Abstract
Honeybees selected for the colony level phenotype of storing large quantities of pollen (pollen hoarding) in the nest exhibit greater walking activity than those selected against pollen hoarding. In this study, we use a simple walking assay to demonstrate that walking activity increases with the proportion of high pollen-hoarding alleles in pure and backcrossed strains of bees (high-strain bees > offspring generated from a high backcross > offspring generated from a low backcross > low-strain bees). The trait is heritable but is not associated with markers linked to three quantitative trait loci (QTL) mapped for their effects on pollen hoarding with demonstrated pleiotropic effects on pollen and nectar foraging and learning behavior. However, locomotion in non-selected bees is correlated with responsiveness to sucrose, a trait that correlates with foraging and learning behavior. We propose that pollen-hoarding behavior involves a syndrome of behavioral traits with complex genetic and regulatory architectures that span sensory sensitivity, foraging behavior, and learning. We propose that locomotor activity is the component of this syndrome and reflects the early maturation of the bees that become pollen foragers.
Keywords: Locomotion, Sucrose responsiveness, QTL, Pollen foraging syndrome
Introduction
A set of behavioral traits linked by pleiotrophic quantitative trait loci (QTLs) constitutes a syndrome associated with the storing of surplus pollen in colonies of Apis mellifera L. Pollen hoarding is a highly heritable trait (Hellmich et al. 1985; Page and Fondrk 1995). Bees from colonies with genotypes that result in more pollen hoarding tend to forage earlier in life (bees usually initiate foraging in about their third week of life; see Winston 1987), are more likely to collect loads of pollen, collect heavier pollen loads and smaller nectar loads, will collect nectar with lower concentrations of sucrose, and perform better in olfactory and tactile-associative learning assays (Calderone and Page 1988, 1991, 1992, 1996; Page and Fondrk 1995; Page et al. 1995, 1998, 2000; Hunt et al. 1995; Scheiner et al. 2001a, b).
Studies of unselected “wild-type” bees have repeatedly confirmed the covariance of these traits (Scheiner et al. 2004). Using the proboscis extension assay (Kuwabara 1957), Page et al. (1998) has showed that pollen-foraging wild-type bees are more responsive to sucrose than nectar foragers. Scheiner et al. (1999) demonstrated that pollen-foraging bees perform better on tactile-associative learning assays than do nectar foragers. The difference in learning performance was explained by differences in the perception of the value of the sucrose reward during conditioning. Perception of the reward is related to sucrose responsiveness. Pankiw and Page (2000) tested one-week old wild-type bees for their PER responses to sucrose and found a highly significant correlation between sucrose responsiveness and the behavior of the bees when they initiated foraging 2–3 weeks later. Bees that responded to the lowest concentrations were more likely to forage for water followed by pollen foragers, foragers that collected both pollen and nectar, and nectar foragers. Bees that were least responsive to sucrose were more likely to return empty from a foraging trip. Pankiw et al. (2004) independently confirmed these results with newly emerged bees.
Pankiw (2003) performed the definitive study linking all of the foraging behavior traits with sucrose responsiveness. She tested newly emerged Africanized and European honey-bees derived from colonies in Texas, USA. Newly emerged Africanized honeybees were more responsive to sucrose than were the European honeybees. Africanized bees foraged earlier in life, collected heavier loads of pollen, and collected nectar with a lower average concentration of sucrose. Within both the Africanized and European honeybee cohorts, she found a significant correlation between sucrose responsiveness and foraging behavior. As in the previous studies (Pankiw and Page 2000; Pankiw et al. 2004), newly emerged bees that were most responsive to low concentrations of sucrose tended to collect water, followed by pollen, pollen and nectar, and nectar, and foraged earlier in life. Again in both cohorts, the bees that were least responsive were more likely to return empty from a foraging trip.
During execution of the studies listed above, we noticed differences in the activities of newly emerged bees from the high and low pollen-hoarding strains. The standard procedure we use for acquiring newly emerged bees from the high and low pollen-hoarding strains is to brush newly emerged bees from combs that are maintained in an incubator into a shallow dishpan. Newly emerged bees are soft bodied and cannot fly out of the pans. It was very apparent to us that the bees in the pans with high-strain bees walked faster and were more distributed in the pan than the bees from the low strain that remained in clumps in the bottom. We had previously reported the activity differences related to foraging (Fewell and Page 2000) and recruitment (Waddington et al. 1998) behavior where bees from the high strain were more active. However, we have not attempted to link any of the activity differences to the other traits involved in the pollen-hoarding behavioral syndrome.
