Abstract
CD26/dipeptidyl peptidase IV (DPPIV) is a cell surface-bound ectopeptidase with important roles in T-cell activation and tumour biology. We now report that CD26/DPPIV enhances sensitivity to apoptosis induced by the antineoplastic agents doxorubicin and etoposide. In particular, CD26/DPPIV presence is associated with increased susceptibility to the mitochondrial pathway of apoptosis, documented by enhanced cleavage of poly (ADP ribose) polymerase (PARP), caspase-3 and caspase-9, Bcl-xl, and Apaf-1, as well as increased expression of death receptor 5 (DR5). We also show that the caspase-9-specific inhibitor z-LEHD-fmk inhibits drug-mediated apoptosis, leading to decreased PARP and caspase-3 cleavage, and reduced DR5 expression. Importantly, through detailed studies that demonstrate the association between topoisomerase II alpha expression and DPPIV activity, our data provide further evidence of the key role played by CD26 in biological processes.
Keywords: CD26/DPPIV, apoptosis, topoisomerase II, caspase-9, DNA damage
CD26 is a 110-kDa type II cell surface glycoprotein with diverse functional properties that is widely expressed on various tissues (Dang and Morimoto, 2002; Sato and Dang, 2003). Its extracellular domain encodes a membrane-associated dipeptidyl peptidase IV (DPPIV) activity capable of processing amino-terminal dipeptides from polypeptides with either L-proline or L-alanine in the penultimate position (Oravecz et al, 1997). In addition, CD26 acts as an alternative pathway of T-cell activation through its physical and functional association with molecules involved in T-cell signal transduction processes, including CD45, mannose 6-phosphate/insulin-like growth factor II receptor and adenosine deaminase (ADA) (Morimoto et al, 1989; Dang et al, 1990a, 1990b, 1990c, 1990d, 1991; Ishii et al, 2001; Dang and Morimoto, 2002; Sato and Dang, 2003). With its deficiency being a cause of severe combined immune deficiency, ADA plays a key role in the development and function of lymphoid tissues and by regulating surface expression of ADA, CD26 potentially has an important role in adenosine metabolism and immune regulation (Sato and Dang, 2003). Meanwhile, recent findings suggest that CD26 has a role in the development of certain types of neoplasms (Morrison et al, 1993; Sedo and Revoltella, 1995; Tanaka et al, 1995; Stecca et al, 1997). B-chronic lymphocytic leukaemia cells have high levels of CD26 protein expression and mRNA transcripts (Bauvois et al, 1999); whereas the more aggressive T-cell malignancies, such as T-cell acute lymphoblastic leukaemia or T-cell CD30+ anaplastic large-cell lymphoma, express higher CD26 level as compared to the more indolent T-cell diseases like mycosis fungoides (Carbone et al, 1995; Jones et al, 2001).
Topoisomerase II inhibitors are widely used agents in cancer treatment (Froelich-Ammon and Osheroff, 1995), exploiting the catalytic activity of topoisomerase II alpha by increasing permanent DNA damage (Beck et al, 1999). Previous findings have shown that DNA damage mediated by topoisomerase II inhibitors induces apoptosis (Beck et al, 1999; Mow et al, 2001), particularly through cytochrome c release (Liu et al, 1996; Kluck et al, 1997), Apaf-1 involvement (Perkins et al, 2000; Lauber et al, 2001), and subsequent caspase-9 activation (Mow et al, 2001). We have previously shown that surface expression of CD26/DPPIV enhances sensitivity of CD26 Jurkat T-cell transfectants to G2/M arrest mediated by the topoisomerase II inhibitors doxorubicin and etoposide (Aytac et al, 2001, 2003). Expanding on these previous findings, we now examine the effect of CD26/DPPIV expression on apoptosis induced by doxorubicin and etoposide. In this paper, we demonstrate that CD26/DPPIV presence enhances sensitivity to apoptosis induced by topoisomerase II inhibitors, associated with increased cleavage of Bcl-xl, Apaf-1, procaspase-9, procaspase-3, and PARP, as well as increased expression of DR5. Meanwhile, pretreatment with the caspase-9-specific inhibitor z-LEHD-fmk significantly reduces etoposide-mediated apoptotic events. Importantly, we present detailed data from different experimental approaches demonstrating the association between DPPIV enzyme activity and topoisomerase II alpha expression. Our findings thus emphasise the increasingly important role of the multifaceted CD26/DPPIV molecule in biological processes, while the functional association between CD26/DPPIV and topoisomerase II alpha may be exploited for future treatments of selected cancers.
MATERIALS AND METHODS
Cells and reagents
Human CD26 Jurkat T-cell leukaemia stable transfectants have been described and characterised previously regarding CD26 surface expression and associated DPPIV enzyme activity (Aytac et al, 2001, 2003). The Jurkat cell lines include: (a) wild-type CD26-transfected Jurkat cell lines (wtCD26); (b) Jurkat cell lines transfected with mutant CD26 containing an alanine at the putative catalytic serine residue at position 630, resulting in a mutant CD26-positive/DPPIV-negative Jurkat transfectant (S630A); (c) Jurkat cell lines transfected with mutant CD26 containing point mutations at ADA-binding site residues 340–343, with amino acid L340, V341, A342, and R343 being replaced by amino acids P340, S341, E342, and Q343, resulting in a mutant CD26-positive/DPPIV-positive Jurkat transfectant incapable of binding ADA (340–344); and (d) nontransfected control Jurkat cells (parental). Jurkat transfectants were maintained in culture media, which consisted of RPMI 1640 supplemented with 10% FCS, penicillin (100 U ml−1), streptomycin (100 μg ml−1), and G418 (0.25 mg ml−1; Life Technologies Inc., NY, USA) Nontransfectant control Jurkat cells were maintained in the same culture media without G418. Annexin V- fluorescein isothiocyanate (FITC) was from BD PharMingen, CA, USA. Anti-PARP, cytochrome c, and caspase-3 Abs were from BD Phar-Mingen; anti-actin was from Sigma Chemical Co, MO, USA; anti-caspase-9 was from Cayman, MI, USA. Anti-DR5 Abs were from Cayman. Anti-Bcl-xl and Apaf-1 Abs were from BD Transduction Laboratories, CA, USA. Anti-topoisomerase II alpha was from Roche, IN, USA. Caspase-9 inhibitor (z-LEHD-fmk) was from BD PharMingen. Diisopropyl fluorophosphate (DFP) was obtained from SIGMA, MO, USA. Substrate for DPPIV, Gly-Pro-p-nitroanilide-tosylate (GPNT), was purchased from WAKO, Japan. Etoposide was purchased from SIGMA and was dissolved in sterile DMSO. Doxorubicin was purchased from Calbiochem, CA, USA and was dissolved in sterile PBS. Soluble CD26 molecules were produced by Chinese hamster ovary cells and purified as described previously (Tanaka et al, 1994).
