Abstract
The heterogeneous nuclear ribonucleoprotein K (hnRNP K), is a ubiquitously expressed protein that interacts with signal transducers, proteins that modulate gene expression and selective RNA and DNA motifs. K protein is modified in response to extracellular signals and directly regulates rates of transcription and translation. We used serum-treated hepatocyte culture, liver after partial hepatectomy and hepatic neoplasms as systems to compare expression, subcellular distribution and tyrosine phosphorylation of K protein in quiescent and dividing cells. The results show that expression of K protein mRNA was increased in states of enhanced proliferation. Levels of nuclear K protein were also higher in proliferating compared to resting cells. In contrast, levels of cytoplasmic K protein were the same or lower in dividing compared to quiescent cells. States of enhanced proliferation were also associated with increased levels of K protein tyrosine phosphorylation. Nuclear shift of K protein in dividing cells may reflect involvement of K protein in signalling multiple events that regulate expression of genes in proliferating cells.
Keywords: hnRNP K protein, proliferation, hepatocyte culture, hepatectomy, hepatic neoplasms
The heterogeneous nuclear ribonucleoprotein K (hnRNP K) protein is an abundant and ubiquitous factor that interacts with a diverse types of molecules (Bomsztyk et al, 1997) including RNA (Dejgaard et al, 1994), DNA (Ostrowski et al, 1994), factors involved in chromatin remodelling (Denisenko and Bomsztyk, 1997; Shnyreva et al, 2000), transcription (Du et al, 1993), RNA splicing (Shnyreva et al, 2000) and translation (Bomsztyk et al, 1997). K protein also interacts with factors involved in signal transduction, including inducible kinases (Weng et al, 1994; Bustelo et al, 1995; Van Seuningen et al, 1995). The diversity of its interactions can be accounted for by K protein's modular structure (Bomsztyk et al, 1997). K protein is modified in response to changes in extracellular environment including cytokines, growth factors and oxidative stress (Ostrowski et al, 1991; 2000a). Changes in K protein phosphorylation regulate its interaction with nucleic acids and protein partners (Schullery et al, 1999; Ostrowski et al, 2000a; Ostareck-Lederer et al, 2002). These properties suggest that K protein bridges signal transduction pathways to nucleic acid-directed processes. In further support of this model, several studies have shown that K protein can alter rates of transcription (Michelotti et al, 1996) and translation (Ostareck et al, 1997).
K protein contains three K homology (KH) domains (Siomi et al, 1993) that are highly conserved in organisms as diverse as yeast and mammals. In Drosophila, the KH domain-containing product, bancal, is most closely related to the mammalian K protein. Null and weak alleles of bancal impair adult appendages morphogenesis (Charroux et al, 1999; Gates and Thummel, 2000). This phenotype reflects decreased cell proliferation in the imaginal disc cells (Charroux et al, 1999). Expression of human K protein rescues these fly phenotypes, suggesting that like the structure, K protein function has also been evolutionarily conserved.
Since bancal has been shown to affect cell proliferation in Drosophila (Charroux et al, 1999; Gates and Thummel, 2000), we wondered if K protein expression is altered in proliferating mammalian cells. To explore the role of K protein in mitogenic responses, we chose hepatic cell lineage because cell cultures and useful models of enhanced proliferative states in intact organs and cancer are available. Our study showed increased expression of K protein mRNA and a nuclear shift of K protein in cultured serum-treated hepatocytes, in regenerating livers postpartial hepatectomy and in hepatic neoplasms.
MATERIAL AND METHODS
Cells
Rat hepatoma cells expressing human insulin receptors, HTC-IR, were grown in plastic cell culture flasks in DME media supplemented with 10% FBS, 2 mM glutamine, penicillin (100 U ml−1), streptomycin (0. 01%), and humidified with 7/93% CO2/air gas mixture.
Animals
Mice were housed under constant room temperature with a 12 : 12-h light–dark and permitted free access to water and to standard food pellets. The animals received humane care in compliance with the regulation of Cancer Center.
Experiments utilised CBA-T6/W mice, which had developed spontaneous hepatocellular neoplasms, and BALB/c mice with liver implanted L1 sarcoma tumors (Ostrowski et al, 2000c). L1 sarcoma cell line was propagated under standard conditions in MEM supplemented with 10% FCS and antibiotics. BALB/c mice, 2 months old, were anaesthetised with ether for laparotomy, and 105 viable L1 cells suspended in 0.05 ml of PBS were injected under the capsule of left lateral lobe of the liver. After 3 weeks, animals with implanted hepatic tumours were used in these studies. Mice anaesthetised by ether were killed, the livers were rapidly resected and classified by gross examination into tumour and hepatic tissue. Two portions of each specimen were frozen in liquid nitrogen and stored in −80°C until use. The remaining portions were fixed in formalin and embedded in paraffin for histological examination.
Partial hepatectomy was performed under ether anaesthesia on 2-month-old BALB/c male mice. Under aseptic conditions animals were subjected to mid-ventral laparatomy and resection of the left lateral lobe which constitutes about one-third of the total liver. The removed lobe was immediately frozen in liquid nitrogen and stored at −80°C until use. After the indicated times, animals were killed and the remnant liver was rapidly collected and frozen. Sham-operated animals underwent mid-ventral laparatomy without resection of liver lobe.
Northern blot analysis and RT–PCR
Total RNA was prepared from tissues and cell pellets using TRIzol reagent. A measure of 10 μg of total RNA was denatured with formaldehyde–formamide and electrophoresed in a 1% agarose/formaldehyde gel. RNA was then transferred to Hybond N nylon membranes in 10 × SSC. The membranes were incubated at 65°C for 20 h with 2 × 107 d.p.m. of the 32P-labelled DNA probes in the hybridisation buffer (6 × SSC, 0.5% Ficoll, 0.5% PVP, 0.5% BSA, 0.5% SDS, 100 μg ml−1 herring sperm DNA). Excess probe was removed from the membrane by serial washes at 65°C in 1 × SSC/0.1% SDS. The hybridised probes were visualised by autoradiography.
A measure of 5 μg of total RNA was reverse transcribed using Superscript II RT (GIBCO-BRL) and oligo-dT in 20 μl volume as per the manufacturer's protocol. RT reactions were diluted 1 : 10 with water, and PCR was carried out as described previously (Ostrowski et al, 2003b) using 2 μl of cDNAs and primers for c-myc. [α-32P]dCTP (NEN) was used to label the PCR products. PCR products were resolved on native 5% polyacrylamide gels, then the gels were dried and the PCR products were quantified using a phosphorimager. Densitometric analysis was performed using OptiQuant™ Image Analysis Software (Packard). The levels of band intensities after background subtraction were expressed in digital light units (DLU).
Cell extracts
Cytoplasmic and nuclear extracts were prepared as described previously (Ostrowski et al, 1991,2000b). Separation of the nuclear fraction from the cytosol was monitored with the cytosolic enzyme marker lactic dehydrogenase and the purity of nuclear extracts was greater than 92%. The protein concentration was measured using MicroBCA protein assay, Pierce Biotechnology (Rockford, IL, USA).
Electrophoresis and immunoblotting
Equal amounts of sample protein (50 μg) were separated by 10% SDS–PAGE and immunostained by standard methods as described previously (Ostrowski et al, 1991,2000b).
Cell proliferation
DNA synthesis was determined by the incorporation of 3H-thymidine. Exponentially growing HTC-IR cells were harvested, seeded at a density of 5 × 103 per well in 96-well plates and then grown in DMEM containing 10% FBS. After 24 h, cells were made quiescent by 48 h serum deprivation and then they were treated with 15% FBS. Cell growth was monitored at 3, 6 and 24 by adding 0.5 μCi of 3H-thymidine to each well for 3 h. Finally, cells were harvested, DNA was collected on GFC filters, and the radioactivity was determined by scintillation counting. Three independent experiments were performed and all assays were repeated in octuplicate.
Mice with spontaneous hepatocellular neoplasms and those after sham or partial hepatectomy were given 25 μCi of 3H-thymidine intraperitoneally 1 h prior to euthanasia. Hepatic DNA was extracted by proteinase K digestion followed by phenol–chloroform extraction, and the quantity of the DNA was measured spectrometrically. The radioactivity of 100-μg samples of total hepatic DNA was determined by scintillation counting.
