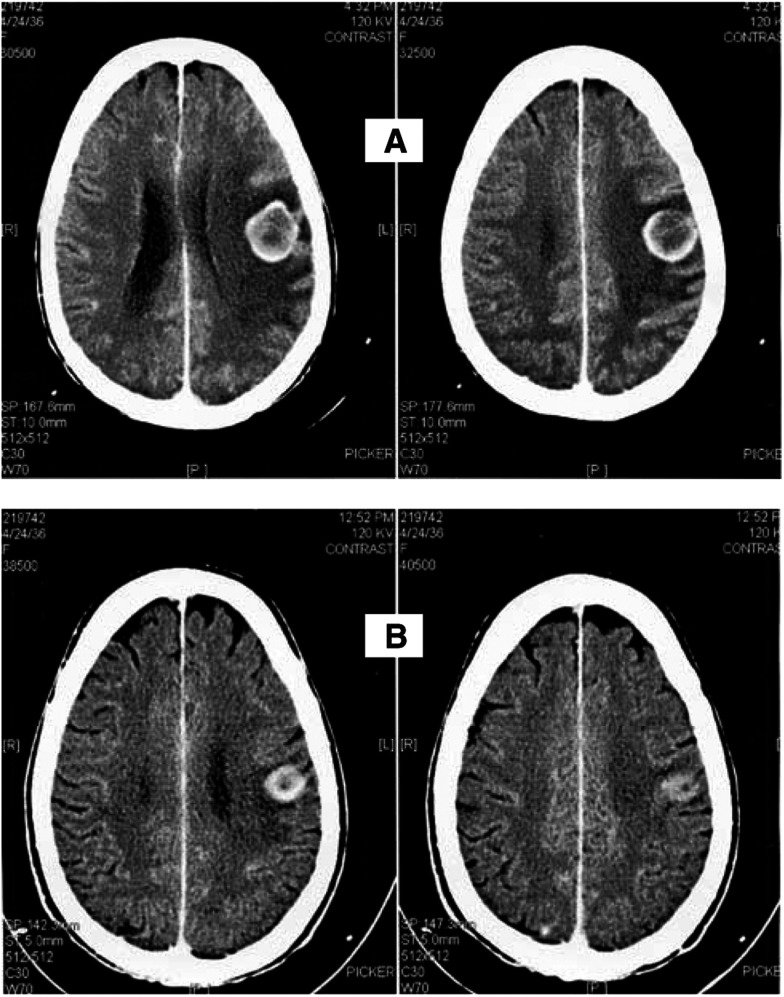
Figure 1.
Case 4: Brain CT-scan at baseline (A) and after 3 months of ZD1839 therapy (B). Brain metastasis from NSCLC responding to ZD 1839 therapy. This patient has been pretreated with three lines of chemotherapy including platinum and taxanes, and received ZD 1839 after whole-brain radiotherapy failure.
This report suggests that ZD 1839 is effective in NSCLC patients with pretreated BM, but further trials are needed to better evaluate the role of this compound in these patients. Based on our results, the presence of BM should not be considered an exclusion criterion in future TKI studies.
References
- Ciardiello F, Caputo R, Bianco R, Damiano V, Pomatico G, De Placido S, Bianco AR, Tortora G (2000) Antitumour effect and potentiation of cytotoxic drug activity in human cancer cells by ZD1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin Cancer Res 6: 2053–2063 [PubMed] [Google Scholar]
- Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Jean-Yves Douillard, Nishiwaki Y, Vansteenkiste JF, Kudo S, Averbuch S, Macleod A, Feyereislova A, José Baselga (2002) Final results from a phase II trial of ZD 1839 (Iressa) for patients with advanced non-small cell lung cancer (IDEAL 1). Proc Am Soc Clin Oncol 21: 298a (abstract 1188) [Google Scholar]
- Giaccone G, Johnson DH, Manegold C, Scagliotti GV, Rosell R, Wolf M, Renne P, Ochs J, Averbuch S, Fandi A (2002) A phase III clinical trial of ZD 1839 (Iressa) in combination with gemcitabine and cisplatin in chemotherapy-naive patients with advanced non-small-cell lung cancer (INTACT 1). Ann Oncol 13: 2 abstract 40 (vocal) [Google Scholar]
- Johnson DH, Herbst R, Giaccone G, Schiller J, Natale RB, Miller V, Wolf M, Helton A, Averbuch S, Grous J (2002) ZD 1839 (Iressa) in combination with paclitaxel & carboplatin in chemotherapy-naive patients with advanced non-small-cell lung cancer (NSCLC): results from a phase III clinical trial (INTACT 2). Ann Oncol 13: 127 abstract 4680 (vocal).12401678 [Google Scholar]
- Kris MG, Herbst R, Rischin D, LoRusso P, Baselga J, Hammond L, Feyereislova A, Ochs J, Averbuch S (2000) Objective regressions in non-small cell lung cancer patients treated in phase I trials of ZD1839 (‘Iressa’), a selective tyrosine kinase inhibitor that blocks the epidermal growth factor receptor (EGFR). Lung Cancer 29: 72 (Suppl 1, poster 233) . [Google Scholar]
- Kris MG, Natale RB, Herbst RS, Lynch TJ Jr., Prager D, Belani CP, Schiller JH, Kelly K, Spiridondis C, Kathy S Albain, Brahmer JR, Sandler A, Crawford J, Lutzker SG, Lilenbaum R, Helms L, Wolf M, Averbuch S, Ochs J, Kay A. (2002) A phase II trial of ZD 1839 (Iressa) in advanced non-small cell lung cancer (NSCLC) patients who had failed platinum- and docetaxel-based regimens (IDEAL 2). Proc Am Soc Clin Oncol 21: 292a (abstract 1166) [Google Scholar]