Abstract
The tolerance and efficacy of oxaliplatin and irinotecan for metastatic colorectal cancer are unknown in elderly patients. Methods. All consecutive patients over 74 years treated with oxaliplatin or irinotecan for metastatic colorectal cancer were enrolled. The tumour response was assessed every 2–3 months and toxicity was collected at each cycle according to World Health Organisation criteria. A total of 66 patients were enrolled from 12 centres. The median age was 78 years (range, 75–88 years); 39 patients had no severe comorbidity according to the Charlson score. In total, 44 and 22 patients received oxaliplatin or irinotecan, respectively, in combination with 5-fluororuracil±folinic acid or raltitrexed in 64 patients. A total of 545 chemotherapy cycles were administered in first (41%), second (51%) or third line (8%). A dose reduction occurred in 190 cycles (35%). Complete response, partial response and stabilisation occurred in 1.5, 20 and 47% of patients, respectively. The median time to progression and overall survival were 6.8 and 11.2 months in first line and 6.3 and 11.6 months in second line, respectively. Grade 3 and 4 toxicity occurred in 42% of patients: neutropenia 17%, diarrhoea 15%, neuropathy 11%, nausea and vomiting 8% and thrombopenia 6%. There was no treatment-related death. In selected elderly patients, chemotherapy with oxaliplatin or irinotecan is feasible with manageable toxicity.
Keywords: colon cancer, elderly, chemotherapy, oxaliplatin, irinotecan
Colorectal cancer is the second most frequent cancer in Europe (Bray et al, 2002) and 40% of patients are of age 74 years and more at diagnosis (Gatta et al, 1998). Nevertheless, patients over 75 years have been usually excluded from randomised clinical trials evaluating chemotherapy for advanced colorectal cancer (Trimble et al, 1994; Kohne et al, 2001). Few data are available on chemotherapy in elderly patients, but it has been suggested that 5-fluorouracil (5-FU)-based chemotherapy efficacy was comparable to that in younger patients (Stein et al, 1995; Chiara et al, 1998; Mabro et al., 1999; Popescu et al., 1999; Magne et al., 2002). Tolerance was comparable except in one study evaluating chemotherapy with bolus 5-FU (Stein et al, 1995). A survival benefit was demonstrated in one unpublished randomised study comparing 5-FU-based chemotherapy to best supportive care in elderly patients (Beretta et al, 1997). In the adjuvant setting, a pooled analysis of randomised trials with 5-FU-based chemotherapy confirmed the improvement of disease-free survival and overall survival (OS) with chemotherapy, even in patients over 70 years (Sargent et al, 2001).
Oxaliplatin (de Gramont et al, 2000) and irinotecan (Douillard et al, 2000), in combination with 5-FU–leucovorin, have demonstrated their efficacy in advanced colorectal cancer with a better tumour response rate than 5-FU and leucovorin alone. After 5-FU failure, irinotecan as second-line chemotherapy has demonstrated a survival advantage compared to best supportive care in advanced colorectal cancer (Cunningham et al, 1998). Combination therapy, however, has an increased toxicity compared to 5-FU alone, especially neutropenia, diarrhoea and neurologic toxicity with oxaliplatin and neutropenia, and diarrhoea with irinotecan.
No data from clinical trials are available about chemotherapy with oxaliplatin or irinotecan in patients aged 75 years and more with advanced colorectal cancer. We report the result of tolerance and efficacy of oxaliplatin- or irinotecan-based chemotherapy in a multicentre observational cohort of patients of age 75 years and more with an advanced colorectal cancer.
PATIENTS AND METHODS
Patients
Data were collected from 12 centres. Each investigator included all consecutives patients over 74 years treated with oxaliplatin or irinotecan for advanced colorectal carcinoma from January 1999 to June 2002. All patients had a histologically proven colorectal adenocarcinoma. All the regimens containing oxaliplatin or irinotecan were considered either in association with 5-FU/leucovorin, oral 5-FU or raltitrexed. Only the first regimen was considered for evaluation in patients receiving both oxaliplatin and irinotecan sequentially. Patients were divided into two groups: group 1 patients 75–79 years old and group 2 patients 80 years old and over. The following clinical and biological variables were recorded: age, sex, body mass index, primary localisation, sites of metastasis, World Health Organisation (WHO) performance status (PS), Charlson comorbidity score (Charlson et al, 1987), haemoglobin, creatinin clearance (according to Cockroft method), alkaline phosphatase and carcinoembryonic antigen.