Quantification
Quantification analysis was performed either by photographing with a digital camera (DC40, Kodak) or scanning the intensity of the bands with an ImageScanner, Amersham Pharmacia Biotech or by scanning 32P radioactivity with a Phosphorimager apparatus. Densitometric analysis was performed using OptiQuant™ Image Analysis Software (Packard).
Statistical analysis
Results are presented as means+s.d. Significant difference between mean values was assessed by means of analysis of variance (ANOVA). P-values for differences from control results were calculated using the Bonferroni method. Means were considered to be different if P<0.05.
RESULTS
Expression and subcellular localisation of K protein from serum-treated hepatocyte culture
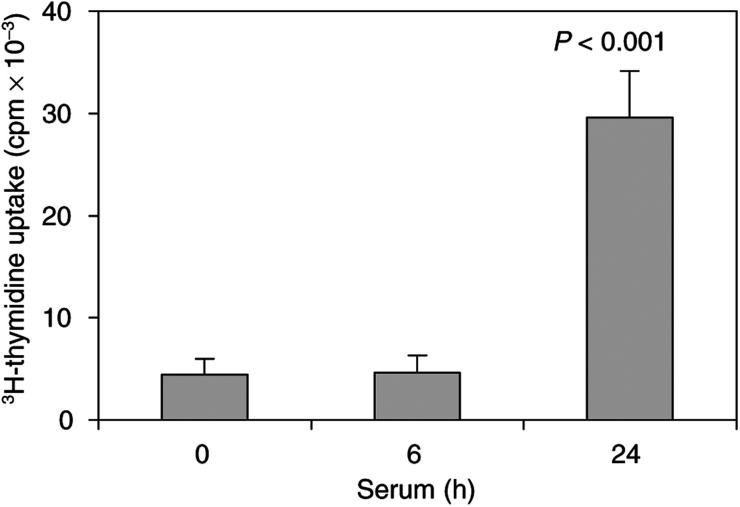
The HTC-IR cells have been a useful system to study signal transduction and gene expression in hepatocyte line grown in culture (Iwamoto et al, 1981; Ostrowski et al, 2001). We used 3H-thymidine uptake to test their proliferative response to serum treatment. Quiescent HTC-IR cells preincubated with 3H-thymidine were treated with 15% serum. At given time points, cells were harvested and 3H counts were measured by scintillation counter. Results shown in Figure 1 illustrate that increased 3H-thymidine uptake into DNA was not seen at 6 h, but there was a strong increase in uptake 24 h after serum treatment. This 3H-thymidine uptake kinetics is similar to studies in other hepatoma cell lines (Kadowaki and Kitagawa, 1988). These results show that the HTC-IR cells are a suitable system to study mitogenic responses to serum treatment.
Figure 1.
3H-thymidine uptake in serum-treated hepatocytes. Cells were grown in 96-well plates in DMEM containing 10% FBS. After 24 h, cells were made quiescent by 48 h serum deprivation and then cells were either untreated (0 time point) or were treated with 15% FBS for 6 or 24 h. Cell proliferation was monitored by adding 0.5 μCi of 3H-thymidine to each well for another 3 h. The radioactivity of collected cellular DNA was determined by scintillation counting. Three independent experiments were performed and the results represent means±s.d. of radioactivity counts expressed in c.p.m.
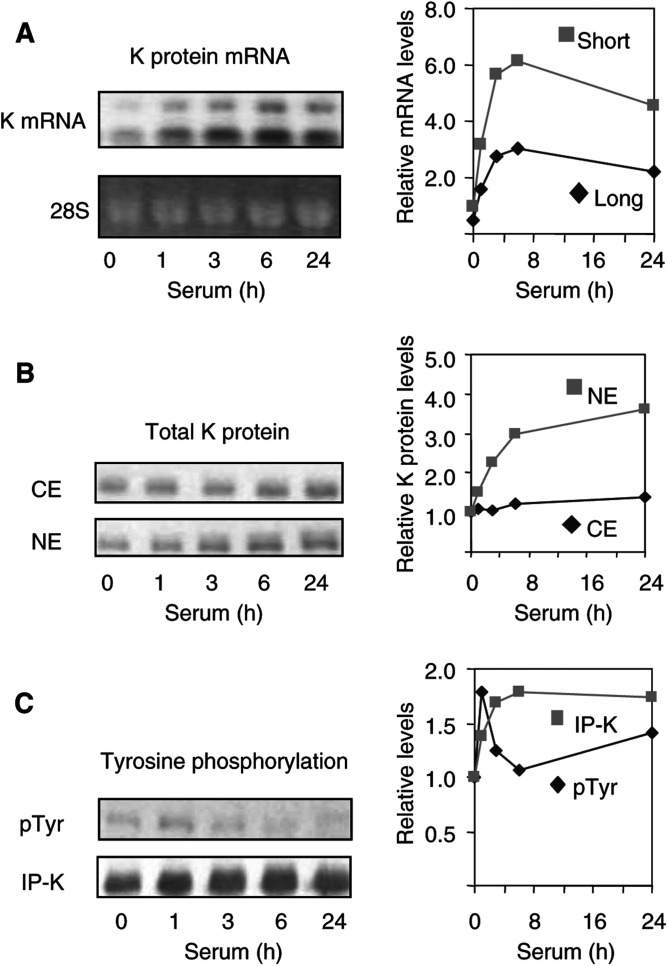
To test if induction of cell cultures to divide alters K protein expression, serum-deprived hepatoma HTC-IR cells were treated with 15% FCS. At given time points, cells were harvested and RNA and protein extracts were prepared (Figure 2). Northern blot analysis revealed two constitutively expressed K protein transcripts, 2.2 and 3.0 kb. The level of expression of the longer transcript was lower. Serum treatment of resting HTC-IR cells increased the level of both transcripts. The increase was first seen after 1 h of stimulation, peaked at 6 h and decreased after 24 h of serum treatment (Figure 2A). At peak levels (6 h), there was a six-fold increase in both the short and long transcript in response to serum treatment. These results demonstrate that induction of cell proliferation is associated with increased K protein gene expression.
Figure 2.
K protein expression in serum-treated hepatocytes. After 48 h of serum deprivation, HTC-IR cells were treated with 15% FCS. At given time points, cells were harvested, and total RNA and proteins as well as nuclear and cytoplasmic proteins were extracted. (A) Total RNA was electrophoresed in a 1% agarose/formaldehyde gel and RNA was visualised with ethidium bromide and photographed with digital camera (DC40, Kodak). After RNA transfer, the nylon membranes were probed with 32P-labelled K protein cDNA. Autoradiographs were photographed with a digital camera (DC40, Kodak). Densitometric analysis was carried out using OptiQuant™ Image Analysis Software (Packard meriden, CT, USA). The mRNA levels shown in the graph were normalised to the levels of 28S. (B) Protein extracts were resolved by SDS–PAGE followed by Western blotting with anti-K protein antibody. Blots were scanned, and densitometric analysis of K protein bands was performed using OptiQuant™ Image Analysis Software. (C) Anti-K protein immunoprecipitates from total cell extracts (300 μg protein) were resolved by SDS–PAGE and immnunobloting was performed with either anti-phosphotyrosine (pTyr) or anti-K protein (K) antibodies. Densitometric analysis was done as in (B).
Total cytoplasmic and nuclear extracts (50 μg each) were analysed by SDS–PAGE and anti-K protein immunoblotting. Western blot analysis revealed that serum treatment induced a sustained increase in K protein levels in both cytoplasmic and nuclear fractions (Figure 2B). The increase was far more pronounced in the nuclear fractions where 24 h following treatment there was a 3.6-fold increase in K protein levels compared to resting cells. At the same time point, K protein level in cytoplasmic extracts increased by 40%. These results suggest that the serum-induced increase in K protein mRNA levels (Figure 2A) results in increased K protein synthesis and that most of the newly synthesised K protein is directed to the nucleus.
K protein is tyrosine phosphorylated by Src-family of kinases (Ostrowski et al, 2000a; Ostareck-Lederer et al, 2002). Serum treatment increases tyrosine phosphorylation of many proteins involved in signal transduction and gene expression (Wang, 1994). To test if K protein is tyrosine phosphorylated in response to a mitogenic signal, K protein was immunoprecipitated with anti-K protein from whole-cell lysates of quiescent and serum-treated HTC-IR cells and the immunoprecipitates were analysed by anti-phosphotyrosine and anti-K protein immunoblots (Figure 2C). There was constitutive tyrosine phosphorylation of K protein in quiescent cells, and following serum treatment the level of tyrosine phosphorylation of K protein increased transiently. Serum treatment increased the total amount of immunoprecipitated K protein, but unlike tyrosine phosphorylation this increase was sustained. The increased level of immunoprecipitated K protein from total cell lysates largely reflects the increase in nuclear K protein (Figure 2B).