Annexin/propidium iodide (PI) assays
Exposure of phosphatidylserine residues was quantified by surface Annexin V staining as previously described (Raynal and Pollard, 1994). Briefly, cells were washed in binding buffer (10 mM HEPES, pH 7.4, 2.5 mM CaCl2, 140 mM NaCl), resuspended in 100 μl and incubated with 0.5 μl ml−1 annexin V-FITC and 2.5 μg ml−1 PI for 15 min in the dark. Cells were then washed again and resuspended in 400 μl of binding buffer, then flow cytometric analysis (FACScan; Becton Dickinson, CA, USA) was performed. A total of 10 000 cells were acquired per sample and data were analysed using Cellquest software (Becton Dickinson).
SDS–PAGE and immunoblotting
After incubation at 37°C in culture media and etoposide or doxorubicin at the concentrations and duration indicated, cells were harvested from wells, washed with PBS, and lysed in lysis buffer consisting of 1% NP-40, 0.5%, deoxycolate, 0.1% SDS, 1 mM phenylmethylsulphonyl fluoride, 1 mM benzamidine, 10 μg ml−1 aprotinin, 50 μg ml−1 leupeptin, 10 μg ml−1 soybean trypsin inhibitor and 1 μg ml−1 pepstatin. After incubating on ice for 5 min, nuclei were removed by centrifugation and supernatants were collected as whole-cell lysates. Sample buffer (4 ×) consisting of 20% glycerol, 4.6% SDS, 0.5 M Tris (pH 6.8), 4% β-mercaptoethanol, and 0.2% bromophenol blue was added to the appropriate aliquots of supernatants. After boiling, protein samples were submitted to SDS–PAGE analysis on an 8% gel under standard conditions using a mini-Protean II system (Bio-Rad, CA, USA). For immunoblotting, the proteins were transferred onto nitrocellulose (Immobilon-P; Millipore, MA, USA). After overnight blocking at 4°C in blocking solution consisting of 0.1% Tween 20 and 5% BSA in Tris-buffered saline, membranes were blotted with the appropriate primary antibodies diluted in blocking solution for 1 h at room temperature. Membranes were then washed with blocking solution, and appropriate secondary antibodies diluted in blocking solution were then applied for 1 h at room temperature. Secondary antibodies were goat anti-mouse or goat anti-rabbit horseradish peroxidase conjugates (Dako). Membranes were then washed with blocking solution, and proteins were subsequently detected by chemiluminescence (Amersham Pharmacia Biotech, NJ, USA).
Dipeptidyl Peptidase IV enzyme activity assays
As previously described (Kajiyama et al, 2002), DPPIV enzyme activity was measured spectrophotometrically using GPNT, a substrate for DPPIV. A 1 × PBS-washed whole-cell suspension was prepared and 5 × 105 cells were resuspended in 200 μl of PBS into 96-well plate, then GPNT was added at a final concentration of 0.24 mM. The absorption was measured at 405 nm using microplate spectrophotometer (BIO-TEK Instruments, inc., VE, USA) twice, just before the addition of the substrate and after 60 min incubation at 37°C. Dipeplidyl peptidase enzyme activity was calculated from the increase of absorption between 0 and 60 min.
Inhibition of DPPIV enzyme activity
As described previously (Koreeda et al, 2001; Kajiyama et al, 2002), DFP was used as the DPPIV chemical inhibitor for inhibition assays. To evaluate effect of continuous exposure to DFP, wtCD26 transfectants or parental Jurkat cells were incubated in culture media alone (DFP−), culture media containing 100 μM DFP for 2 h or for 6 h (DFP+). A representative sample of cells reflecting each treatment condition was obtained for DPPIV enzyme activity assays or to examine topoisomerase II alpha expression. Alternatively, wtCD26 Jurkat transfectants were incubated in culture media; or in culture media with 100 μM DFP for 4 h; or they were incubated in culture media with 100 μM DFP for 4 h, then washed twice in PBS to ensure removal of DFP followed by incubation in culture media for 2 or 8 h. A representative sample of cells reflecting each treatment condition was obtained for DPPIV enzyme activity assays or to examine topoisomerase II alpha expression. For all treatment conditions, trypan blue uptake assays consistently showed >90% cell viability (data not shown).
Preparation of cytosol fractions
As previously described (Haridas et al, 2001; Nishimura et al, 2001), Jurkat cells (4.0 × 107) were suspended in 1 ml sucrose buffer (250 mM sucrose in 30 mM Tris HCl, pH 7.4) and transferred into an N2 cavitation chamber (PARR Instruments, Moline, IL, USA). The cells were subjected to N2 cavitation (250 psi for 5 min) according to the manufacturer's instructions. Under these conditions, most of the cell membrane was disrupted with no change in the mitochondrial respiratory activity. Next, DNA and the nuclear fraction were removed by centrifugation (1500 g for 2 min). The supernatant was further centrifuged (16 000 g for 10 min), and the supernatant was used as the cytosol fraction.
Preparation of nuclear extracts
Cells (10 × 106) were harvested and allowed to swell for 15 min on ice in cytoplasmic extraction buffer (10 mM HEPES, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 1 mM PMSF, 2 μg ml−1 leupeptin, 2 μg ml−1aprotinin, and 0.5 mg ml−1 benzamidine). Then NP-40 (final concentration 0.3%) was added into that cell suspension and vortexed for 10 s. After 2 min-centrifugation at 16 000 g, the supernatant was discarded. The pellet was then incubated with nuclear extraction buffer (20 mM HEPES, 400 mM KCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.5 mM PMSF, 2 μg ml−1 leupeptin, 2 μg ml−1 aprotinin, and 0.5 mg ml−1 benzamidine) for 30 min on ice with intermittent vortexing. The suspension was centrifuged at 16000 g for 6 min, and the supernatant was saved as the nuclear extract.