Based upon our observation that high-strain bees seem to be more advanced in their sensory and motor development than low-strain bees, we hypothesized (1) that high-strain bees would be more active walkers than low-strain bees and this difference would be heritable (Experiment 1). We also hypothesized (2) that this behavioral difference was linked into the pollen-hoarding syndrome and was a consequence of pleiotropy with the three pollen-hoarding quantitative trait loci mapped by Hunt et al. (1995) and Page et al. (2000) that have demonstrated pleiotropy with the foraging behavioral traits discussed above (Experiment 2). To validate the locomotor activity as a component of the pollen-hoarding behavioral syndrome, we hypothesized (3) that walking activity would correlate with sucrose responsiveness in newly emerged wild-type bees (Experiment 3).
Methods
Experiment 1: measuring locomotion in high and low pollen-hoarding strains of bees
Honeybee sources and rearing techniques
Bees were derived from the high and low pollen-hoarding strains of Page and Fondrk (1995) and their reciprocal backcrosses. For the backcross sources, low-strain queens were mated by instrumental insemination (Laidlaw 1977) to high-strain drones to produce F1 hybrid queens. Each of these hybrid queens was backcrossed to either a high (HBC)- or low (LBC)-strain drone. In combination, we were able to test the bees with 100 (high strain), 75 (high backcross), 25 (low backcross) and 0 percent of their genotypes derived from the high strain.
Three high- and three low-strain queens were caged on empty brood frames containing worker-sized cells and allowed to lay eggs for 15–24 h. The queens were then caged on the opposite side of their combs for an additional 15–24 h. After 48 h, the queens were released and all six frames containing eggs and newly hatched larvae were removed and placed into a single wild-type rearing colony to control for colony level variation in the brood-rearing environment. The host colony was a populous three-story Langstroth hive with adequate pollen and honey stores. Approximately 15–24 h prior to adult emergence (development from egg to adult takes 21 days), the six brood frames were removed from the rearing colony and placed in individual cages into a humidified incubator. High and low backcross bees were produced in the same way from three HBC and three LBC queens.
Locomotion assay
Workers from the high and low strains were tested together followed by workers from the reciprocal backcrosses. A total of 76 high- and 79 low-strain bees were tested. Groups of 10–12 bees were collected from the frames immediately upon emergence and each placed into a plastic cup. The queen source (indicating genotype) of the bee was recorded on the bottom of the cup. The cups were then shuffled, so the observer was blind to the source of the bee being assayed and bees from each source were tested in an arbitrary order. Each bee was removed from her holding cup and placed into the center of a clean polystyrene Petri dish (14 cm diameter) which was placed upon a paper grid (1.5 cm2 pattern). For 2 min, the number of grid marks crossed by the bee was recorded with a manual hand-tally counter. Only grids fully crossed by the center of the bee’s thorax were recorded. Grid marks crossed twice due to a reversal in direction were not recounted. All measurements were obtained over a 3-day period in a room with ambient lighting. All the bees were assayed within 40 min of emergence. The same procedures were followed for workers from the reciprocal backcrosses. A total of 402 bees (201 of each cross) were assayed in groups of 15–20, each within 90 min of emergence.
Experiment 2: effects of pollen-hoarding QTLs on locomotion
DNA extraction and marker screening
Bees from the high backcross were assayed for marker alleles linked to the three pollen-hoarding QTLs that have previously been mapped and confirmed in high backcrosses of these same strains (Hunt et al. 1995; Page et al. 2000). These bees were derived from a hybrid queen backcrossed to a high-strain male. The male is haploid with just one set of chromosomes that he contributes to the worker offspring. The diploid queens undergo meiosis and recombine the high- and low-strain QTLs and their linked markers and randomly distribute them among their worker offspring. As a consequence, the effects of QTLs on locomotion can be tested by correlating differences in behavior with differences in marker alleles inherited from the queen.