Expression and subcellular distribution of K protein in injured livers
Partial hepatectomy leads to hepatic regeneration resulting in almost complete restoration of the liver mass (Taub, 1996). The mechanisms initiating and controlling hepatocyte ‘priming’ involve activation of several nonspecific factors in the initiating phase. These cellular events include increased Na+ flux into the cells, elevation in cAMP content, activation of ornithine decarboxylase and Na+/K+-ATPase, expression of and response to growth factors and cytokines, activation of transcription factors (NF-κB, Stat-3, AP-1, C/EBP-β) and induction of immediate-early genes (e.g. c-fos, c-jun, c-myc) (Taub, 1996; Rozga, 2002; Zimmermann, 2002).
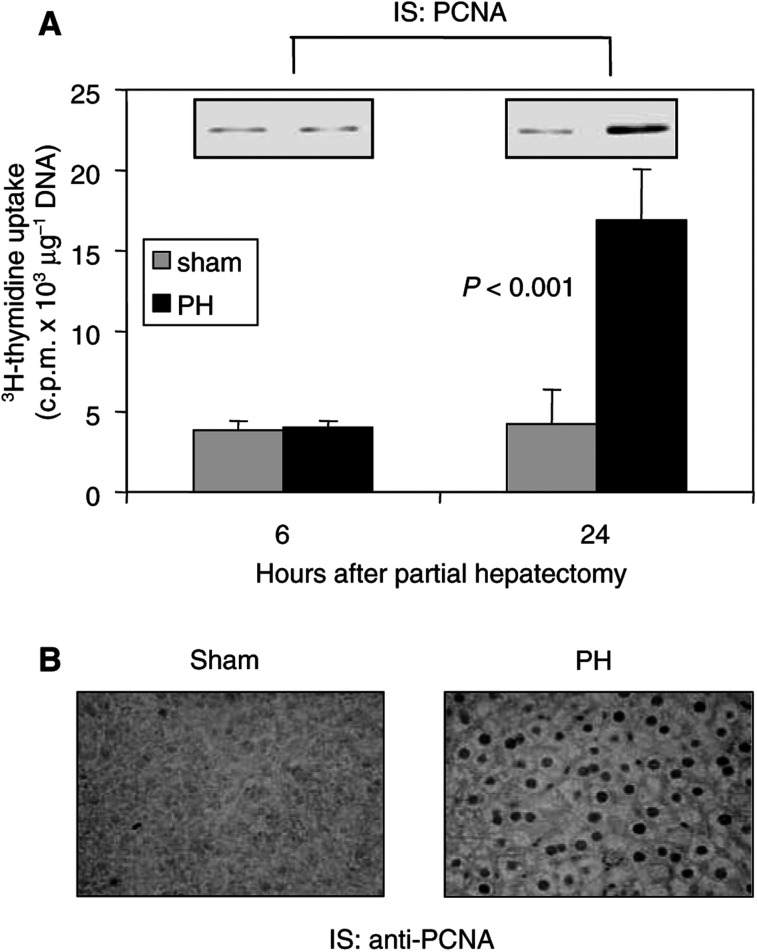
3H-thymidine uptake into the DNA was used to assess hepatocyte proliferation following partial hepatectomy. Mice were anaesthetised, and after laparatomy in one group of animals the livers were left alone (sham operated), while in the other group the left lobe of the liver was removed (30% hepatectomy)–partial hepatectomy group. Abdominal cavities were closed and the animals were allowed to recover from the ether anaesthesia. At 1 h prior to harvesting the livers, mice were injected with 3H-thymidine, animals were again ether anaesthetised and the livers were removed. As shown in Figure 3A, at 6 h following surgery, no differences in 3H-thymidine uptake into the livers were seen between sham and partial hepatectomy animals. In contrast, hepatic DNA synthesis was induced 24 h after partial hepatectomy, but no increase was seen in the control sham-operated mice. Expression of proliferating cell nuclear antigen (PCNA) correlates well with hepatocyte proliferation (Wolf and Michalopoulos, 1992). Proliferating cell nuclear antigen protein levels were determined in liver lysates at 6 and 24 h following surgery by immunoblot analysis. A representative blot is presented as an inset in Figure 3A. Not unexpectedly, the increase in the partial hepatectomy-induced DNA synthesis was mirrored by the increased PCNA expression. Liver lysates contain proteins from hepatocytes and other cell types. Thus, next we did PCNA immunocytochemistry of the livers from either sham-operated animals or animals following partial hepatectomy that allows direct visualisation of hepatocytes. In sham-operated mice there was little or no PCNA detected. In contrast, there was very strong nuclear PCNA staining of hepatocytes 24 h following partial hepatectomy (Figure 3B). These observations are consistent with previous partial hepatectomy studies carried out in rats and mice where DNA synthesis occurred in a single peak 18–24 h after resection (Lambotte et al, 1997; Freeman et al, 1999).
Figure 3.
Cell proliferation indices in liver after partial hepatectomy of anaesthetised mice. (A) Mice after sham or partial hepatectomy (PH) (three animals in each group at 6 and 24 h after surgery) were given 25 μCi of 3H-thymidine intraperitoneally 1 h prior to euthanasia. The livers were harvested, hepatic DNA was extracted and the radioactivity of 100-μg samples of total hepatic DNA was determined by scintillation counting. Total DNA was measured using a spectrophotometer. The results represent means±s.d. of radioactivity counts expressed in c.p.m. μg−1 DNA. The insets represent Western blot analysis of PCNA in liver lysates. (B) Immunocytochemistry of the liver tissues was performed as previously described (Kupryjanczyk et al, 2003). Briefly, portions of the liver tissues from sham-operated mice and animals following PH (24 h) were embedded in paraffin. After heat-induced epitope retrieval, sections were incubated with anti-PCNA antibody (1 : 4000, Santa Cruz) for 1 h at room temperature. Biotinylated goat anti-mouse IgG was used as a detection system.
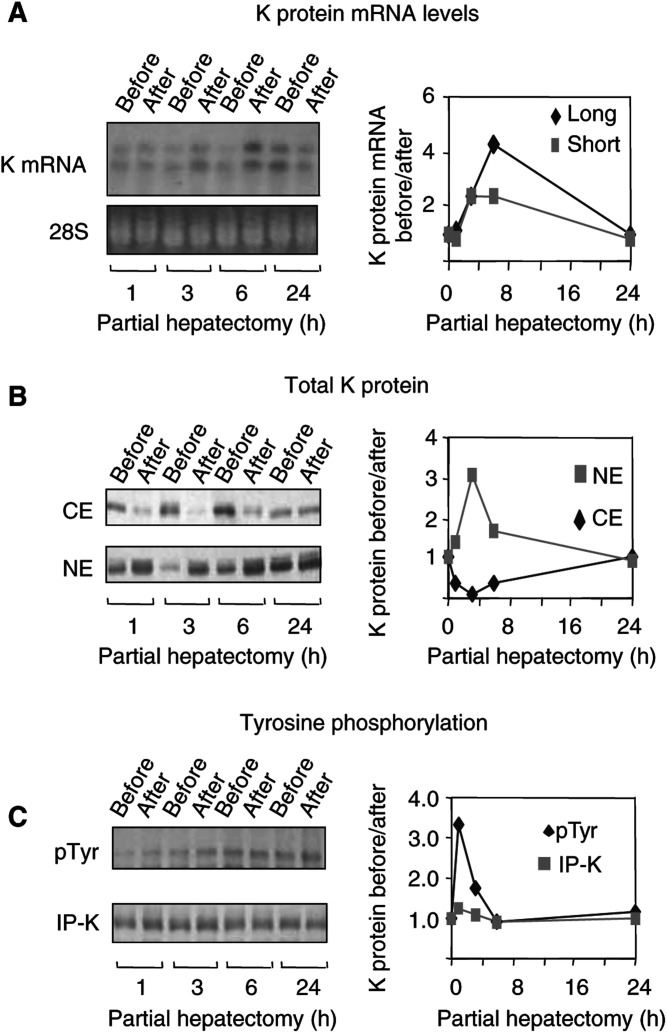
As partial hepatectomy is an excellent model of cellular proliferation in an intact organ, we used it to correlate changes in cell proliferation with K protein expression and subcellular distribution in this system (Figure 4). Mice were anaesthetised, and after laparatomy the left lobe of the liver was removed (30% hepatectomy) and rapidly frozen. Abdominal cavities were closed and the animals were allowed to recover from the ether anaesthesia. At given time points, mice were again ether anaesthetised and the remnant livers were harvested and rapidly frozen. Total RNA, total cell lysates, cytoplasmic and nuclear extracts were prepared from the resected lobes and from the remnant livers. All analyses presented were carried out by comparing levels in the remnant liver to the levels measured in the resected lobe of the same animal (Figure 4).