Chemotherapy
The chemotherapy regimens used were: FOLFOX 4 (leucovorin 200 mg m−2 day−1 as a 2-h infusion followed by bolus 5-FU 400 mg m−2 day−1 and a 22-h 5-FU infusion of 600 mg m−2 day−1, repeated for 2 consecutive days every 2 weeks. Oxaliplatin 85 mg m−2 was given on day 1 as a 2-h infusion concurrent with leucovorin) (de Gramont et al, 2000), FOLFOX 6 (oxaliplatin 100 mg m−2 was infused with leucovorin 400 mg m−2 as a 2-h infusion on day 1, followed by bolus 400 mg m−2 and a 46-h infusion 2400 mg m−2 of 5-FU, every 2 weeks) (Maindrault-Goebel et al, 2000), FOLFOX 7 consisted of the same regimen with oxaliplatin 130 mg m−2 (Maindrault-Goebel et al, 2001), ELOXFU-3 (leucovorin 200 mg m−2 as a 2-h infusion followed by bolus 5FU 400 mg m−2 and a 48-h infusion of 2400 mg m−2 and oxaliplatin 130 mg m−2 on day 1 every 3 weeks) (Ducreux et al, 1999), LV5FU2-irinotecan (leucovorin 200 mg m−2 day−1 as a 2-h infusion followed by bolus 5-FU 400 mg m−2 day−1 and a 22-h 5-FU infusion of 600 mg m−2 day−1, repeated for 2 consecutive days every 2 weeks. Irinotecan 180 mg m−2 was given on day 1 as a 1.5-h infusion concurrent with leucovorin) (Douillard et al, 2000).
According to evidence-based medicine, treatment was continued until disease progression or unacceptable toxicity occurred or until a patient chose to discontinue it.
Study parameters
When measurable, tumour response was assessed with computed tomography (CT) examination every 2–3 months according to WHO criteria. A complete response was defined as the complete disappearance of all clinically assessable disease, and a partial response was defined as a decrease of at least 50% of the sum of the products of the diameters of measurable lesions. Stable disease was defined as a decrease of less than 50% or an increase of less than 25% of measurable lesions, and progressive disease was defined as an increase of at least 25% of measurable lesions or the appearance of new malignant lesion(s). Progression-free survival (PFS) was defined as the time interval from the beginning of oxaliplatin- or irinotecan-based chemotherapy to the date of disease progression or, if the patient died without evidence of progression, to the date of death. OS lasted from the date of the beginning of oxaliplatin- or irinotecan-based chemotherapy to the date of death or the date when the patient was seen for the last time. Toxicities were collected at each cure according to WHO criteria.
Statistical analysis
Since this was not a randomised study, only patient characteristics at inclusion were statistically compared, using χ2 test for categorical variables and Student's t-test for continuous variables. OS and PFS were calculated using the Kaplan–Meier method. For all statistical tests, P-values less than or equal to 0.05 were considered significant.
RESULTS
Patients and treatment
Data from 66 consecutive patients treated from January 1999 to June 2002 were collected. Patient characteristics are presented in Table 1 . The median age was 78 years (range, 75–88 years). In all, 48 patients were 75–79 years old (group 1) and 18 were aged 80 or over (group 2). For groups 1 and 2, the median ages were, respectively, 77 years (range, 74–79 years) and 81 years (range, 80–88 years). Of the 66 patients, 39 had no severe comorbidity according to the Charlson index. No significant difference for sex, site of primary tumour, number of metastasic sites and comorbidity were observed between patients of groups 1 and 2. Group 2 patients had a significantly poorer PS. Creatinin clearance was significantly lower in group 2 than in group 1 (Table 1). There was no significant difference in baseline characteristics between patients treated in first line and patients treated in second line (Table 1).
Table 1. Patient characteristics.