After each bee was assayed for its locomotion rate, it was immediately frozen on dry ice and stored at −80°C. The DNA was phenol–chloroform extracted (Hunt and Page 1995) and quantified on a spectrophotometer. DNA was selectively amplified with PCR using specific primers that amplified STS markers which were known to flank each of the three pollen-hoarding QTL (Table 1). The amplified DNA fragments from each marker were electrophoresed on 0.8% agarose gels and those fragment polymorphisms segregating in the experimental population were scored for each individual.
Table 1.
Running conditions and primer sequences for markers used to test the effects of QTLs
| Genome region | Sts-marker (with reference) | PCR reagents | PCR thermoprofile |
|---|---|---|---|
| “pln1” | stsD8-.33f (Hunt et al. 1995b) | 200 μM dNTPs | Denaturation: 94°C, 60 s |
| Forward primer: | 0.3 μM primers | Annealing: 51°C, 60 s | |
| ACAACCAGAGAGCAAACGCC | 2 mM MgCl2 | Elongation: 73°C, 120 s | |
| Reverse primer: | 0.5 U Taq | 35 cycles | |
| CGGTGCAACGGTATATTATTATTACC | |||
| “pln2” | sts-tyr (cut with TaqI) | 200 μM dNTPs | Den.: 94°C, 60 s |
| Forward primer: | 0.7 μM primers | Anl.: 60°C (5×)/50°C (30×), 60 s | |
| GTGTCACCAATGATCAAGGATT | 2 mM MgCl2 | Elo.: 72°C, 120 s | |
| Reverse primer: AGCCTGTCATGTCGTAGTCCTC | 0.5 U Taq | ||
| “pln3” | sts-q4-ecap (cut with MboI) | 200 μM dNTPs | Den.: 94°C, 60 s |
| forward primer: | 0.5 μM primers | Anl.: 68°C (8×), | |
| GCGCTGTTCAAAAATTCCACATATCTG | 2 mM MgCl2 | 63LC (30×) 60 s | |
| reverse primer:GTATTTATTACCACGTTGTGACAAATCG | 0.5 U Taq | Elo: 72°C, 120 s |
Experiment 3: walking activity and sucrose responsiveness in wild-type bees
Honeybee sources and rearing techniques
Bees used in this experiment were derived from “wild type” commercial colonies. Combs containing capped brood with emerging workers were removed from the colony and taken directly into the lab. As the bees emerged from the comb, they were immediately removed to prevent the consumption of honey and placed individually into pre-numbered Petri dishes. The bees were collected in groups of 20 and assayed for walking activity (see Experiment 1) within one hour of emergence. Immediately after the locomotion assay, the bees were mounted in brass tubes with their bodies immobilized (Page et al. 1998). The tubes were then numbered in a location not visible to the observer and placed randomly in an upright orientation into the wells of polystyrene ELISA plates (24-well). The bees were then left for an additional 3 h at room temperature before being assayed for sucrose sensitivity (at 22–26°C). A total of 192 bees were assayed.
Sucrose sensitivity assay
In this assay, we used the proboscis extension reflex (PER) (Kuwabara 1957) to report sucrose responsiveness to increasing concentrations of solution. Before initiating the assay, the bees were satiated with water by letting them imbibe ad lib from water droplets suspended from a syringe. When the bees no longer responded to the water droplets, they were tested for PER with a drop of water touched to each antenna. Bees responding to water were excluded from the analyses. The bees’ antennae were then stimulated successively with drops of sucrose solution of the following concentrations: 0.1%, 0.3%, 1.0%, 3.0%, 10.0%, 30.0%. These trials were followed by a final presentation of honey to the antennae. Bees not responding to the honey were also excluded from the analysis. The total number of responses to sucrose was recorded for each bee (only a full extension of the proboscis was recorded as a positive response). Bees were tested in groups of 20, allowing a 4- min interval between the presentations of sucrose stimuli.
Statistical analysis
Single factor ANOVA was performed on walking activity data for workers from the high and low strains and between workers of the two backcrosses. Although the data were not normally distributed, ANOVA is a robust method against departures from normality (Kallenberg and Ledwina 1997). The number of grid crossings of each individual was regressed on its genotype represented as the proportion of its genotype inherited from the high strain. Single factor ANOVA was used to determine the effects of segregating QTLs on the walking activity phenotype. A Spearman rank correlation test was used to determine whether walking activity scores correlated with sucrose responsiveness (Sokal and Rohlf 1995).