Figure 4.
K protein expression after partial hepatectomy in mice. (A) Total RNA was extracted from resected lobes (before) and from the remnant livers (after). Northern blots were probed with 32P-labelled K protein cDNA. The slower (long) and the faster (short) K protein mRNA transcripts (upper gel) were analysed by densinometry. The intensity of the K protein mRNA bands was normalised to the levels of 28S RNA (lower gel). The results in the graph are expressed as a ratio of levels after (after) to before (before) partial hepatectomy. (B and C) Protein extracts prepared from livers before and after partial hepatectomy were analysed by anti-K protein Western blots as in Figure 1. Results are expressed as a ratio of levels measured after (after) and before (before) partial hepatectomy of the same liver. (B) Levels of K protein in cytoplasmic (CE) and nuclear (NE) extracts. (C) Anti-K protein immunoprecipitates from whole-cell lysates analysed with anti-phosphotyrosine (pTyr) and anti-K protein (K) antibody.
As in case of HTC-IR cells (Figure 2A), Northern blot analysis showed that in an intact liver there is constitutive expression of the two transcripts of K protein (Figure 4A). The level of both transcripts increased after resection, a change first seen in the remnant liver 3 h after resection; the peak effect occurred at 6 h and the mRNA levels came down to baseline 24 h postpartial hepatectomy. These results show transient increase in K protein mRNA levels that is associated with cell proliferation in vivo.
Western blot analysis revealed that there was a transient increase in nuclear K protein, with peak levels (three-fold increase) seen 3 h postpartial hepatectomy (Figure 4B). Concurrently, there was a decrease in cytoplasmic K protein levels. At 24 h postpartial hepatectomy, the levels of K protein in the cytoplasmic and nuclear extracts from remnant livers returned to baseline. These results revealed that following partial hepatectomy, there is nuclear translocation of K protein from the cytoplasm.
Liver injury activates tyrosine kinases leading to phosphorylation of such key factors as the cyclins (Diehl and Rai, 1996; Spiewak Rinaudo and Thorgeirsson, 1997). We used immunoprecipitation assays to assess whether partial hepatectomy alters the level of tyrosine phosphorylation of K protein (Figure 4C). Anti-phosphotyrosine blots of K protein immunoprecipitations showed that in the intact liver there is constitutive tyrosine phosphorylation of K protein. Liver resection induced a transient increase in the level of K protein tyrosine phosphorylation, with the peak effect (3.5-fold increase) observed in the remnant liver 1 h after resection (Figure 4C, pTyr). Since K protein transcripts were transiently induced postpartial hepatectomy (Figure 4A), it was surprising to see that the level of K protein immunoprecipitated from total cell lysates from remnant livers did not change appreciably (Figure 4C, IP-K).
Expression and subcellular distribution of K protein in hepatic neoplasms
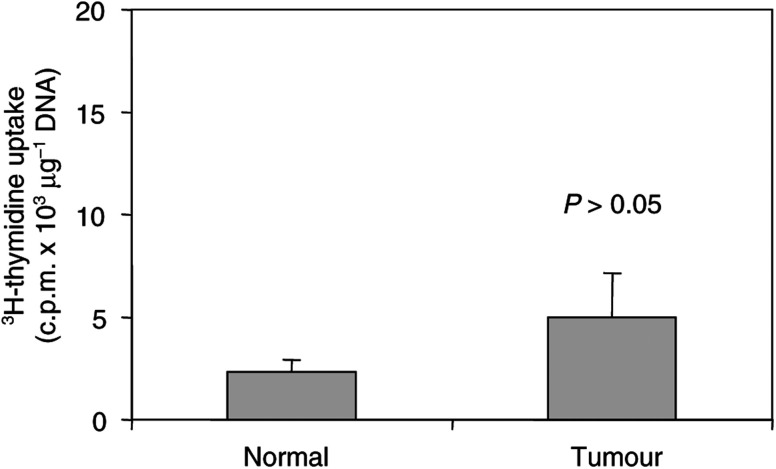
CBA-T6/W mice develop spontaneous liver tumours including hepatocellular adenoma and carcinoma (Ostrowski et al, 2000c). To assess the rates of proliferation of these tumours, we again used 3H-thymidine incorporation (Figure 5). At 1 h after injecting 3H-thymidine, mice were ether anaesthetised, the livers were resected and carcinomas were dissected out from the normal liver parenchyma. DNA was purified from normal and cancer tissue and 3H counts were assessed as before. A part of the resected tissue was used for histology. Although 3H-thymidine incorporation tended to be higher in hepatocellular carcinomas than in the surrounding histologically normal hepatic tissue, these differences were not statistically significant. This is not surprising since these tumours are slow growing (Szymanska, 1991).
Figure 5.
3H-thymidine uptake in mouse spontaneous hepatic adenocarcinoma hepatocellular carcinomas. Mice harbouring spontaneous hepatocellular carcinomas (five animals) were given 25 μCi of 3H-thymidine intraperitoneally 1 h prior to euthanasia. The livers were harvested and hepatic DNA was extracted from the liver tumours and from the normal surrounding tissues. Radioactivity of 100-μg samples of total hepatic DNA was determined by scintillation counting. The results represent means±s.d. (n=5) of radioactivity counts expressed in c.p.m μg−1 DNA.
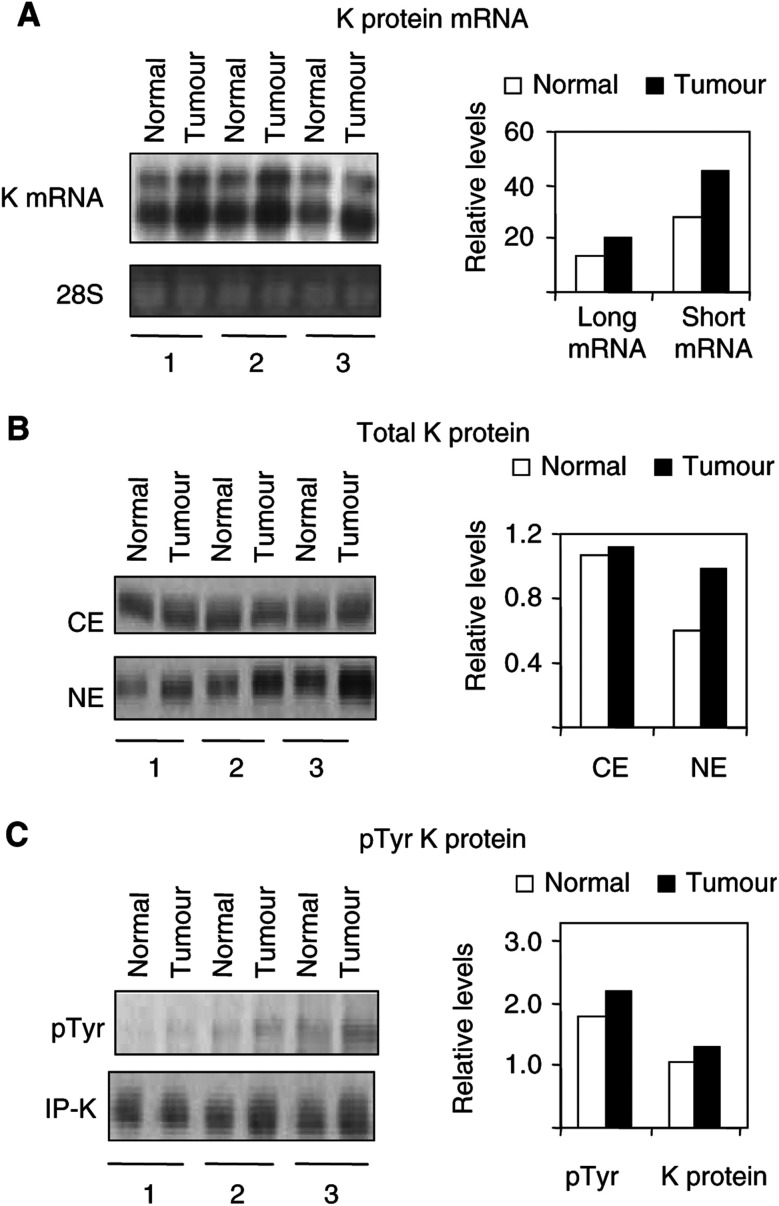
We used the mice with hepatocellular carcinoma to assess K protein expression and its subcellular distribution. The Livers harbouring tumours were resected from anaesthetised mice and carcinomas were dissected out from the normal liver parenchyma. As before, a part of the resected tissue was used for histology and the rest was rapidly frozen. Figure 6 shows finding in three animals with hepatocellular carcinoma. Northern blot analysis revealed two transcripts, and in all three animals the levels of K protein mRNA were higher in the tumour than in the surrounding normal parenchyma (Figure 6A). The increased K protein mRNA levels in cancer is consistent with the findings in serum-treated HTC-IR (Figure 2A) and in livers postpartial hepatectomy (Figure 4A).