| Patients characteristics | All patients, n=66 (%) | Group 1, n=48 (%) | Group 2, n=18 (%) | First line, n=27 (%) | Second line, n=34 (%) |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 39 (59) | 25 (52) | 14 (78)* | 15 (56) | 20 (59) |
| Female | 27 (41) | 23 (48) | 4 (22) | 12 (44) | 14 (41) |
| Site of primary tumour | |||||
| Colon | 52 (79) | 38 (79) | 14 (78) | 20 (74) | 27 (79) |
| Rectum | 14 (21) | 10 (21) | 4 (22) | 7 (26) | 7 (21) |
| No. of metastatic site(s) | |||||
| 1 | 38 (58) | 30 (62) | 8 (44) | 16 (59) | 19 (56) |
| 2 | 19 (29) | 11 (23) | 8 (44) | 8 (30) | 10 (29) |
| >2 | 9 (14) | 7 (15) | 2 (12) | 3 (11) | 5 (15) |
| Charlson comorbidity index | |||||
| 0 | 39 (59) | 29 (60) | 10 (55) | 16 (59) | 21 (62) |
| 1–2 | 18 (27) | 13 (27) | 5 (28) | 6 (22) | 10 (29) |
| >2 | 9 (14) | 6 (13) | 3 (17) | 5 (19) | 3 (9) |
| Performance status | |||||
| 0 | 13 (20) | 11 (23) | 2 (11) | 3 (11) | 8 (24) |
| 1 | 31 (47) | 22 (46) | 9 (50) | 14 (52) | 14 (41) |
| 2 | 19 (29) | 15 (31) | 4 (22) | 8 (30) | 11 (32) |
| 3 | 3 (4) | 0 (0) | 3 (17)** | 2 (7) | 1 (3) |
| Mean creatinin clearance (ml min−1) | 58.9±1.8 | 61.4±2.1 | 52±3.5*** | 62±2.5 | 57±2.6 |
| Mean haemoglobin (g dl−1) | 12±0.2 | 11.8±0.2 | 12.5±0.3 | 11.8±0.2 | 12.1±0.3 |
| CEA <5 (ng ml−1) | 11 (17) | 9 (19) | 2 (11) | 5 (18) | 6 (18) |
| >5–50 ng ml−1 | 19 (29) | 13 (27) | 6 (33) | 7 (26) | 9 (26) |
| >50 ng ml−1 | 31 (47) | 22 (46) | 9 (50) | 15 (56) | 15 (44) |
| Not done | 5 (7) | 4 (8) | 1 (6) | 0 | 4 (12) |
| Alkaline phosphatase | |||||
| <N | 42 (64) | 34 (71) | 8 (45) | 19 (70) | 20 (59) |
| >N–2N | 11 (17) | 5 (10) | 6 (33) | 4 (15) | 5 (15) |
| >2N | 10 (15) | 6 (13) | 4 (22) | 4 (15) | 6 (17) |
| Not done | 3 (4) | 3 (6) | 0**** | 0 | 3 (9) |
P=0.058.
P=0.025.
P=0.04.
P=0.044.
Out of the 66 patients, 44 received an oxaliplatin-based regimen and 22 received an irinotecan-based regimen. A total of 60 patients were treated with a combination of oxaliplatin or irinotecan with 5-FU/leucovorin (Table 2 ). Patients received oxaliplatin- or irinotecan-based regimen mainly as second-line treatment (51%) after failure of 5-FU (Table 3 ).
Table 2. Chemotherapy characteristics.
| Chemotherapy regimens | All patients, n=66 (%) | Mean number of cyclesa | Group 1, n=48 (%) | Mean number of cycle | Group 2, n=18 (%) | Mean number of cycle |
|---|---|---|---|---|---|---|
| LV5FU2-irinotecan | 21 (32) | 10.9±1.3 | 15 (31) | 10.7±1.4 | 6 (33) | 11.5±3.2 |
| FOLFOX 4 | 20 (30) | 8.2±0.7 | 17 (36) | 8.8±0.8 | 3 (17) | 5.3±1.4 |
| FOLFOX 6 | 12 (18) | 7.8±1 | 8 (17) | 8±1.4 | 4 (22) | 7.5±1.8 |
| ELOXFU 3 | 5 (8) | 3.4±0.8 | 4 (8) | 3.5±1 | 1 (6) | 3.0 |
| FOLFOX 7 | 2 (3) | 7.0±1 | 2 (4) | 7.0±1 | — | — |
| Oxaliplatin monotherapy | 2 (3) | 5.0±2 | 2 (4) | 5.0±2 | — | — |
| Othersb | 4 (6) | 4.0±1 | — | — | 4 (22) | 4.0±1 |
| Overall | 10.8±0.8 | 10.2±0.8 | 12.5±2.2 |
LV5FU2-irinotecan, FOLFOX 4, FOLFOX 6 and FOLFOX 7 are biweekly regimens; ELOXFU 3, oxaliplatin monotherapy and other regimens are three-weekly regimens.
One patient was treated with an association of raltitrexed and oxaliplatin, two patients with an association of capecitabine and oxaliplatin and one patient with an association of raltitrexed and irinotecan.