Results
Experiment 1
High-strain bees were significantly more active than lowstrain bees (F1,153 = 172.93, P < 0.0001; Fig. 1). Fifty-three percent of the phenotypic variance was explained by the regression on genotype (r2 = 0.531, P < 0.0001; Fig 1). This estimate is analogous to the broad sense heritability of this trait (Falconer 1981). Bees derived from the high backcross were also more active than the bees derived from the low backcross (F1,401 = 22.32, P < 0.0001). The estimate of r2 when considering just the backcross bees was 0.053 (P < 0.0001). This lower estimate was expected due to the decreased difference in genotypic value between the backcrosses and because the backcrosses were expected to be genetically more heterogeneous. Genetic heterogeneity results in a reduced estimate of r2 because it contributes to the unexplained variance. The estimate of heritability using all four genotypes is r2 = 0.196 (F1,555 = 134.97, P < 0.0001).
Fig. 1.
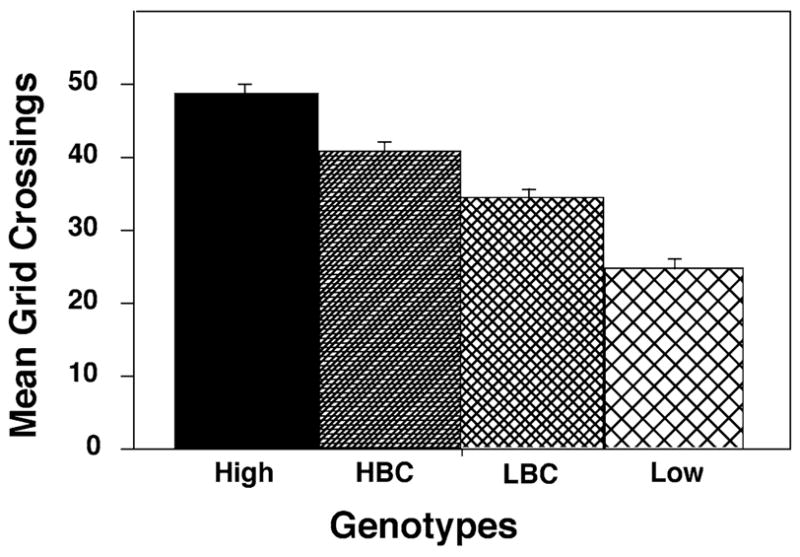
Mean number of times (±S.E.) bees from the four genotypic groups, high strain (HIGH), high backcross (HBC), low backcross (LBC), and low strain (LOW) crossed grid lines during the locomotion activity assay
Experiment 2
We did not detect any QTL effects on walking activity (pln1: F1,194 = 0.447, P = 0.504; pln2: F1,177 = 0.898, P = 0.345; pln3: F1,181 = 0.330, P = 0.566). Comparable non-parametric tests yielded similar results.
Experiment 3
The number of grid crossings in newly emerged workers correlated significantly with the total number of responses to sucrose (rho = 0.234, Z = 3.237, P = 0.0012).
Discussion
Increased walking activity at emergence is a component of the pollen-hoarding behavioral syndrome, though we do not yet have direct evidence of genetic pleiotropy. Walking activity was strongly correlated with responsiveness to sucrose. Responsiveness to sucrose is correlated with all of the behavioral traits so far identified: pollen and nectar foraging load sizes, concentration of nectar collected, age of onset of foraging, and tactile and olfactory associative learning (Hunt et al. 1995; Page et al. 1998; Scheiner et al. 1999, 2001a, b, 2004; Page et al. 2000; Pankiw and Page 2000; Pankiw et al. 2004; Pankiw 2003). The trait demonstrated high heritability in comparisons of high-and low-strain bees and the reciprocal backcrosses, however, we found no evidence for effects of allelic variation for mapped pollen-hoarding QTLs. This suggests that the component traits of the syndrome have different genetic architectures with as yet unmapped QTLs linking walking activity with the behavioral syndrome.