Figure 6.
K protein expression in spontaneous hepatic adenocarcinoma in mice. (A) Total RNA was extracted from hepatic adenocarcinoma and the livers harbouring these tumours. Northern blots were probed with 32P-labelled K protein cDNA. The slower (long) and the faster (short) K protein mRNA transcripts (upper gel) were analysed by densinometry. The intensity of the K protein mRNA bands was normalised to the levels of 28S RNA (lower gel). The results in the graph are expressed as a ratio of levels measured in the adenocarcinoma (tumour) and those measured in the liver (normal) harbouring the tumour. (B and C) Protein extracts prepared from liver adenocarcinomas (tumour) and normal liver tissue (normal) surrounding these tumours were analysed by anti-K protein Western blots as in Figure 1. Results are expressed as the ratio of levels measured in the tumour (tumour) and those in the normal parenchyma (normal) of the same liver. (B) Levels of K protein in cytoplasmic (CE) and nuclear (NE) extracts. (C) Anti-K protein immunoprecipitates from whole-tissue extracts analysed with anti-phosphotyrosine (pTyr) and anti-K protein (K) antibody.
In further agreement with the other results (Figures 2 and 4), Western blots showed that in all the three animals the level of K protein in nuclear extracts was higher in the tumour than in the normal liver harbouring these cancers (an average 66% higher in the tumour) (Figure 4B). In contrast, the levels of K protein in cytoplasmic extracts from cancer were not different from the normal parenchyma. In all the three animals, the level of tyrosine phosphorylation of K protein in the tumours was higher than in the normal liver (average 20% difference), but in part this might be explained by the slightly higher levels of K protein immunoprecipitated from the cancer (Figure 6C).
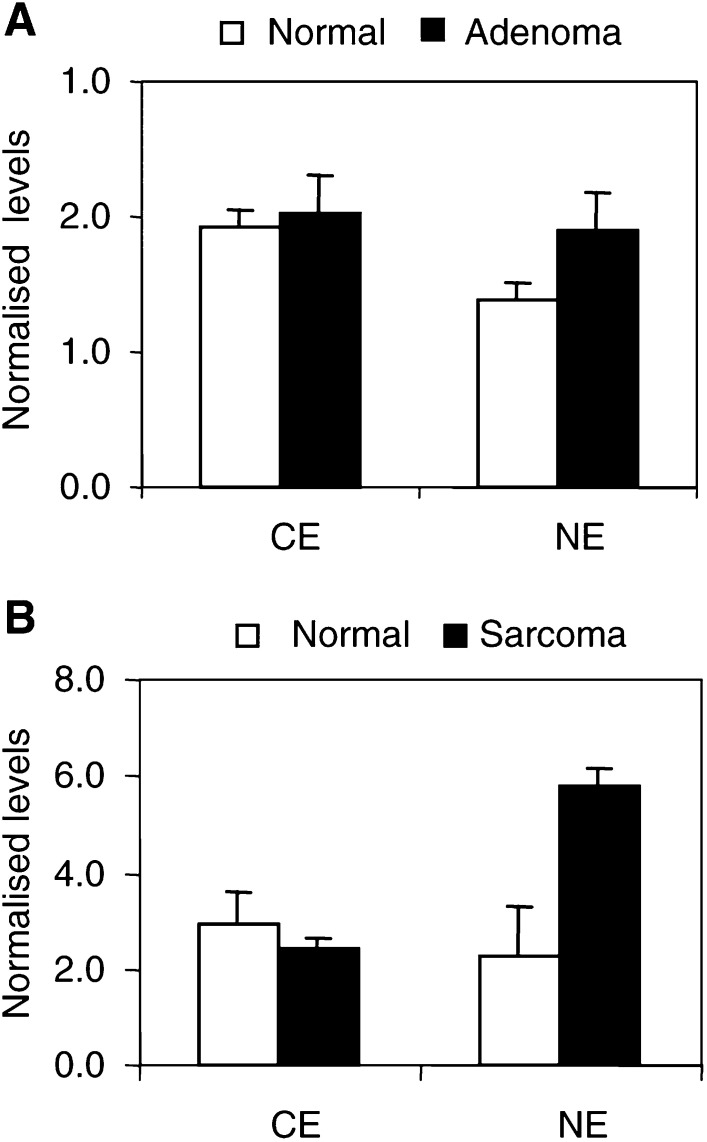
We also assessed K protein expression and subcellular distribution in hepatic adenoma (seven animals) and in L1 sarcoma implanted into liver (six animals) (Figure 7). The level of K protein in the cytoplasmic extracts from liver adenomas was the same as in the adjacent normal parenchyma (Figure 7A, CE). In six out of seven animals, the level of nuclear K protein was higher in the hepatic adenomas compared to the normal liver parenchyma surrounding the tumours (Figure 7A, NE). On an average, the level of K protein in the adenomas was 38% higher than in normal tissues. In the implanted sarcomas, which are more aggressive tumours, in all the six animals the level of nuclear K protein was higher than in the nuclear extracts from livers harbouring these tumours (Figure 7B). On an average, K protein levels in the nuclear extracts from sarcomas was 2.5-fold higher than those measured in nuclear extracts from normal liver parenchyma adjacent to these tumours. As in the hepatocellular neoplasms (Figures 6 and 7A), the levels of K protein in cytoplasmic extracts from sarcomas and normal liver tissues were not different (Figure 7B).
Figure 7.
Expression of K protein in spontaneous adenomas and implanted sarcomas in mouse livers. Cytoplasmic (CE) and nuclear (NE) extracts were prepared from spontaneous liver adenomas (adenoma), implanted sarcomas (sarcoma) and normal liver parenchyma (normal) surrounding these tumours. Levels of K protein were analysed in Western blots as in Figure 1. Results are expressed as the ratio of levels measured in the tumour (tumour) and those in the normal tissue (normal) of the same liver.
Activation of c-myc gene expression in serum-treated HTC-IR cells, in injured livers and liver tumours
Mitogenic stimulation leads to rapid induction of immediate-early genes (Herschman, 1991). Immediate-early genes are activated in a protein synthesis-independent manner and are involved in cell proliferation (Jochum et al, 2001). They regulate later phases in G1 of the cell cycle and represent diverse classes of genes including those encoding transcription factors, c-myc is one of the immediate-early genes, whose expression is transiently increased in G1. c-myc encodes a transcription factor that targets a host of genes that regulate cell proliferation (Bouchard et al, 1998). With regard to the liver, antisense oligomer to c-myc can reduce cell proliferation in the regenerating rat liver, suggesting that expression of this gene plays a role in the process of regeneration (Arora et al, 2000). In response to serum treatment, K protein is recruited to multiple sites along the c-myc locus (Ostrowski et al, 2003a). In gene reporter systems, K protein was shown to regulate c-myc promoter (Michelotti et al, 1995,1996) and translational elements activity (Evans et al, 2003). Studies in breast carcinoma lines suggested that increased expression of K protein enhanced c-Myc levels (Mandal et al, 2001). Taken together, these studies suggest that K protein regulates c-myc gene expression on both transcriptional and post-transcriptional levels.