RESULTS
Effect of CD26/DPPIV expression on apoptosis of Jurkat cells mediated by topoisomerase II inhibitors
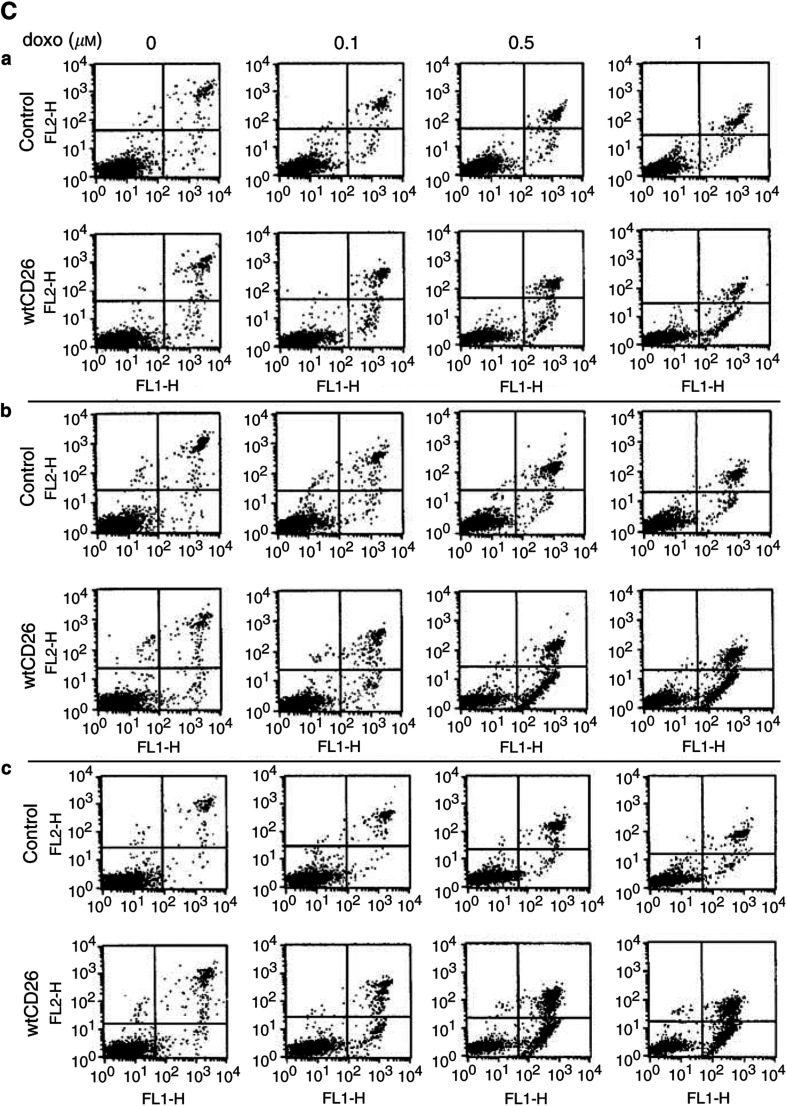
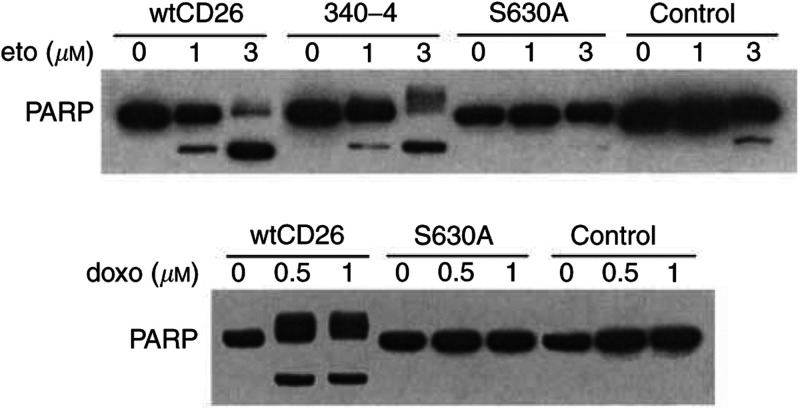
Annexin V/PI assays show that wtCD26 Jurkat transfectants are more sensitive to the apoptotic effect of etoposide than S630A or parental control Jurkat cells. Meanwhile, 340–4 transfectants (340–4) exhibit higher level of drug-induced apoptosis, similar to that of wtCD26 transfectants (Figure 1A). Furthermore, wtCD26 and 340–4 cells display greater apoptosis when treated with doxorubicin as compared with parental or S630A Jurkat cells (Figure 1B). Time course studies to evaluate the effect of doxorubicin on apoptosis in these CD26 Jurkat transfectants are also performed. Our data show that wtCD26 Jurkat transfectants consistently exhibit greater drug-induced apoptosis over the time intervals and drug concentrations tested than parental Jurkat (Figure 1C), strongly suggesting that the presence of CD26 directly enhances cellular sensitivity to topoisomerase II inhibitor-induced apoptosis. Similar results are obtained with etoposide treatment (data not shown). Similarly, both the wtCD26 and 340–4 Jurkat transfectants are more sensitive to etoposide-mediated PARP cleavage (Figure 2), as compared with parental cells and S630A transfectants. Similar results are seen when cells are treated with doxorubicin. These data hence suggest that the presence of CD26, especially its associated DPPIV enzymatic activity, enhances apoptosis mediated by topoisomerase II inhibitors.
Figure 1.
Enhancing effect of CD26/DPPIV surface expression on apoptosis induced by topoisomerase II inhibitors. CD26 Jurkat transfectants were incubated at 37°C in culture media alone or culture media containing etoposide (A) for 14 h or doxorubicin (B) for 16 h at the concentrations indicated. Cells were then harvested and Annexin V/PI assays were performed as described in Materials and Methods. wtCD26: wild-type CD26 Jurkat transfectant; S630A: Jurkat cells transfected with mutant CD26 containing an alanine at the putative catalytic serine residue at position 630, resulting in a mutant CD26-positive/DPPIV-negative Jurkat transfectant; control: nontransfected parental Jurkat; 340–4: Jurkat cells transfected with mutant CD26 containing point mutations at the ADA-binding site residues 340–343, with amino acids L340, V341, A342, and R343 being replaced by amino acids P340, S341, E342, and Q343, resulting in a mutant CD26-positive/DPPIV-positive mutant CD26 Jurkat transfectant incapable of binding ADA. Data are representative of three separate experiments. (C) wtCD26 Jurkat transfectants and parental cells were treated with doxorubicin over the indicated time intervals and drug concentrations. a: 12 h, b: 24 h, c: 36 h. Data are representative of three separate experiments.
Figure 1.
Figure 2.