Table 3. Repartition of oxaliplatin or irinotecan chemotherapy line.
| Chemotherapy line | All patients, n=66 (%) | Group 1, n=48 (%) | Group 2, n=18 (%) |
|---|---|---|---|
| First line | 27 (41) | 20 (42) | 7 (39) |
| Oxaliplatin | 19 | 14 | 5 |
| Irinotecan | 8 | 6 | 2 |
| Second line | 34 (51) | 26 (54) | 8 (44) |
| Oxaliplatin | 21 | 17 | 4 |
| Irinotecan | 13 | 9 | 4 |
| Third line | 5 (8) | 2 (4) | 3 (17) |
| Oxaliplatin | 4 | 2 | 2 |
| Irinotecan | 1 | — | 1 |
Among the 545 cycles of chemotherapy administered, 190 cycles (35%) were carried out with a dose reduction of 126 (31%) in group 1 and 64 (48%) in group 2.
Toxicity
In all, 28 (42%) patients experienced grade 3 and 4 toxicity (Table 4 ). The main severe toxicities were neutropenia in 11 patients and diarrhoea in 10 patients. The toxicity pattern was similar in groups 1 and 2. Definitive treatment interruption for toxicity occurred in four of the nine (44%) patients with a Charlson score higher than 2 and in seven of the 57 (12%) patients with a Charlson score equal or less than 2.
Table 4. Maximum toxicity per patient.
| Grade 3 and 4 toxicitya | All patients, n=66 (%) | Oxaliplatin-based regimen, n=44 (%) | Irinotecan-based regimen, n=22 (%) | Group 1, n=48 (%) | Group 2, n=18 (%) |
|---|---|---|---|---|---|
| Any grades 3 and 4 (except alopecia) | 28 (42) | 16 (36) | 12 (54) | 20 (42) | 8 (44) |
| Neutropenia | 11 (17) | 6 (14) | 5 (23) | 8 (17) | 3 (17) |
| Thrombopenia | 4 (6) | 4 (9) | 0 | 2 (4) | 2 (11) |
| Anaemia | 1 (1.5) | 1 (2) | 0 | 1 (2) | 0 |
| Nausea and vomiting | 5 (8) | 3 (7) | 2 (9) | 4 (8) | 1 (6) |
| Diarrhoea | 10 (15) | 4 (9) | 6 (27) | 7 (15) | 2 (11) |
| Stomatitis | 2 (3) | 2 (5) | 0 | 2 (4) | 0 |
| Neurologic | 7 (11) | 7 (16) | 0 | 5 (10) | 2 (11) |