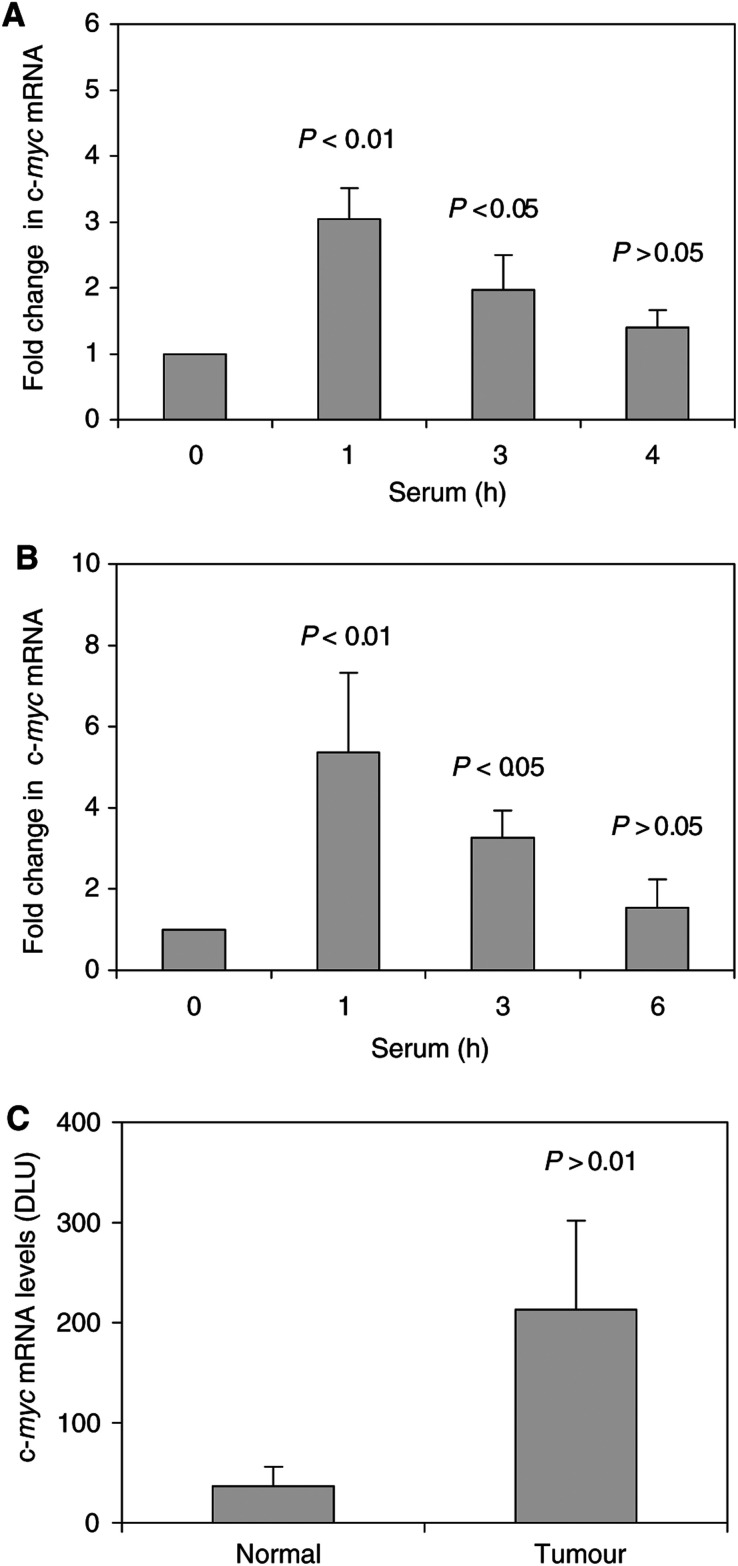
We next compared semiquantitative RT–PCR analysis of RNA in serum-treated HTC-IR cells, in resected livers and in hepatic tumours to correlate c-myc transcript levels with K protein expression in these models (Figure 8). Treatment of serum-starved HTC-IR cells rapidly induced the c-myc expression (Figure 8A), with a peak in c-myc expression seen at 1 h after serum treatment. This time course is similar to the kinetics of serum-induced transient recruitment of K protein to the promoter and transcribed region of the c-myc locus in these cells (Ostrowski et al, 2003a). The kinetics of c-myc mRNA expression after partial hepatectomy (Figure 8B) was similar to that seen in cultured serum-treated hepatocytes in vitro; a large peak in c-myc transcript levels was seen at 1 h following partial resection, and its level declined nearly to baseline at 6 h. These c-myc results are similar to the studies published by others (Taub et al, 1987). Similarly, there was also increased c-myc transcript expression in hepatocellular carcinoma compared to normal tissue, that is, an eight-fold higher levels (Figure 8C).
Figure 8.
c-myc mRNA transcript levels in serum-treated hepatocyte cultures (A), postpartial hepatectomy (B) and in hepatic tumours (C). (A) Serum-deprived HTC-IR cells were treated with 15% FBS for 0, 1, 3, 6 and 24 h. After harvesting the cells, total RNA was isolated and used in RT. PCR was carried out using [α32P]dCTP (0.5 μCi per reaction) and c-myc primers (forward: GCAAATGCTCCAGCCCCAGGTC; reverse: AGTCCCAAAGCCCCAGCCAAGGTT). PCR products were resolved by native PAGE and were quantified using a phosphorimager (Cyclone, Packard meriden, CT, USA). Results are expressed as fold increase in the mRNA level at each time point of serum treatment compared to the levels seen in untreated cells. The values represent means±s.d. (n=3). (B) Partial hepatectomy and control sham operation were carried out as in Figure 3. At given time points following surgery, the livers were harvested from each group and total RNA was isolated (three animals for each time point), c-myc mRNA levels were determined by RT–PCR as above. Results are expressed as fold increase in mRNA levels compared to sham-operated mice. (C) Comparison of c-myc mRNA levels in spontaneous hepatocellular carcinoma and the normal surrounding liver tissue, c-myc mRNA levels were determined by RT–PCR and the 32P signal of the labelled products is expressed in DLU. The results represent means±s.d. (n=5 animals).
DISCUSSION
Treatment of resting HTC-IR cells with serum resulted in sustained increase in nuclear K protein with little changes in cytoplasmic K protein (Figure 2B). Similar observations were made in hepatic tumours (Figures 6B and 7). While the levels of K protein in nuclear extracts were higher in the neoplasms compared to the adjacent normal parenchyma, the levels of K protein in the cytoplasmic fractions were the same in the neoplastic and the normal surrounding tissues. Serum-treated HTC-IR cells and tumours represent sustained states of enhanced cell proliferation. In contrast, following partial hepatectomy, the number of cycles of cell division is limited. Within minutes after partial hepatectomy, quiescent liver cells in the remaining lobes enter a state of replicative competence (an initiation phase), followed by a proliferation phase and, finally, by a termination phase (Zimmermann, 2002). Thus, it is not surprising that postpartial hepatectomy there was only a transient nuclear shift of K protein that peaked at 3 h following liver injury and by 6 h it was near baseline. The kinetics of the nuclear shift of K protein in response to serum treatment and following liver injury provides some clues regarding the consequences of this effect. In response to serum treatment, K protein is recruited to multiple sites along the c-myc locus including both the promoter and the transcribed regions (Ostrowski et al, 2003a). K protein binds in vitro CT-rich DNA from the c-myc promoter (Michelotti et al, 1995) and activates c-myc promoter activity in several types of cells including breast cancer (Michelotti et al, 1996; Mandal et al, 2001). Here, we have shown that c-myc expression is activated in hepatocyte culture in response to mitogens and following liver injury (Figure 8A and B). Taken together, these results suggest that at an early time point following liver injury K protein may play a role in the induction of c-myc gene. Expression of c-fos and egr-1 is also activated in remnant liver following partial hepatectomy (Taub, 1996). Promoters of both c-fos and egr-1 genes contain putative CT-like K protein-binding motifs, and in response to serum treatment of hepatocyte culture K protein is recruited to both egr-1 (Ostrowski et al, 2003a) and c-fos (unpublished observations) loci. It is, therefore, plausible that the role of the K protein fraction that is newly directed to the nucleus in remnant livers is to regulate transcription of these immediate-early genes and other factors that mediate the enhanced cell proliferation during an initiation (prereplicative) phase. Here, the increase in the level of tyrosine phosphorylation of K protein (Figure 4C) may play a role in its nuclear translocation and/or its recruitment to inducible transcribed gene loci such as the immediate-early genes (Ostrowski et al, 2003a).