CD26/DPPIV-associated enhancement in PARP cleavage induced by topoisomerase II inhibitors. CD26 Jurkat transfectants were incubated at 37°C with media containing etoposide for 16 h or doxorubicin for 18 h at the indicated doses. Cells were then harvested, and whole-cell lysates were obtained. Following SDS–PAGE of lysates, immunoblotting studies for PARP and β-actin were performed as described in Materials and Methods. The cleaved product of PARP was detected at ∼85 kDa. Each lane was loaded with 30 μg of protein.
Effect of CD26/DPPIV surface expression on the mitochondrial pathway of apoptosis induced by etoposide
Previous work demonstrated that DNA damage mediated by topoisomerase II inhibitors induces apoptosis through the mitochondrial pathway. Our time course analyses (Figure 3) show that etoposide treatment results in enhanced cleavage of PARP and procaspase-3, leading to increased levels of the cleaved 17 kDa caspase-3 bands in wtCD26 transfectants, as compared with S630A and parental Jurkat cells. Moreover, our work shows that etoposide treatment leads to significantly greater cleavage of procaspase-9 in wtCD26 cells compared to S630A and parental control Jurkat. We also demonstrate greater cleavage of the 130 kDa proform of Apaf-1 in etoposide-treated wtCD26 Jurkat transfectants as compared with parental control or S630A Jurkat. Furthermore, the increase in sensitivity to etoposide-induced apoptosis in wtCD26 transfectants is accompanied by greater cleavage of the full-length antiapoptotic molecule Bcl-xl (Fujita et al, 1998) and a resultant rise in the 18 kDa cleaved band. Taken together, our results indicate that CD26/DPPIV enhances etoposide-mediated apoptosis of Jurkat cells by affecting cellular processes known to be involved in drug-mediated apoptosis, including those involving caspase-9 processing and the mitochondrial pathway, as well as processing of bcl-2-related molecules. Similarly, time course analyses demonstrate that wtCD26 Jurkat transfectants exhibit greater apoptosis through caspase-9 processing when treated with doxorubicin, as demonstrated by greater cleavage of PARP and procaspase-9.
Figure 3.
Time course study of the effect of CD26/DPPIV surface expression on etoposide-induced apoptosis. Jurkat cells were incubated at 37°C with media containing 3 μM etoposide or 1 μM doxorubicin for the indicated time periods at the indicated doses. Cells were then harvested, and cytosol fractions were obtained as described in Materials and Methods. Following SDS–PAGE of lysates, immunoblotting studies with specific antibodies for PARP, caspase-9, caspase-3, Apaf-1, Bcl-xl, and β-actin were performed as described in Materials and methods (*): caspase-3 cleaved products; (**): Bcl-xl cleaved products. Each lane was loaded with 30 μg of protein.
Effect of the caspase-9 inhibitor z-LEHD-fmk on etoposide-induced apoptosis in CD26 Jurkat transfectants
To further confirm our findings that CD26 affects etoposide-induced apoptosis through caspase-9-related events, we evaluated the effect of the caspase-9 inhibitor z-LEHD-fmk on this process. Western blot analyses show that pretreatment with z-LEHD-fmk significantly abrogates the effect of etoposide on wtCD26 Jurkat transfectants. As shown in Figure 4, etoposide-mediated cleavage of procaspase-9 is inhibited by z-LEHD-fmk in a dose-dependent manner. Furthermore, cleavage of procaspase-3 and PARP, events downstream of caspase-9 processing, is significantly reduced following pretreatment with the caspase-9 inhibitor. Our data therefore indicate CD26 augments etoposide-induced apoptosis in CD26 Jurkat transfectants through caspase-9-related events. Similarly, pretreatment with z-LEHD-fmk significantly decreases the effect of doxorubicin on wtCD26 Jurkat transfectants, as measured by cleavage of PARP and procaspase-9.
Figure 4.
Effect of caspase-9 inhibitor z-LEHD-fmk on etoposide-induced apoptosis in wtCD26 Jurkat transfectant. wtCD26 Jurkat transfectants were incubated at 37°C for 2 h of preincubation with z-LEHD-fmk at varying doses, and then treated with 3 μM etoposide or 1 μM doxorubicin for 16 h. Cells were then harvested, and whole-cell lysates were obtained as described in Materials and Methods. Following SDS–PAGE of lysates, immunoblotting studies for PARP, caspase-3, caspase-9, and β-actin were performed as described in Materials and Methods (*): caspase-3 cleaved products. Each lane was loaded with 30 μg of protein.
Effect of the DPPIV enzyme inhibitor DFP on topoisomerase II alpha expression
We previously showed that topoisomerase II alpha expression and catalytic activity are higher in wtCD26 Jurkat transfectant than S630A or parental cells (Aytac et al, 2003) (Figure 5A). To further evaluate in detail the effect of DPPIV activity on topoisomerase II alpha expression, we examined the effect of the DPPIV chemical inhibitor DFP (Koreeda et al, 2001; Kajiyama et al, 2002) on topoisomerase II alpha expression. Continuous treatment with DFP results in inhibition of DPPIV enzyme activity in wtCD26 Jurkat transfectants (Figure 5B), associated with decreased expression of topoisomerase II alpha in these cells (Figure 5C). On the other hand, expression of topoisomerase II alpha in parental Jurkat cells is not significantly affected by continuous exposure to DFP, as expected. Additionally, we examined the status of DPPIV enzyme activity and topoisomerase II alpha expression following DFP treatment. For this purpose, following treatment with DFP, wtCD26 cells were washed and incubated in culture media for the indicated time periods. We demonstrate that recovery of DPPIV enzyme activity is associated with recovery of topoisomerase II expression (Figure 5D and E). These results further corroborate and expand on our earlier findings regarding the importance of DPPIV enzyme activity in topoisomerase II alpha expression in CD26 Jurkat transfectants.
Figure 5.