Not all QTLs affect all traits and not all effects are demonstrable in all crosses. Hunt et al. (1995) showed that pln1 and pln2 affected foraging choice for pollen or nectar but only pln2 affected the concentration of nectar collected by nectar foragers. Page et al. (2000) mapped a new QTL (pln3) for pollen hoarding which was shown also to affect pollen and nectar load sizes and the sugar concentration of nectar collected. In that study, pln2 had no demonstrable effect on nectar concentration but did affect the size of pollen loads collected. Subsequent studies of QTL effects in backcross populations have demonstrated direct effects of pln1 on sucrose responsiveness (R. Page, unpublished data) and age of initiation of foraging (Rueppell et al. 2004a, b), pln2 effects on nectar load size, and complex interactions among the three pollen-hoarding QTLs for foraging onset and nectar and pollen-foraging variables (Rueppell et al. 2004a, b; R. Page, unpublished data).
Differences in walking activity could reflect differences in motoneuronal activity, motor-response differences to ambient light, or both. Newly emerged bees do not orient directly to or away from light (R. Page, personal observation). However, they may be sensitive to light and exhibit non-oriented increases in activity in response to light stimulus.
Pollen foragers, 7-day-old preforaging high-strain bees, and “wild-type” bees that are more responsive to sucrose orient to lower intensity light sources (J. Tsuruda and R. Page, unpublished; J. Erber and R. Scheiner, unpublished), demonstrating that oriented motor responsiveness to light is an additional component of the pollen-hoarding behavioral syndrome. It is plausible that newly emerged bees with greater sensitivity to sucrose are more light sensitive and perform more undirected locomotion in response to light. A connection between sucrose sensitivity and motor activity was demonstrated by Pribbenow and Erber (1996). Sucrose stimulation of the antenna increased antennal motor activity. In addition, injection of octopamine (OA) into the dorsal lobes of the brain increased antennal scanning movements. OA has been shown to be important in the elicitation of the proboscis extension in response to sucrose stimulation (Erber et al. 1993; Menzel et al. 1994; Blenau and Erber 1998; Pankiw and Page 2003; Scheiner et al. 2002).
High-strain bees are in general more responsive and active when they emerge as adults. Young adult bees go through a process of maturation during their first 5 days of adult life, they become more sensitive to light (unpublished) and sucrose stimuli (Pankiw and Page 1999), and demonstrate an ability to perform on associative learning tests (Ray and Ferneyhough 1997; Maleszka and Halliwell 2001). Also during this time, changes occur in brain structure and biochemistry. Synaptic maturation continues past emergence (Masson and Arnold 1987; Farris et al. 2001) with corresponding increases in biogenic amines (Schulz and Robinson 1999; Wagener-Hulme et al. 1999), protein kinases PKA, PKC, and synapsin (Humphries et al. 2003a), and receptors for the biogenic amines dopamine (Kokay and Mercer 1997; Humphries et al 2003b) and tyramine (Humphries et al., unpublished). When the high-strain bees emerge as adults, they have higher titres of PKA, PKC (Humphries et al. 2003a), tyr1 receptor mRNA (M. Humphries and R. Page, unpublished data), and higher titres of vitellogenin (an egg storage protein) and vitellogenin mRNA (Amdam et al. 2004). This suggests that high-strain bees are developmentally more mature at emergence.
Selection for pollen hoarding, a colony level phenotype, resulted in bees that emerge as more behaviorally mature adults. Differences in behavior persist throughout life resulting in an early onset of foraging, differences in learning performance, and pronounced differences in foraging behavior. Developmental differences that result in these differences in behavior must occur prior to adult emergence. There is now mounting evidence that the c-AMP signaling cascade is involved (Page and Erber 2002; Scheiner et al. 2002, 2003, 2004; Pankiw and Page 2003). Tyramine, octopamine, PKA and PKC (indirectly) are all involved in the c-AMP second messenger signaling cascade (see Humphries et al. 2003a for review; Blenau et al. 2000; Scheiner et al. 2002, 2003) and have been shown to affect responses to sucrose, learning, age of onset of foraging, and motor activity. Differences in components of this cascade are detectable at emergence and persist for at least 5–15 days post-emergence (Humphries et al. 2003a; M. Humphries and R. Page, unpublished data).