| Treatment interruption for toxicity | 11 (17) | 8 (18) | 3 (14) | 9 (19) | 2 (11) |
| Number of delayed cycles for toxicity/total number of cycles | 38/545 (7) | 24/309 (8) | 14/236 (6) | 27/411 (7) | 11/134 (8) |
According to WHO grade.
Objective tumoral responses
Three patients (4.5%) were not evaluable for tumoral response. Objective responses were observed in 14 patients (21%) (Table 5 ). Tumour control defined as an objective response or stabilisation was obtained in 44 patients (67%).
Table 5. Tumoral responses.
| All patients, n=66 (%) | First line, n=27 (%) | Second line, n=34 (%) | Group 1, n=48 (%) | Group 2, n=18 (%) | |
|---|---|---|---|---|---|
| Complete response | 1 (1.5) | 1 (4) | 0 | 0 | 1 (6) |
| Partial response | 13 (20) | 9 (33) | 4 (12) | 11 (23) | 2 (11) |
| Stable disease | 31 (47) | 12 (44) | 17 (50) | 20 (42) | 11 (61) |
| Progression | 18 (27) | 4 (15) | 12 (35) | 14 (29) | 4 (22) |
| Not evaluable | 3 (4.5) | 1 (4) | 1 (3) | 3 (6) | 0 |
PFS and OS
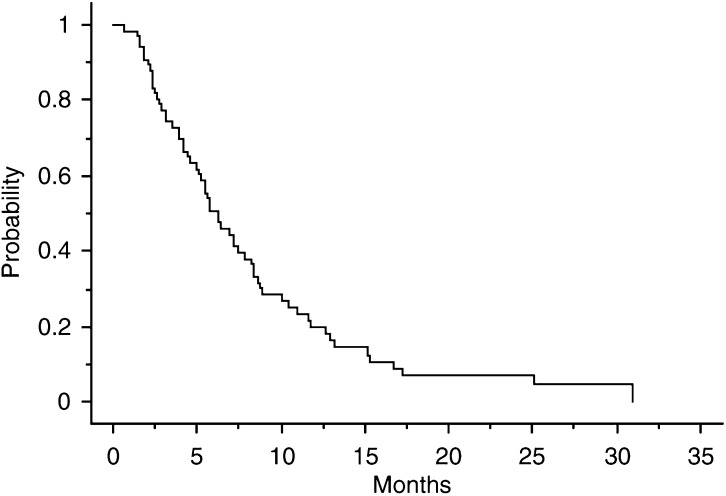
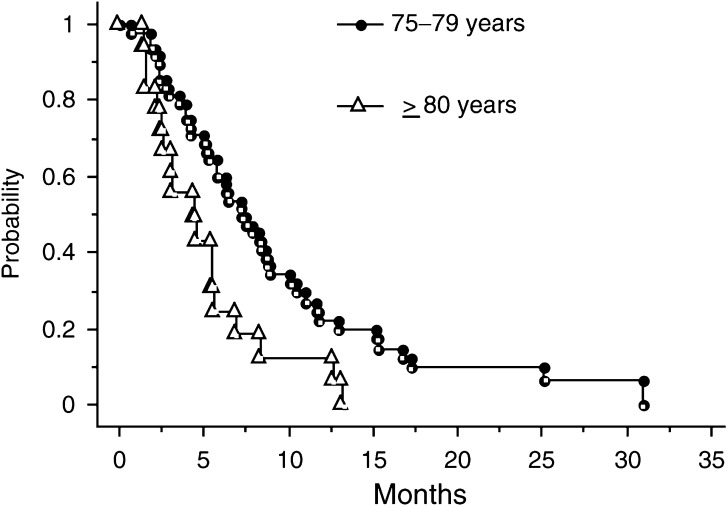
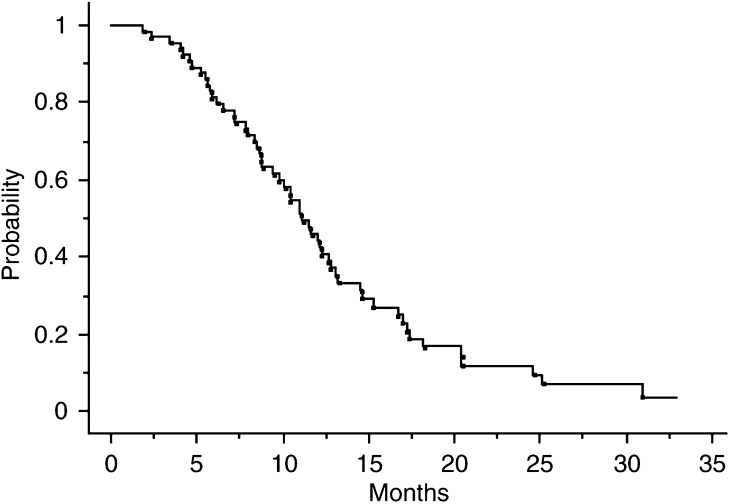
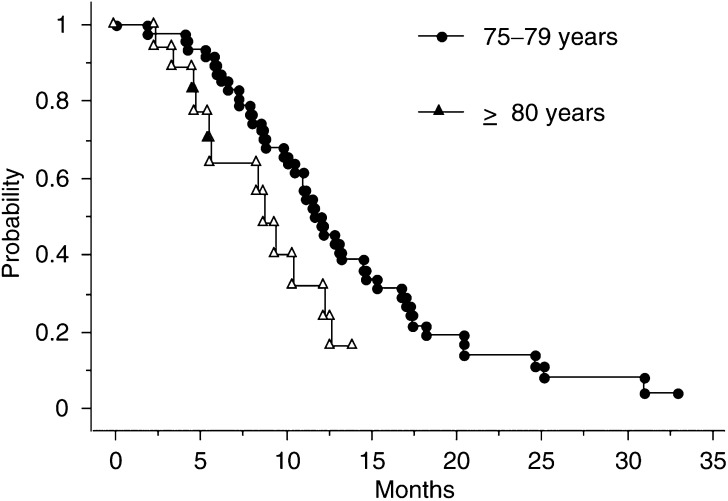
For all patients, the median PFS was 6.3 (range, 1–30.9) months (Figure 1). PFS was 4.5 (range, 1.4–13.2) months for group 2 patients and 7.3 (range, 1–30.9) months for group 1 patients (Figure 2). The median OS was 11.8 (range, 1.8–32.8) months (Figure 3). The median OS was 9.9 (range, 2.3–13.9) months for group 2 patients and 12.1 (range, 1.8–32.8) months for group 1 patients (Figure 4). The median OS from the beginning of the first-line chemotherapy was 16.7 (range, 4–42.3) months. The survival rate from the beginning of the first-line chemotherapy was 72% at 1 year and 34% at 2 years.