Increased K protein expression has previously been found in breast cancer cells. In that study, the authors provide evidence that the increased K protein levels contribute to the enhanced c-myc gene expression in these tumours (Mandal et al, 2001). The current study demonstrates for the first time that there is a nuclear shift of K protein in hepatic neoplasm and other states of enhanced proliferation. As discussed above, there is considerable evidence that K protein regulates c-myc gene expression, so the increased level of K protein in the nucleus seen in tumours (Figures 6 and 7) may play a role in enhanced expression of this immediate-early gene in malignancy. K protein binding appears to be genomewide, where it exhibits both constitutive and inducible interactions with chromatin (Ostrowski et al, 2003a). Some of these interactions may reflect direct binding of K protein to DNA. However, K protein binds many proteins, DNA and RNA (Bomsztyk et al, 1997). Some of the K protein partners are recruited to transcribed loci by interacting with DNA, transcriptionally active complexes or with nascent RNA. One or more of these K protein interactions could be responsible for the recruitment of K protein to chromatin by an indirect mechanism. Thus, the nuclear shift of K protein in tumours (Figures 6 and 7) may reflect its involvement in multiple DNA- and/or RNA-directed processes that are altered in malignancies. With respect to nuclear K shift in cancer, two nuclear processes seem relevant to consider. First, cisplatin cross-linking in human breast cancer cells revealed that K protein is one of the nuclear matrix proteins (Samuel et al, 1998). The key role of nuclear matrix is to organise the chromatin topology so that it can serve as a conducive template for transcription and replication. Although much remains to be learned, abnormal nuclear matrix is one of the morphologic hallmarks of cancer (Leman and Getzenberg, 2002). Thus, the nuclear shift of K may reflect, in part, altered protein composition of nuclear matrix in cancer. If so, the ability of cisplatin to cross-link K protein to DNA may be one of the mechanisms by which this chemotherapeutic agent inhibits processes that compose transcription and replication (Samuel et al, 1998). Second, K and other hnRNP proteins bind mammalian telomeric sequences (Lacroix et al, 2000; Ford et al, 2002) and Saccharomyces cerevisiae K protein-like genes regulate telomeric processes (Denisenko and Bomsztyk, 2002). These observations suggest that K protein may be involved in maintaining the integrity of mammalian telomeres. Preservation of telomere length which, in part, results from reactivation of telomerase is another hallmark of continuous cells growth, especially of advanced malignancies (Neumann and Reddel, 2002). The increased nuclear levels of K protein may play a role in the altered telomeric processes seen in cancer.
In summary, we have demonstrated that in several states of enhanced cell proliferation, there are increased K protein levels in the nucleus. Induction of cell proliferation results in the activation of a large repertoire of genes (Iyer et al, 1999). K protein is an abundant factor involved in transcription, mRNA processing and other events that compose gene expression. It is likely that the increased K protein levels seen in the nuclei of the proliferating cells serve to support nuclear process that not only composes inducible expression of a very large number genes but also maintains conducive chromatin topology in growing cells.
Acknowledgments
We thank Piotr Kowalczyk and Marek Woszczynski for expert technical assistance. We wish to thank Dr Jolanta Kupryjanczyk for the PCNA immunocytochemistry. The study was supported by NIH Fogarty International Research Collaboration Award (FIRCA) TW05685, the CMKP Grant 501-2-2-08-94/00 and by the Foundation for Polish Science grant (IMMUNO 6/99).
References
- Arora V, Knapp DC, Smith BL, Statdfield ML, Stein DA, Reddy MT, Weller DD, Iversen PL (2000) c-Myc antisense limits rat liver regeneration and indicates role for c-Myc in regulating cytochrome P-450 3A activity. J Pharmacol Exp Ther 292: 921–928 [PubMed] [Google Scholar]
- Bomsztyk K, Van Seuningen I, Suzuki H, Denisenko O, Ostrowski J (1997) Diverse molecular interactions of the hnRNP K protein. FEBS Lett 403: 113–115 [DOI] [PubMed] [Google Scholar]
- Bouchard C, Staller P, Eilers M (1998) Control of cell proliferation by Myc. Trends Cell Biol 8: 202–206 [DOI] [PubMed] [Google Scholar]
- Bustelo XR, Suen K-I, Michael WM, Dreyfuss G, Barbacid M (1995) Association of the vav proto-oncogene product with poly(rC)-specific RNA-binding proteins. Mol Cell Biol 15: 1324–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charroux B, Angelats C, Fasano L, Kerridge S, Vola C (1999) The levels of the bancal product, a Drosophila homologue of vertebrate hnRNP K protein, affect cell proliferation and apoptpsis in imaginal disc cells. Mol Cell Biol 19: 7846–7856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejgaard K, Leffers H, Rasmussen HH, Madsen P, Kruse TA, Gesser B, Nilesen H, Celis JE (1994) Identification, molecular cloning, expression and chromosome mapping of a family of transformation upregulated hnRNP-K proteins derived by alternative splicing. J Mol Biol 236: 33–48 [DOI] [PubMed] [Google Scholar]
- Denisenko ON, Bomsztyk K (1997) The product of murine homolog of the Drosophila extra sex comb gene displays transcriptional repressor activity. Mol Cell Biol 17: 4707–4717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denisenko ON, Bomsztyk K (2002) Yeast hnRNP K-like genes are involved in regulation of the telomeric position effect and telomere length. Mol Cell Biol 22: 286–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl AM, Rai RM (1996) Liver regeneration 3: regulation of signal transduction during liver regeneration. FASEB J 10: 215–227 [DOI] [PubMed] [Google Scholar]
- Du W, Thanos D, Maniatis T (1993) Mechanism of transcriptional synergism between distinct virus-inducible enhancer elements. Cell 74: 887–898 [DOI] [PubMed] [Google Scholar]
- Evans JR, Mitchell SA, Spriggs K, Ostrowski J, Bomsztyk K, Ostarek D, Willis AE (2003) Members of the poly (rC) binding protein family stimulate the activity of the c-myc internal ribosome entry segment in vitro and in vivo. Oncogene, (in press) [DOI] [PubMed]
- Ford LP, Wright WE, Shay JW (2002) A model for heterogeneous nuclear ribonucleoproteins in telomere and telomerase regulation. Oncogene 21: 580–583 [DOI] [PubMed] [Google Scholar]
- Freeman TL, Ngo HQ, Mailliard ME (1999) Inhibition of system A amino acid transport and hepatocyte proliferation following partial hepatectomy in the rat. Hepatology 30: 437–444 [DOI] [PubMed] [Google Scholar]
- Gates J, Thummel CS (2000) An enhancer trap screen for ecdysone-inducible genes required for Drosophila adult leg morphogenesis. Genetics 156: 1765–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschman HR (1991) Primary response genes induced by growth factors and tumor promoters. Annu Rev Biochem 60: 281–319 [DOI] [PubMed] [Google Scholar]
- Iwamoto Y, Wong KY, Goldfine ID (1981) Insulin action in cultured HTC and H35 rat hepatoma cells: receptor binding and hormone sensitivity. Endocrinology 108: 44–51 [DOI] [PubMed] [Google Scholar]
- Iyer VR, Eisen MB, Ross DT, Schuler G, Moore T, Lee JCF, Trent JM, Staudt LM, Hudson Jr J, Boguski MS, Lashkari D, Shalon D, Botstein D, Brown PO (1999) The transcriptional program in the response of human fibroblasts to serum. Science 283: 83–87 [DOI] [PubMed] [Google Scholar]