QTL studies suggest overlapping genetic architectures associated with the different components of the pollen-hoarding behavioral syndrome. We were unable, in this study, to directly demonstrate pleiotropy of the mapped pollen-hoarding QTLs with locomotor activity. However, the correlation between locomotor activity and sucrose responsiveness, and the demonstrated heritability of both traits, suggest that they share common, but still undetected, genetic components. Certainly, other undetected QTLs exist. How these different architectures overlap and involve the c-AMP cascade remains to be demonstrated.
Acknowledgments
We thank Tanya Pankiw, Mindy Nelsen, Jennifer Tsuruda, and Amanda Ruby for observations and comments on general activity differences in high and low strains of the bees. Research was funded by NSF IBN 0090482 and IBN0076811, and NIH POI AG022500-01 to REP.
Abbreviations
- QTL
Quantitative trait locus
References
- Amdam GV, Norberg K, Page RE. Reproductive ground plan may mediate colony-level selection effects on individual foraging behavior in honeybees. Proc Natl Acad Sci USA. 2004;101:11350–11355. doi: 10.1073/pnas.0403073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenau W, Erber J. Behavioral pharmacology of dopamine, serotonin, and putative aminergic ligands in the mushroom bodies of the honeybee (Apis mellifera) Behav Brain Res. 1998;96:115–124. doi: 10.1016/s0166-4328(97)00201-5. [DOI] [PubMed] [Google Scholar]
- Blenau W, Balfanz S, Baumann A. Am tyr 1: characterization of a gene from honeybee (Apis mellifera) brain encoding a functional tyramine receptor. J Neurochem. 2000;74:900–908. doi: 10.1046/j.1471-4159.2000.0740900.x. [DOI] [PubMed] [Google Scholar]
- Calderone NW, Page RE. Genotypic variability in age polyethism and task specialization in the honeybee, Apis mellifera (Hymenoptera: Apidae) Behav Ecol Sociobiol. 1988;22:17–25. [Google Scholar]
- Calderone NW, Page RE. Evolutionary genetics of division of labor in colonies of the honeybee (Apis mellifera) Am Nat. 1991;138:69–92. [Google Scholar]
- Calderone NW, Page RE. Effect of interactions among genotypically diverse nestmates on task specialization by for-aging honeybees (Apis mellifera) Behav Ecol Sociobiol. 1992;30:219– 226. [Google Scholar]
- Calderone NW, Page RE. Temporal polyethism and behavioural canalization in the honeybee, Apis mellifera. Anim Behav. 1996;51:631–643. [Google Scholar]
- Erber J, Kloppenburg P, Scheidler A. Neuromodulation by serotonin and octopamine in the honeybee: behavior, neuroanatomy and electrophysiology. Experientia. 1993;49:1073–1083. [Google Scholar]
- Falconer DS. Introduction to quantitative genetics. 2. Longman; London: 1981. [Google Scholar]
- Farris SM, Robinson GE, Fahrback SE. Experience- and age-related outgrowth of intrinsic neurons in the mushroom bodies of the adult worker honeybee. J Neurosci. 2001;21:6395–6404. doi: 10.1523/JNEUROSCI.21-16-06395.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewell JH, Page RE. Colony-level selection effects on individual and colony foraging task performance in honeybees, Apis mellifera L. Behav Ecol Sociobiol. 2000;48:173–181. [Google Scholar]
- Hellmich RL, Kulincevic JM, Rothenbuhler WC. Selection for high and low pollen-hoarding honeybees. J Heredity. 1985;76:155–158. [Google Scholar]
- Humphries MA, Müller U, Fondrk MK, Page RE., Jr PKA and PKC content in the honeybee central brain differs in genotypic strains with distinct foraging behavior. J Comp Physiol A. 2003a;189:555–562. doi: 10.1007/s00359-003-0433-z. [DOI] [PubMed] [Google Scholar]