Effect of inhibition of DPPIV activity on topoisomerase II alpha expression. (A) After incubation of Jurkat cells at 37°C for 24 h in culture media, cells were harvested and nuclear extracts were obtained. Following SDS–PAGE of lysates, immunoblotting studies were performed for topoisomerase II alpha or β-actin as described in Materials and Methods. Each lane was loaded with 30 μg of protein. Lane 1: wtCD26 Jurkat transfectant, lane 2: S630A mutant transfectant, lane 3: parental Jurkat. (B) wtCD26 Jurkat transfectants or parental Jurkat were incubated in culture media alone (DFP−), culture media containing 100 μM DFP for 2 or 6 h (DFP+). A representative sample of cells reflecting each treatment condition was obtained, and DPPIV enzyme activity assays were then performed as described in Materials and Methods. (C) wtCD26 Jurkat transfectants (lanes 1–3) or parental Jurkat (lanes 4–6) were incubated in culture media alone (lanes 1, 3), culture media containing 100 μM DFP for 2 h (lanes 2, 5) or for 6 h (lanes 3, 6). Cells were harvested, and nuclear extracts were obtained. Following SDS–PAGE of lysates, immunoblotting studies for topoisomerase II alpha or β-actin were performed as described in Materials and Methods. Each lane was loaded with 30 μg of protein. (D) wtCD26 Jurkat transfectants were incubated in culture media (bar I), or in culture media with 100 μM DFP for 4 h (bar II), or they were incubated in culture media with 100 μM DFP for 4 h, then washed twice in PBS to ensure removal of DFP followed by incubation in culture media for 2 h (bar III) or 8 h (bar IV). A representative sample of cells reflecting each treatment condition was obtained, and DPPIV enzyme activity assays were then performed as described in Materials and Methods. (E) wtCD26 Jurkat transfectants were incubated in culture media (lane 1), or in culture media with 100 μM DFP for 4 h (lane 2), or they were incubated in culture media with 100 μM DFP for 4 h, then washed twice in PBS to ensure removal of DFP followed by incubation in culture media for 2 h (lane 3) or 8 h (lane 4). Cells were then harvested and nuclear extracts were obtained. Following SDS–PAGE of lysates, immunoblotting studies for topoisomerase II alpha or β-actin were performed as described in Materials and Methods. Each lane was loaded with 30 μg of protein.
Effect of soluble CD26 molecules on topoisomerase II alpha expression and sensitivity to doxorubicin
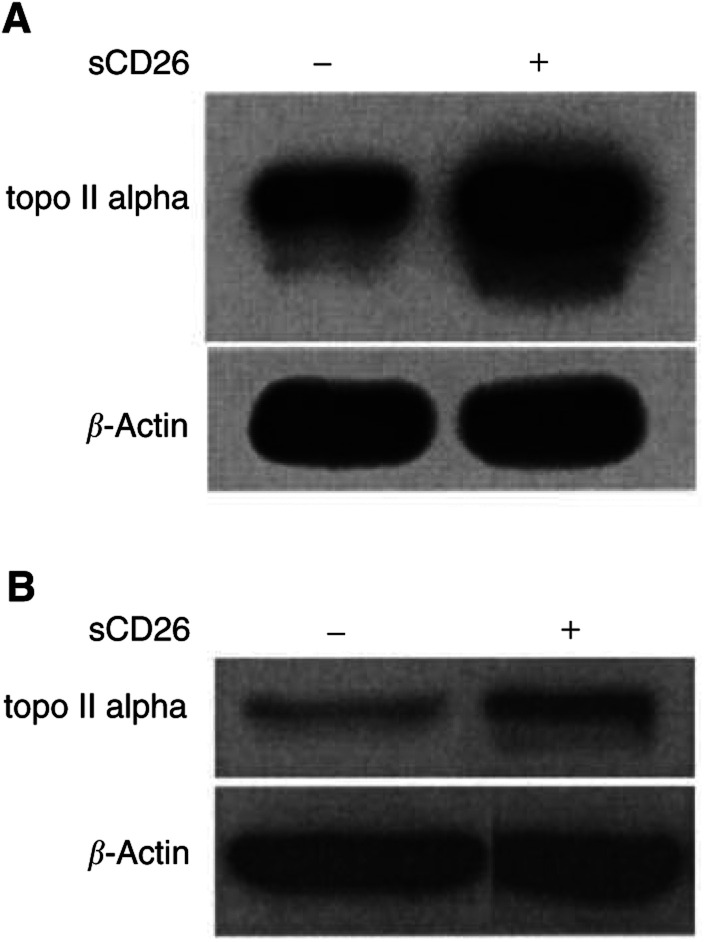
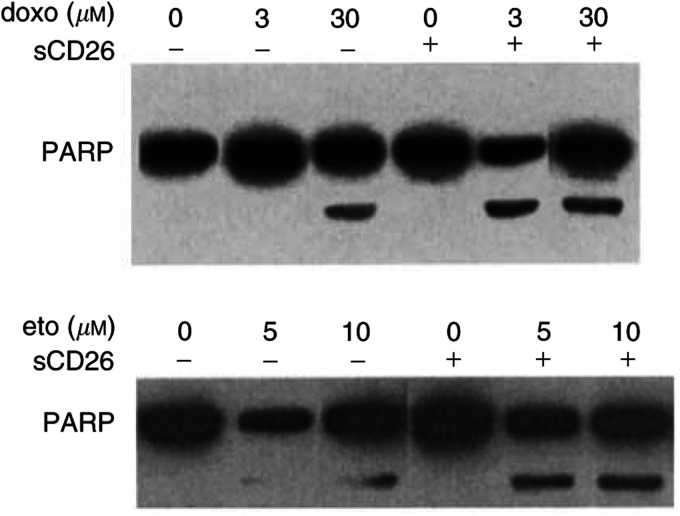
It is theoretically possible that DPPIV effect is dependent on surface expression of the intact CD26/DPPIV molecule. To address this issue, we evaluate the effect of soluble CD26 (sCD26) molecules on topoisomerase II alpha expression. As shown in Figure 6, incubation with sCD26 molecules results in a significant increase in topoisomerase II alpha protein expression in parental control Jurkat cells (A) or Jiyoye cells (B). Along with an increase in topoisomerase II alpha expression, incubation of parental Jurkat cells with sCD26 molecules also results in enhanced doroxubicin-induced or etoposide-induced PARP cleavage (Figure 7). Our findings further confirm that the presence of DPPIV activity itself, and not necessarily surface expression of CD26/DPPIV, augments topoisomerase II alpha expression, leading to a resultant increase in sensitivity to topoisomerase II inhibitors.
Figure 6.
Effect of soluble CD26 molecules on topoisomerase II alpha expression. Parental Jurkat cells (A) or Jiyoye cells (B) were incubated overnight in culture media alone (−) or culture media containing soluble CD26 (sCD26) molecules (300 μg ml−1) (+) at 37°C. Cells were then harvested and nuclear extracts were obtained. Following SDS–PAGE of lysates, immunoblotting studies for topoisomerase II alpha or β-actin were performed as described in Materials and Methods. Each lane was loaded with 30 μg of protein.