- Jochum W, Passegue E, Wagner EF (2001) AP-1 in mouse development and tumorigenesis. Oncogene 20: 2401–2412 [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Kitagawa Y (1988) Enhanced transcription of mitochondrial genes after growth stimulation and glucocorticoid treatment of Reuber hepatoma H-35. FEBS Lett 233: 51–56 [DOI] [PubMed] [Google Scholar]
- Kupryjanczyk J, Szymanska T, Madry R, Timorek A, Stelmachow J, Karpinska G, Rembiszewska A, Ziolkowska I, Kraszewska E, Debniak J, Emerich J, Ulanska M, Pluzanska A, Jedryka M, Goluda M, Chudecka-Glaz A, Rzepka-Gorska I, Klimek M, Urbanski K, Breborowicz J, Zielinski J, Markowska J (2003) Evaluation of clinical significance of TP53, BCL-2, BAX and MEK1 expression in 229 ovarian carcinomas treated with platinum-based regimen. Br J Cancer 88: 848–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix L, Lienard H, Labourier E, Djavaheri-Mergny M, Lacoste J, Leffers H, Tazi J, Helene C, Mergny JL (2000) Identification of two human nuclear proteins that recognise the cytosine-rich strand of human telomeres in vitro. Nucleic Acids Res 28: 1564–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambotte L, Saliez A, Triest S, Tagliaferri EM, Barker AP, Baranski AG (1997) Control of rate and extent of the proliferative response after partial hepatectomy. Am J Physiol 273: G905–G1112 [DOI] [PubMed] [Google Scholar]
- Leman ES, Getzenberg RH (2002) Nuclear matrix proteins as biomarkers in prostate cancer. J Cell Biochem 86: 213–223 [DOI] [PubMed] [Google Scholar]
- Mandal M, Vadlamudi R, Nguyen D, Wang RA, Costa L, Bagheri-Yarmand R, Mendelsohn J, Kumar R (2001) Growth factors regulate heterogeneous nuclear ribonucleoprotein K expression and function. J Biol Chem 276: 9699–9704 [DOI] [PubMed] [Google Scholar]
- Michelotti EF, Michelotti GA, Aronsohn AI, Levens D (1996) Heterogenous nuclear ribonucleoprotein K is a transcription factor. Mol Cell Biol 16: 2350–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelotti EF, Tomonaga T, Krutzsch H, Levens D (1995) Cellular nucleic acid binding protein regulates the CT element of the human c-myc protooncogene. J Biol Chem 270: 9494–9499 [DOI] [PubMed] [Google Scholar]
- Neumann AA, Reddel RR (2002) Telomere maintenance and cancer–look, no telomerase. Nat Rev Cancer 2: 879–884 [DOI] [PubMed] [Google Scholar]
- Ostareck-Lederer A, Ostareck DH, Neubauer G, Bomsztyk K, Superti-Furga G, Hentze MW (2002) c-Src mediated phosphorylation of hnRNP K drives translational activation of specifically silenced mRNAs. Mol Cell Biol 22: 4535–4543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostareck DH, Ostareck-Lederer A, Wilm M, Thiele BJ, Mann M, Hentze MW (1997) mRNA silencing in erythroid differentiation: hnRNP K nad hnRNP E1 regulate 15-lipogenase translation from the 3′ end. Cell 89: 597–606 [DOI] [PubMed] [Google Scholar]
- Ostrowski J, Kawata Y, Schullery DS, Denisenko ON, Bomsztyk K (2003a) Transient recruitment of the hnRNP K protein to inducibly transcribed gene loci. Nucleic Acids Res 14: 3954–3962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski J, Kawata Y, Schullery D, Denisenko ON, Higaki Y, Abrass CK, Bomsztyk K (2001) Insulin alters heterogeneous ribonucleoprotein K protein binding to DNA and RNA. Proc Natl Acad Sci USA 98: 9044–9049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski J, Schullery DS, Denisenko ON, Higaki Y, Watts J, Aebersold R, Stempka L, Gschwendt M, Bomsztyk K (2000a) Role of tyrosine phosphorylation in the regulation of the interaction of heterogenous nuclear ribonucleoprotein K protein with its protein and RNA partners. J Biol Chem 275: 3619–3628 [DOI] [PubMed] [Google Scholar]
- Ostrowski J, Sims JE, Sibley CH, Valentine MA, Dower SK, Meier KE, Bomsztyk K (1991) A serine/threonine kinase activity is closely associated with a 65-kDa phosphoprotein specifically recognized by the kappa B enhancer element. J Biol Chem 266: 12722–12733 [PubMed] [Google Scholar]
- Ostrowski J, Van Seuningen I, Seger R, Rouch CT, Sleath PR, McMullen BA, Bomsztyk K (1994) Purification, cloning, and expression of a murine phosphoprotein that binds the κB motif in vitro identifies it as the homolog of the human hnRNP K protein. Description of a novel DNA-dependent phosphorylation process. J Biol Chem 269: 17626–17634 [PubMed] [Google Scholar]
- Ostrowski J, Wocial T, Skurzak H, Bartnik W (2003b) Do altering in ornithine decarboxylase activity and gene expression contribute to antiproliferative properties of COX inhibitors? Br J Cancer 88: 1143–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski J, Woszczynski M, Kowalczyk P, Trzeciak L, Hennig E, Bomsztyk K. (2000b) Treatment of mice with EGF and orthovanadate activates cytoplasmic and nuclear MAPK, p70S6k, and p90rsk in the liver. J Hepatol 32: 965–974 [DOI] [PubMed] [Google Scholar]
- Ostrowski J, Woszcznski M, Kowalczyk P, Wocial T, Henning E, Trzeciak L, Janik P, Bomsztyk K (2000c) Increased activity of MAP, p70S6 and p90rs kinases is associated with AP-1 activation in spontaneous liver tumours, but not in adjacent tissue in mice. Br J Cancer 82: 1041–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozga J (2002) Hepatocyte proliferation in health and in liver failure. Med Sci Monit 8: RA32–RA38 [PubMed] [Google Scholar]
- Samuel SK, Spencer VA, Bajno L, Sun JM, Holth LT, Oesterreich S, Davie JR (1998) In situ cross-linking by cisplatin of nuclear matrix-bound transcription factors to nuclear DNA of human breast cancer cells. Cancer Res 58: 3004–3008 [PubMed] [Google Scholar]
- Shnyreva M, Schullery DS, Suzuki H, Higaki Y, Bomsztyk K (2000) Interaction of two multifunctional proteins. Heterogeneous nuclear ribonucleoprotein K and Y-box binding protein. J Biol Chem 275: 15498–15503 [DOI] [PubMed] [Google Scholar]
- Schullery DS, Ostrowski J, Denisenko ON, Stempka L, Shnyreva M, Suzuki H, Gschwendt M, Bomsztyk K (1999) Regulated interaction of protein kinase Cδ with the heterogeneous nuclear ribonucleoprotein K protein. J Biol Chem 274: 15101–15109 [DOI] [PubMed] [Google Scholar]
- Siomi H, Matunis MJ, Michael WM, Dreyfuss G (1993) The pre-mRNA binding protein contains a novel evolutionary conserved motif. Nucleic Acids Res 21: 1193–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiewak Rinaudo JA, Thorgeirsson SS (1997) Detection of a tyrosine-phosphorylated form of cyclin A during liver regeneration. Cell Growth Differ 8: 301–309 [PubMed] [Google Scholar]
- Szymanska H (1991) Morphological classification of spontaneous liver tumours in 4 inbred mouse strains. Zwierz Lab 28: 77–86 [Google Scholar]
- Taub R (1996) Transcriptional control of liver regeneration. FASEB J 10: 413–427 [PubMed] [Google Scholar]
- Taub R, Roy A, Dieter R, Koontz J (1987) Insulin as a growth factor in rat hepatoma cells. Stimulation of protooncogene expression. J Biol Chem 262: 10893–10897 [PubMed] [Google Scholar]
- Van Seuningen I, Ostrowski J, Bustelo X, Sleath P, Bomsztyk K (1995) The K protein domain that recruits the IL-1-responsive K protein kinase lies adjacent to a cluster of Src- and Vav-SH3-binding sites. Implications that K protein acts as a docking platform. J Biol Chem 270: 26976–26985 [DOI] [PubMed] [Google Scholar]
- Wang JYJ (1994) Nuclear protein tyrosine kinases. TIBS 19: 373–376 [DOI] [PubMed] [Google Scholar]
- Weng Z, Thomas SM, Rickles RJ, Taylor JA, Brauer AW, Seidel-Dugan C, Michael WM, Dreyfuss G, Brugge JS (1994) Identification of Scr, Fyn, Lyn SH3-binding proteins: implications for a function of SH3 domains. Mol Cell Biol 14: 4509–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf HK, Michalopoulos GK (1992) Hepatocyte regeneration in acute fulminant and nonfulminant hepatitis: a study of proliferating cell nuclear antigen expression. Hepatology 15: 707–713 [DOI] [PubMed] [Google Scholar]
- Zimmermann A (2002) Liver regeneration: the emergence of new pathways. Med Sci Monit 8: RA53–RA63 [PubMed] [Google Scholar]