- Humphries MA, Mustard JA, Hunter SJ, Mercer A, Ward V, Ebert PR. Invertebrate D2 type dopamine receptor exhibits age-based plasticity of expression in the mushroom bodies of the honeybee brain. J Neurobiol. 2003b;55:315–330. doi: 10.1002/neu.10209. [DOI] [PubMed] [Google Scholar]
- Hunt GJ, Page RE. Linkage map of the honeybee, Apis mellifera, based on RAPD markers. Genetics. 1995;139:1371–1382. doi: 10.1093/genetics/139.3.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt GJ, Page RE, Fondrk MK, Dullum CJ. Major quantitative trait loci affecting honeybee foraging behavior. Genetics. 1995;141:1537–1545. doi: 10.1093/genetics/141.4.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenberg WCM, Ledwina T. Data-driven smooth tests when the hypothesis is composite. J Am Stat Assoc. 1997;92:1094–1104. [Google Scholar]
- Kokay IC, Mercer AR. Age-related changes in dopamine receptor densities in the brain of the honeybee, Apis mellifera. J Comp Physiol A. 1997;181:415–423. [Google Scholar]
- Kuwabara M. Bildung des bedingten Reflexes von Pavlovs Typu bei der Honnigvene, Apis mellica. J Fac Sci Hokkaido Univ Zool. 1957;13:458–464. [Google Scholar]
- Laidlaw HH. Instrumental insemination of honeybee queens. Dadant and Sons; Hamilton: 1977. [Google Scholar]
- Maleszka R, Helliwell P. Effect of juvenile hormone on short-term olfactory memory in young honeybees (Apis mellifera) Horm Behav. 2001;40:403–408. doi: 10.1006/hbeh.2001.1705. [DOI] [PubMed] [Google Scholar]
- Masson C, Arnold G. Organization and plasticity of the olfactory system of the honeybee, Apis mellifera. In: Menzel R, Mercer A, editors. Neurobiology and behavior of honeybees. Springer; Berlin Heidelberg New York: 1987. pp. 280–295. [Google Scholar]
- Menzel R, Durst C, Erber J, Eichmüller S, Hammer M, Hildebrandt, Mauelshagen J, Müller U, Rosenboom H, Rybak J, Schäfer S, Scheidler A. The mushroom bodies in the honeybee: From molecules to behaviour. In: Schildberger K, Elsner N, editors. Neural basis of behavioural adaptations (Fortschritte der Zoologie) Vol. 19. Fischer; Stuttgart: 1994. pp. 81–102. [Google Scholar]
- Page RE, Jr, Erber J, Fondrk MK. The effect of genotype on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.) J Comp Physiol A. 1998;182:489–500. doi: 10.1007/s003590050196. [DOI] [PubMed] [Google Scholar]
- Page RE, Erber J. Levels of behavioral organization and the evolution of division of labor. Naturwissenschaften. 2002;89:91–106. doi: 10.1007/s00114-002-0299-x. [DOI] [PubMed] [Google Scholar]
- Page RE, Jr, Fondrk K. The effects of colony-level selection on the social organization of honey bee (Apis mellifera L.) colonies: colony-level components of pollen hoarding. Behav Ecol Sociobiol. 1995;36:135–144. [Google Scholar]
- Page, Waddington KD, Hunt GJ, Fondrk MK. Genetic determinants of honey bee foraging behaviour. Anim Behav. 1995;50:1617–1625. [Google Scholar]
- Page RE, Jr, Fondrk MK, Hunt GJ, Guzman-Novoa E, Humphries MA, Nguyen K, Greene AS. Genetic dissection of honeybee (Apis mellifera L.) foraging behavior. J Hered. 2000;91:474–479. doi: 10.1093/jhered/91.6.474. [DOI] [PubMed] [Google Scholar]
- Pankiw T. Directional change in a suite of foraging behaviors in tropical and temperate evolved honeybees (Apis mellifera L.) Behav Ecol Sociobiol. 2003;54:458–464. [Google Scholar]
- Pankiw T, Page RE., Jr The effect of genotype, age, sex, and caste on response thresholds to sucrose and foraging behavior of honeybees (Apis mellifera L.) J Comp Physiol A. 1999;185:207–213. doi: 10.1007/s003590050379. [DOI] [PubMed] [Google Scholar]
- Pankiw T, Page RE. Response thresholds to sucrose predict foraging division of labor in honeybees. Behav Ecol Sociobiol. 2000;47:265–267. [Google Scholar]