Figure 1.
PFS curve.
Figure 2.
PFS curves according to age group.
Figure 3.
OS curve.
Figure 4.
OS curves according to age group.
For the 27 patients treated in first line, the median PFS was 6.8 months (range, 1–29.2) and the median OS was 11.2 months (range, 4–29.2). For the 34 patients treated in second line, the median PFS was 6.3 months (range, 1–30.9) and the median OS was 11.6 months (range, 1.8–32.8). The median OS from the beginning of the first-line chemotherapy was 20.6 months (range, 5.8–42.3) in these 34 patients.
PS and weight improvement
Among the 53 patients with an initial PS >1, six were not evaluable for PS evolution and 15 (32%) had a PS improvement. A total of 28% of all the patients had a gain in weight during the treatment.
DISCUSSION
This is the first study of tolerance and efficacy of oxaliplatin- and irinotecan-based chemotherapy in elderly patients. Our results show that oxaliplatin- or irinotecan-based chemotherapy in elderly patients is feasible and safe.
A total of 42% of the patients experienced severe toxicity without treatment-related death. Previous retrospective studies evaluating 5-FU+leucovorin regimens in an elderly population reported severe toxicity in 20% of patients treated with the LV5FU2 regimen (Mabro et al, 1999) and in 58% of patients treated with the 5-FU bolus regimen (Stein et al, 1995). We report severe neutropenia in 14 and 23% of patients treated with oxaliplatin and irinotecan, respectively. Previous studies in nonelderly patients reported severe neutropenia in 36–41% (Andre et al, 1999; de Gramont et al, 2000) with oxaliplatin and 29–54% (Douillard et al, 2000; Saltz et al, 2000) with irinotecan. In our study, severe neuropathy occurred in 16% of patients treated with oxaliplatin; previous studies reported severed neuropathy in 18 and 20% (Andre et al, 1999; de Gramont et al, 2000). Severe diarrhoea occurred in 27% of patients treated with irinotecan. Previous studies reported severe diarrhoea in 22–44% (Douillard et al, 2000; Saltz et al, 2000). A full dose of chemotherapy was administered in 65% of the cycles. Previous studies in nonelderly patients reported a dose intensity of 73% for oxaliplatin (de Gramont et al, 2000) and 72–93% for irinotecan (Douillard et al, 2000; Saltz et al, 2000) in first-line chemotherapy. The frequent dose reduction observed in our report, especially in group 2, could partly explain the mild toxicity.
The overall objective response rate was 22%; 38% in first-line treatment. The response rates reported in previous studies in elderly patients treated with a 5-FU- or raltitrexed-based regimen were 18% (Chiara et al, 1998), 22% (Feliu et al, 2002), 24% (Popescu et al, 1999), 26% (Magne et al, 2002) and 44% (Mabro et al, 1999). The response rate in nonelderly patients treated in first line with an oxaliplatin- or irinotecan-based regimen is about 50% (de Gramont et al, 2000; Douillard et al, 2000; Saltz et al, 2000). In second line, the response rates ranged from 17% (Rougier et al, 1997) to 46% (de Gramont et al, 1997). The response rates observed in our study in first-line treatment seem to be higher than those generally obtained with 5-FU alone in elderly patients and comparable to those obtained with oxaliplatin or irinotecan in nonelderly patients. In second line, the response rates seem to be lower than those published with oxaliplatin or irinotecan in nonelderly patients.
We observed a median OS of 11.2 months in first line. It was 9.6 (Feliu et al, 2002), 9.7 (Popescu et al, 1999), 16.4 (Mabro et al, 1999) and 14 months (Magne et al, 2002) in previous studies where elderly patients were treated with a 5-FU- or raltitrexed-based regimen. Nevertheless, although we did not observe a better OS with oxaliplatin or irinotecan than in previous studies with 5-FU or raltitrexed, it must be pointed out that in all these studies the median age was younger and only few patients over 79 years were considered. We have suggested that age over 79 years is associated with poor survival. This population represents 27% of the patients in our study and could explain the poor OS observed. Nevertheless, the survival observed in our elderly patients is poorer than the one reported in prospective studies in younger patients in first-line treatment (de Gramont et al, 2000; Douillard et al, 2000; Saltz et al, 2000). In patients treated in second line with oxaliplatin or irinotecan, we observed an 11.6 month median survival from the beginning of second line and a 20.6 month median survival from the beginning of first line. These data prompted us to use a second-line chemotherapy in the elderly after first-line failure. Nevertheless, the discrepancy between OS of patients treated in first line with oxaliplatin or irinotecan and OS from the beginning of chemotherapy in patients treated in second line with oxaliplatin or irinotecan suggest that tumours of patients treated in second line had a slower growth rate.