Figure 7.
CD26-associated enhancement of doxorubicin or etoposide-induced PARP cleavage. Parental Jurkat cells were incubated overnight in culture media alone (−) or culture media containing soluble CD26 (sCD26) molecules (300 μg ml−1) (+) at 37°C, followed by incubation with doxorubicin or etoposide at the indicated concentrations for 16 h. Cells were then harvested, and whole-cell lysates were obtained as described in Materials and Methods. Following SDS–PAGE of lysates, immunoblotting studies for PARP or β-actin were performed as described in Materials and Methods. Each lane was loaded with 30 μg of protein.
Caspase-9-dependent involvement of DR5 in etoposide-induced apoptosis in CD26 Jurkat transfectants
Expression of death receptor 5 (DR5), a member of the TRAIL (tumour necrosis factor-related apoptosis-inducing ligand) family, is upregulated following treatment with such DNA-damaging agents as doxorubicin and etoposide (Gibson et al, 2000). We now demonstrate that etoposide treatment leads to a greater increase in the levels of the 58 kDa DR5 in wtCD26 transfectants as compared with S630A or parental Jurkat cells (Figure 8A). Interestingly, Western blotting analyses with anti-DR5 mAb also detect the expression of a smaller 32 kDa band with etoposide treatment, with its levels again being significantly higher in wtCD26 Jurkat than S630A or parental cells. In time course studies, the appearance of the 32 kDa band consistently precedes the observed increase in expression levels of the 58 kDa band.
Figure 8.
Effect of CD26/DPPIV on DR5 expression induced by etoposide treatment. (A) Jurkat cells were incubated at 37°C in culture media containing etoposide (3 μM) for the indicated time periods at the indicated doses. Cells were then harvested, and whole-cell lysates were obtained as described in Materials and Methods. Following SDS–PAGE of lysates, immunoblotting studies for DR5 and β-actin were performed as described in Materials and Methods. Each lane was loaded with 30 μg of protein. Anti- DR5 mAb detects two bands of 58 and 32 kDa. (B) Following 2 h of preincubation at 37°C with varying doses of z-LEHD-fmk, wtCD26 Jurkat transfectants were treated with 3 μM etoposide or 1 μM doxorubicin for 48 h. Cells were then harvested, and whole cell lysates were obtained as described in Materials and Methods. Following SDS–PAGE of lysates, immunoblotting studies for DR5, caspase-9, and β-actin were performed as described in Materials and Methods. Each lane was loaded with 30 μg of protein.
We have already demonstrated that etoposide-induced apoptosis in wtCD26 Jurkat transfectant involves caspase-9 processing. To determine whether the enhancement in DR5 expression in etoposide-treated wtCD26 Jurkat is dependent on caspase-9-related events, we examined DR5 expression in cells treated with etoposide following preincubation with the caspase-9-specific inhibitor z-LEHD-fmk. As demonstrated in Figure 8B, the increase in the 58 kDa band seen in etoposide-treated wtCD26 cells is significantly attenuated when cells are preincubated with z-LEHD-fmk. In addition, the 32 kDa band induced by etoposide is no longer detectable with z-LEHD-fmk preincubation. Concordant with the findings with etoposide, pretreatment with z-LEHD-fmk similarly inhibits doxorubicin effect on DR5 status.
DISCUSSION
Through experiments involving CD26 Jurkat transfectants, DPPIV chemical inhibitor and soluble CD26/DPPIV molecules, we provide conclusive evidence that presence of DPPIV enzyme activity results in enhanced topoisomerase II alpha expression, associated with increased sensitivity to apoptosis induced by topoisomerase II inhibitors. Topoisomerase II enzyme plays an important role in the metabolism of DNA topoisomers and is essential for cellular proliferation (Wang, 1996). Two topoisomerase II isoforms, alpha and beta, exist in eukaryotes, coded by two different genes (Drake et al, 1989; Goswami et al, 1996). In particular, the 170 kDa topoisomerase II alpha isoform is closely associated with the cell cycle, being highly expressed during cellular proliferation, and is the primary target of such topoisomerase II inhibitors as doxorubicin or etoposide (Burden and Osheroff, 1998). These drugs selectively exploit the catalytic activity of topoisomerase II alpha to create DNA damage by increasing the frequency and duration of DNA cleavage sites, causing permanent double-stranded breaks and leading to apoptosis (Froelich-Ammon and Osheroff, 1995; Beck et al, 1999; Mow et al, 2001). Owing to this mechanism of toxicity, increased enzyme level is associated with enhanced sensitivity, and drug resistance is related with reduced topoisomerase II alpha level (Beck et al, 1993, Oloumi et al, 2000). Our current findings that the enhanced topoisomerase II alpha expression associated with CD26/DPPIV presence results in greater sensitivity to apoptosis induced by doxorubicin and etoposide are therefore consistent with the known mechanism of action of these topoisomerase II inhibitors.
Etoposide or doxorubicin engages the caspase-9-related mitochondrial pathway of apoptosis (Sun et al, 1999). It is also known that perturbation of Bcl-2-related proteins such as Bcl-xl is important for apoptotic processes associated with drug-induced DNA damage, potentially augmenting death signals from the mitochondrial pathway (Liu et al, 1996; Kluck et al, 1997; Fujita et al, 1998). Our results, including those demonstrating increased drug-induced Bcl-xl cleavage associated with CD26/DPPIV expression and the effect of the caspase-9-specific inhibitor z-LEHD-fmk, are consistent with the conclusion that the CD26/DPPIV-associated increase in apoptosis induced by the topoisomerase II inhibitors is mediated through caspase-9 processing and the mitochondrial pathway. Nevertheless, it is theoretically possible that CD26 exerts its influence on cell growth inhibition via other additional pathway(s).
Our results also indicate that CD26/DPPIV expression is associated with enhancement of not only the 58 kDa DR5 protein, but also the smaller 32 kDa form following topoisomerase II inhibitor treatment of Jurkat cells. While being consistent with previous reports demonstrating that DR5 expression is upregulated following treatment with DNA-damaging agents (Gibson et al, 2000), our work also demonstrates the existence of the smaller 32 kDa band. While the exact relationship between the 32 kDa band and the full-length 58 kDa band remains to be elucidated, one potential explanation from our work would be that the smaller band represents a precursor form of DR5. Our time course experiments show that while the 32 kDa band is not detected in untreated cells, its appearance in cells treated with etoposide precedes the detectable increase in the expression levels of the 58 kDa band. Additionally, the pretreatment with the caspase-9 inhibitor z-LEHD-fmk completely inhibits topoisomerase II inhibitor-induced expression of the 32 kDa band while abrogating the increased expression of the 58 kDa band. Besides our demonstration of the existence of the 32 kDa band, our work also reveals a functional relationship between caspase-9 and DR5. Previous work has indicated that death signals related with DR5 are subsequently transmitted downstream to caspase-9 processing events (Gibson et al, 2000). However, our findings suggest that caspase-9 processing also affects DR5 expression following drug-induced DNA damage, since pretreatment with the caspase-9 inhibitor z-LEHD-fmk negatively affects expression levels of both the 58 and 32 kDa bands in topoisomerase II inhibitor-treated cells.