- Pankiw T, Page RE. The effect of pheromones, hormones, and handling on sucrose response thresholds of honeybees (Apis mellifera L.) J Comp Physiol A. 2003;189:675–684. doi: 10.1007/s00359-003-0442-y. [DOI] [PubMed] [Google Scholar]
- Pankiw T, Nelson M, Page RE, Fondrk MK. The communal crop: modulation of sucrose response thresholds of pre-foraging honeybees with incoming nectar quality. Behav Ecol Sociobiol. 2004;55:286–292. [Google Scholar]
- Pribbenow B, Erber J. Modulation of antennal scanning in the honeybee by sucrose stimuli, serotonin, and octopamine: behavior and electrophysiology. Neurobiol Learn Mem. 1996;66:109– 120. doi: 10.1006/nlme.1996.0052. [DOI] [PubMed] [Google Scholar]
- Ray S, Ferneyhough B. The effects of age on olfactory learning and memory in the honeybee Apis mellilfera. Neuro-Report. 1997;8:789–793. doi: 10.1097/00001756-199702100-00042. [DOI] [PubMed] [Google Scholar]
- Rueppell O, Pankiw T, Nielson D, Beye M, Page RE. The genetic architecture of the behavioral ontogeny of honeybee workers. Genetics. 2004a;167:1767–1779. doi: 10.1534/genetics.103.021949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueppell O, Pankiw T, Page RE. Pleiotropy, epistasis and new QTL: the genetic architecture of honeybee foraging behavior. J Hered. 2004b;95:481–491. doi: 10.1093/jhered/esh072. [DOI] [PubMed] [Google Scholar]
- Scheiner R, Erber J, Page RE. Tactile learning and the individual evaluation of the reward in honeybees (Apis mellifera L.) J Comp Physiol A. 1999;185:1–10. doi: 10.1007/s003590050360. [DOI] [PubMed] [Google Scholar]
- Scheiner R, Page RE, Erber J. Responsiveness to sucrose affects tactile and olfactory learning in preforaging honeybees of two genetic strains. Behav Brain Res. 2001a;120:67–73. doi: 10.1016/s0166-4328(00)00359-4. [DOI] [PubMed] [Google Scholar]
- Scheiner R, Page RE, Erber J. The effects of genotype, foraging role, and sucrose responsiveness on the tactile learning performance of honeybees (Apis mellifera L.) Neurobiol Learn Mem. 2001b;76:138–150. doi: 10.1006/nlme.2000.3996. [DOI] [PubMed] [Google Scholar]
- Scheiner R, Plückhahn S, Öney B, Blenau W, Erber J. Behavioural pharmacology of octopamine, tyramine and dopamine in honeybees. Behav Brain Res. 2002;136:545–553. doi: 10.1016/s0166-4328(02)00205-x. [DOI] [PubMed] [Google Scholar]
- Scheiner R, Müller U, Heimburger S, Erber J. Activity of protein kinase A and gustatory responsiveness in the honeybee (Apis mellifera L.) J Comp Physiol A. 2003;189:427–434. doi: 10.1007/s00359-003-0419-x. [DOI] [PubMed] [Google Scholar]
- Scheiner R, Page RE, Erber J. Sucrose responsiveness and behavioral plasticity in honeybees. Apidologie. 2004;35:133–142. [Google Scholar]
- Schulz DJ, Robinson GE. Biogenic amines and division of labor in honeybee colonies: behaviorally related changes in the antennal lobes and age-related changes in the mushroom bodies. J Comp Physiol A. 1999;184:481–488. doi: 10.1007/s003590050348. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. W.H. Freeman and Co; New York: 1995. [Google Scholar]
- Waddington KD, Nelson MC, Page RE. Effects of pollen quality and genotype on the dance of foraging honeybees. Anim Behav. 1998;56:35–39. doi: 10.1006/anbe.1998.0736. [DOI] [PubMed] [Google Scholar]
- Wagener-Hulme C, Kuehn JC, Schulz DJ, Robinson GE. Biogenic amines and division of labor in honeybee colonies. J Comp Physiol A. 1999;184:471–479. doi: 10.1007/s003590050347. [DOI] [PubMed] [Google Scholar]
- Winston ML. The biology of the honeybee. Harvard University Press; Cambridge: 1987. [Google Scholar]