The median PFS in first line was 6.8 months, which is comparable with PFS reported in younger patients: 9 months with oxaliplatin (de Gramont et al, 2000) and 6.7–7 months with irinotecan (Douillard et al, 2000; Saltz et al, 2000). An improvement of PS was noted in 32% of the patients with initial degraded PS; this is comparable with the 42% of improvement of PS reported with oxaliplatin in younger patients (de Gramont et al, 2000). In elderly patients treated with 5-FU alone, PS improvement varied from 26 to 56% (Mabro et al, 1999; Magne et al, 2002). Nevertheless, it is necessary to evaluate the influence of chemotherapy on quality of life and patient autonomy in a prospective study on elderly patients. In our study, the majority of the patients had no or little severe comorbidity. This suggests that the population is selected. This is the first study using the Charlson comorbidity score for elderly patients treated for metastatic colorectal carcinoma. A prognostic value of Charlson >2 was suggested for the occurrence of definitive treatment interruption for toxicity. A stratification according to the Charlson comorbidity score should be considered in prospective studies in elderly patients.
We conclude that chemotherapy with oxaliplatin or irinotecan in selected elderly patients is feasible with manageable toxicity. Improvements of PS and prolonged PFS and OS were obtained, but the benefit is weaker after 79 years. Moreover, second-line chemotherapy should be considered whenever it is possible in elderly patients. Prospective studies are needed to establish the benefit of the different chemotherapy regimens for quality of life and autonomy in elderly patients.
References
- Andre T, Bensmaine MA, Louvet C, Francois E, Lucas V, Desseigne F, Beerblock K, Bouche O, Carola E, Merrouche Y, Morvan F, Dupont-Andre G, de Gramont A (1999) Multicenter phase II study of bimonthly high-dose leucovorin, fluorouracil infusion, and oxaliplatin for metastatic colorectal cancer resistant to the same leucovorin and fluorouracil regimen. J Clin Oncol 17: 3560–3568 [DOI] [PubMed] [Google Scholar]
- Beretta G, Bolina R, Cozzi C (1997) Should we consider the weekly (w) chemotherapy (CT) with fluorouracil (F)+racemic folinic acid (f) a standard treatment for advanced/metastatic carcinoma of digestive tract (DTC) in elderly patients (pts)? Proc Am Soc Clin Oncol 16: 259a [Google Scholar]
- Bray F, Sankila R, Ferlay J, Parkin DM (2002) Estimates of cancer incidence and mortality in Europe in 1995. Eur J Cancer 38: 99–166 [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40: 373–383 [DOI] [PubMed] [Google Scholar]
- Chiara S, Nobile MT, Vincenti M, Lionetto R, Gozza A, Barzacchi MC, Sanguineti O, Repetto L, Rosso R (1998) Advanced colorectal cancer in the elderly: results of consecutive trials with 5-fluorouracil-based chemotherapy. Cancer Chemother Pharmacol 42: 336–340 [DOI] [PubMed] [Google Scholar]
- Cunningham D, Pyrhonen S, James RD, Punt CJ, Hickish TF, Heikkila R, Johannesen TB, Starkhammar H, Topham CA, Awad L, Jacques C, Herait P (1998) Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet 352: 1413–1418 [DOI] [PubMed] [Google Scholar]
- de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18: 2938–2947 [DOI] [PubMed] [Google Scholar]
- de Gramont A, Vignoud J, Tournigand C, Louvet C, Andre T, Varette C, Raymond E, Moreau S, Le Bail N, Krulik M (1997) Oxaliplatin with high-dose leucovorin and 5-fluorouracil 48-hour continuous infusion in pretreated metastatic colorectal cancer. Eur J Cancer 33: 214–219 [DOI] [PubMed] [Google Scholar]
- Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P (2000) Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 355: 1041–1047 [DOI] [PubMed] [Google Scholar]