Previously published work suggested that CD26/DPPIV expression renders human T cells more responsive to activation signals from various stimuli (Dang and Morimoto, 2002). In addition, CD26 expression on selected human tumours are associated with aggressive tumour behaviour (Carbone et al, 1995; Sato and Dang, 2003). Our present findings of the association between CD26/DPPIV and topoisomerase II alpha expression may potentially provide an explanation for these previous observations. In view of the role played by topoisomerase II alpha in cellular proliferation, it is possible that the biological behaviour of these CD26-bearing cells reflects in part the higher levels of topoisomerase II alpha. Our present work thus provides additional evidence of the essential role of the multifaceted CD26 molecule in cellular processes. Furthermore, along with our recent study indicating an antitumour effect of anti-CD26 mAb (Ho et al, 2001), our findings may provide insights into the design of future novel treatments against selected human tumours based on our knowledge of CD26 biology.
Acknowledgments
NH Dang is supported by grants from the MD Anderson Cancer Center Physician-Scientist Program, the V Foundation, and the Gillson Longenbaugh Foundation. C Morimoto is supported by National Institutes of Health Grant AR33713. K. Sato is supported by a grant from the Eli Lilly Japan International Fellowship.
References
- Aytac U, Claret F-X, Ho L, Sato K, Ohmura K, Mills GB, Cabanillas F, Morimoto C, Dang NH (2001) Expression of CD26 and its associated depeptidyl peptidase IV enzyme activity enhances sensitivity to doxorubicin-induced cell cycle arrest at the G2/M checkpoint. Cancer Res 61: 7204–7210 [PubMed] [Google Scholar]
- Aytac U, Sato K, Yamochi T, Ohnuma K, Mills GB, Morimoto C, Dang NH (2003) Effect of CD26/dipeptidyl peptidase IV on Jurkat sensitivity to G2/M arrest induced by topoisomerase II inhibitors. Br J Cancer 88: 455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauvois B, De Meester I, Dumont J, Rouillard D, Zhao HX, Bosmans E (1999) Costitutive expression of CD26/dipeptidyl peptidase IV on peripheral blood B lymphocytes of patients with B chronic lymphocytic leukeamia. Br J Cancer 79: 1042–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck WT, Danks MK, Wolverton, JS, Kim R, Chen M (1993) Drug resistance associated with altered DNA topoisomerase II. Adv Enzyme Regul 33: 113–127 [DOI] [PubMed] [Google Scholar]
- Beck WT, Morgan SE, Mo YY, Bhat UG (1999) Drug resist. Tumor cell resistance to DNA topoisomerase II inhibitors: new developments. Drug Resist Update 2: 382–389 [DOI] [PubMed] [Google Scholar]
- Burden DA, Osheroff N (1998) Mechanism of action of eukaryotic topoisomerase II and drugs targeted to the enzyme. Biochim Biophys Acta 1400: 139–154 [DOI] [PubMed] [Google Scholar]
- Carbone A, Gloghini A, Zagonel V, Aldinucci D, Gattei V, Degan M, Improta S, Sorio R, Monfardini S, Pinto A (1995) The expression of CD26 and CD40 ligand is mutually exclusive in human T-cell non-Hodgkin's lymphomas/leukemias. Blood 86: 4617–4626 [PubMed] [Google Scholar]
- Dang NH, Hafler DA, Schlossman SF, Breitmeyer JB (1990a) FcR-mediated crosslinking of Ta1 (CDw26) induces human T lymphocyte activation. Cell Immunol 125: 42–57 [DOI] [PubMed] [Google Scholar]
- Dang NH, Morimoto C (2002) CD26: an expanding role in immune regulation and cancer. Histol Histopathol 17: 1213–1226 [DOI] [PubMed] [Google Scholar]
- Dang NH, Torimoto Y, Deusch K, Schlossman SF, Morimoto C (1990b) Comitogenic effect of solid-phase immobilized anti-1F7 on human CD4 T cell activation via CD3 and CD2 pathways. J Immunol 144: 4092–4100 [PubMed] [Google Scholar]
- Dang NH, Torimoto Y, Schlossman SF, Morimoto C (1990c) Human CD4 helper T cell activation: functional involvement of two distinct collagen receptors, 1F7 and VLA integrin family. J Exp Med 172: 649–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang NH, Torimoto Y, Shimamura K, Tanaka T, Daley JF, Schlossman SF, Morimoto C (1991) 1F7 (CD26): a marker of thymic maturation involved in the differential regulation of the CD3 and CD2 pathways of human thymocyte activation. J Immunol 147: 2825–2832 [PubMed] [Google Scholar]
- Dang NH, Torimoto Y, Sugita K, Daley JF, Schow P, Prado C, Schlossman SF, Morimoto C (1990d) Cell surface modulation of CD26 by anti-1F7 monoclonal antibody. Analysis of surface expression and human T cell activation. J Immunol. 145: 3963–3971 [PubMed] [Google Scholar]
- Drake FH, Hofmann GA, Bartus HF, Mattern MR, Crooke ST, Mirabelli CK (1989) Biochemical and pharmacological properties of p170 and p180 forms of topoisomerase II. Biochemistry 28: 8154–8160 [DOI] [PubMed] [Google Scholar]
- Froelich-Ammon SJ, Osheroff N (1995) Topoisomerase poisons: harnessing the dark side of enzyme mechanism. J Biol Chem 270: 21429–21432 [DOI] [PubMed] [Google Scholar]
- Fujita N, Nagahashi A, Nagashima K, Rokudai S, Tsuruo T (1998) Acceleration of apoptotic cell death after the cleavage of Bcl-XL protein by caspase-3-like proteases. Oncogene 17: 1295–1304 [DOI] [PubMed] [Google Scholar]