- Ducreux M, Rougier P, Polus M, Ould Kaci M, Ychou M, Conroy T (1999) Résultats de l'association oxaliplatine-LV5FU2 toutes les 3 semaines dans les cancers colorectaux avancés progressant sous 5FU. Gastroentérol Clin Biol 23: A108 [Google Scholar]
- Feliu J, Mel JR, Camps C, Escudero P, Aparicio J, Menendez D, Garcia GC, Rodriguez MR, Sanchez JJ, Gonzalez BM (2002) Raltitrexed in the treatment of elderly patients with advanced colorectal cancer: an active and low toxicity regimen. Eur J Cancer 38: 1204–1211 [DOI] [PubMed] [Google Scholar]
- Gatta G, Faivre J, Capocaccia R, Ponz dL (1998) Survival of colorectal cancer patients in Europe during the period 1978–1989. EUROCARE Working Group. Eur J Cancer 34: 2176–2183 [DOI] [PubMed] [Google Scholar]
- Kohne CH, Grothey A, Bokemeyer C, Bontke N, Aapro M (2001) Chemotherapy in elderly patients with colorectal cancer. Ann Oncol 12: 435–442 [DOI] [PubMed] [Google Scholar]
- Mabro M, Gilles-Amar V, Louvet C, Carola E, Maindrault-Goebel F, de Gramont A, Krulik M (1999) Bimonthly 5-fluorouracil in elderly patients with metastatic colorectal cancer study of 50 patients. Rev Med Intern 20: 863–868 [DOI] [PubMed] [Google Scholar]
- Magne N, Francois E, Broisin L, Guardiola E, Ramaioli A, Ferrero JM, Namer M (2002) Palliative 5-fluorouracil-based chemotherapy for advanced colorectal cancer in the elderly: results of a 10-year experience. Am J Clin Oncol 25: 126–130 [DOI] [PubMed] [Google Scholar]
- Maindrault-Goebel F, de Gramont A, Louvet C, Andre T, Carola E, Gilles V, Lotz JP, Tournigand C, Mabro M, Molitor JL, Artru P, Izrael V, Krulik M (2000) Evaluation of oxaliplatin dose intensity in bimonthly leucovorin and 48-hour 5-fluorouracil continuous infusion regimens (FOLFOX) in pretreated metastatic colorectal cancer. Oncology Multidisciplinary Research Group (GERCOR). Ann Oncol 11: 1477–1483 [DOI] [PubMed] [Google Scholar]
- Maindrault-Goebel F, de Gramont A, Louvet C, Andre T, Carola E, Mabro M, Artru P, Gilles V, Lotz JP, Izrael V, Krulik M (2001) High-dose intensity oxaliplatin added to the simplified bimonthly leucovorin and 5-fluorouracil regimen as second-line therapy for metastatic colorectal cancer (FOLFOX 7). Eur J Cancer 37: 1000–1005 [DOI] [PubMed] [Google Scholar]
- Popescu RA, Norman A, Ross PJ, Parikh B, Cunningham D (1999) Adjuvant or palliative chemotherapy for colorectal cancer in patients 70 years or older. J Clin Oncol 17: 2412–2418 [DOI] [PubMed] [Google Scholar]
- Rougier P, Bugat R, Douillard JY, Culine S, Suc E, Brunet P, Becouarn Y, Ychou M, Marty M, Extra JM, Bonneterre J, Adenis A, Seitz JF, Ganem G, Namer M, Conroy T, Negrier S, Merrouche Y, Burki F, Mousseau M, Herait P, Mahjoubi M (1997) Phase II study of irinotecan in the treatment of advanced colorectal cancer in chemotherapy-naive patients and patients pretreated with fluorouracil-based chemotherapy. J Clin Oncol 15: 251–260 [DOI] [PubMed] [Google Scholar]
- Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL (2000) Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343: 905–914 [DOI] [PubMed] [Google Scholar]
- Sargent DJ, Goldberg RM, Jacobson SD, Macdonald JS, Labianca R, Haller DG, Shepherd LE, Seitz JF, Francini G (2001) A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 345: 1091–1097 [DOI] [PubMed] [Google Scholar]
- Stein BN, Petrelli NJ, Douglass HO, Driscoll DL, Arcangeli G, Meropol NJ (1995) Age and sex are independent predictors of 5-fluorouracil toxicity. Analysis of large scale phase III trial. Cancer 75: 11–17 [DOI] [PubMed] [Google Scholar]
- Trimble EL, Carter CL, Cain D, Freidlin B, Ungerleider RS, Friedman MA (1994) Representation of older patients in cancer treatment trials. Cancer 74: 2208–2214 [DOI] [PubMed] [Google Scholar]