- Gibson SB, Oyer R, Spalding AC, Anderson SM, Johnson GL (2000) Increased expression of death receptor 4 and 5 synergizes the apoptosis response to combined treatment with etoposide and TRAIL. Mol Cell Biol 20: 205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami PC, Roti JL, Hunt CR (1996) The cell cycle-coupled expression of topoisomerase II alpha during S phase is regulated by mRNA stability and is disrupted by heat shock or ionizing radiation. Mol Cell Biol 16: 1500–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haridas V, Higuchi M, Jayatilak GS, Bailey D, Mujoo K, Blake ME, Arntzen CJ, Gutterman JU (2001) Avicins: triterpenoid saponins from Acacia victoriae (Bentham) induce apoptosis by mitochondrial perturbation. Proc Natl Acad Sci USA 98: 5821–5826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L, Aytac U, Stephens LC, Ohnuma K, Mills GB, McKee KS, Neumann C, LaPushin R, Cabanillas F, Abbruzzese JL, Morimoto C, Dang NH (2001) In vitro and in vivo antitumor effect of the anti-CD26 monoclonal antibody 1F7 on human CD30+ anaplastic large cell T-cell lymphoma Karpas 299. Clin Cancer Res 7: 2031–2040 [PubMed] [Google Scholar]
- Ishii T, Ohnuma K, Murakami A, Takasawa N, Kobayashi S, Dang NH, Schlossman SF, Morimoto C (2001) CD26-mediated signaling for T cell activation occurs in lipid rafts through its association with CD45RO. Proc Natl Acad Sci USA 98: 12138–12143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D, Dang NH, Duvic M, Washington LT, Huh, YO (2001) Absence of CD26 expression is a useful marker for diagnosis of T-cell lymphoma in peripheral blood. Am J Clin Pathol 115: 885–892 [DOI] [PubMed] [Google Scholar]
- Kajiyama H, Kikkawa F, Suzuki T, Shibata K, Ino K, Mizutani S (2002) Prolonged survival and decreased invasive activity attributable to dipeptidyl peptidase IV overexpression in ovarian carcinoma. Cancer Res 62: 2753–2757 [PubMed] [Google Scholar]
- Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD (1997) The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275: 1132–1136 [DOI] [PubMed] [Google Scholar]
- Koreeda Y, Hayakawa M, Ikemi T, Abiko Y, Arch (2001) Isolation and characterisation of dipeptidyl peptidase IV from Prevotella loescheii ATCC 15930. Oral Biol 46: 759–766 [DOI] [PubMed] [Google Scholar]
- Lauber K, Appel HA, Schlosser SF, Gregor M, Schulze-Osthoff K, Wesselborg S (2001) The adapter protein apoptotic protease-activating factor-1 (Apaf-1) is proteolytically processed during apoptosis. J Biol Chem 276: 29772–29781 [DOI] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86: 147–157 [DOI] [PubMed] [Google Scholar]
- Morimoto C, Torimoto Y, Levinson G, Rudd CE, Schrieber M, Dang NH, Letvin N, Schlossman SF (1989) 1F7, a novel cell surface molecule, involved in helper function of CD4 cells. J Immunol 143: 3430–3439 [PubMed] [Google Scholar]
- Morrison ME, Vijayasaradhi S, Engelstein D, Albino AP, Houghton AN (1993) A marker for neoplastic progression of human melanocytes in a cell surface ectopeptidase. J Exp Med 177: 1135–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mow BM, Blajeski AL, Chandra J, Kaufmann SH (2001) Apoptosis and the response to anticancer therapy. Curr Opin Oncol 13: 453–462 [DOI] [PubMed] [Google Scholar]
- Nishimura G, Proske RJ, Doyama H, Higuchi M (2001) Regulation of apoptosis by respiration: cytochrome c release by respiratory substrates. FEBS Lett 505: 399–404 [DOI] [PubMed] [Google Scholar]
- Oloumi A, MacPhail SH, Johnston PJ, Banath JP, Olive PL (2000) Changes in subcellular distribution of topoisomerase II alpha correlate with etoposide resistance in multicell spheroids and xenograft tumors. Cancer Res 60: 5747–5753 [PubMed] [Google Scholar]
- Oravecz T, Pall M, Roderiquez G, Gorrell MD, Ditto M, Nguyen NY, Boykins R, Unsworth E, Norcross MA (1997) Regulation of the receptor specificity and function of the chemokine RANTES (regulated on activation, normal T cell expressed and secreted) by dipeptidyl peptidase IV (CD26)-mediated cleavage. J Exp Med 186: 1865–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins CL, Fang G, Kim CN, Bhalla KN (2000) The role of Apaf-1, caspase-9, and bid proteins in etoposide- or paclitaxel-induced mitochondrial events during apoptosis. Cancer Res 60: 1645–1653 [PubMed] [Google Scholar]
- Raynal P, Pollard HB (1994) Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta 1197: 63–93 [DOI] [PubMed] [Google Scholar]
- Sato K, Dang NH (2003) CD26: a novel treatment target for T-cell lymphoid malignancies? Int J Oncol 22: 481–497 [PubMed] [Google Scholar]
- Sedo A, Revoltella RP (1995) Detection of dipeptidyl peptidase IV in glioma C6 and neuroblastoma SK-N-SH cell lines. Biochem Cell Biol 73: 113–115 [DOI] [PubMed] [Google Scholar]
- Stecca BA, Nardo B, Chieco P, Mazziotti A, Bolondi L, Cabaralli AJ (1997) Aberrant dipeptidyl peptidase IV (DPPIV/CD26) expression in human hepatocellular carcinoma. Hepatology 27: 337–345 [DOI] [PubMed] [Google Scholar]
- Sun XM, MacFarlane M, Zhuang J, Wolf BB, Green DR, Cohen GM (1999) Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J Biol Chem 274: 5053–5060 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Duke-Cohan JS, Kameoka J, Yaron A, Lee I, Schlossman SF, Morimoto C (1994) Enhancement of antigen-induced T-cell proliferation by soluble CD26/dipeptidyl peptidase IV. Proc Natl Acad Sci USA 91: 3082–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Umeki K, Yamamoto I, Sakamoto M, Noguchi S, Ohtaki S (1995) CD26 (dipeptidyl peptidase IV/DPPIV) as a novel molecular marker for differentiated thyroid carcinoma. Int J Cancer 64: 326–333 [DOI] [PubMed] [Google Scholar]
- Wang JC (1996) DNA topoisomers. Annu Rev Biochem 65: 635–692 [DOI] [PubMed] [Google Scholar]