Abstract
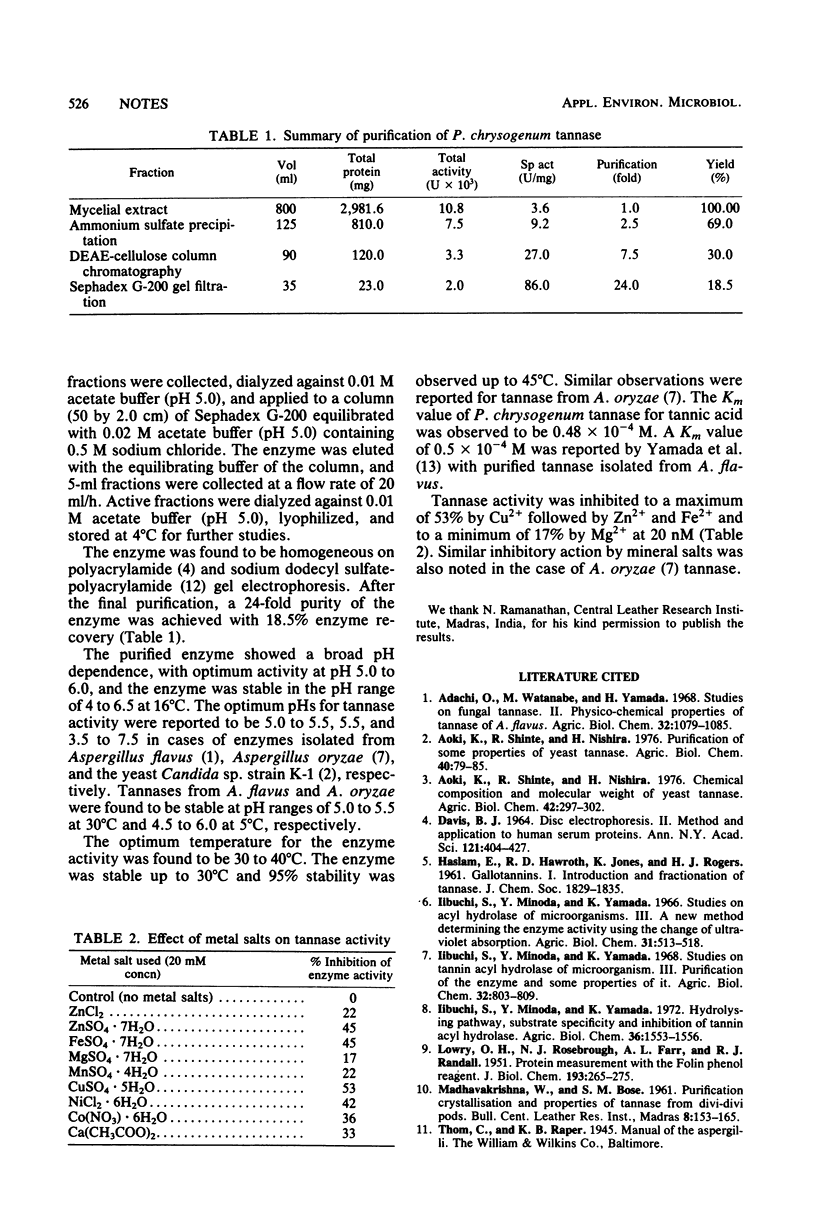
Tannase isolated from Penicillium chrysogenum was purified 24-fold with 18.5% recovery after ammonium sulfate precipitation, DEAE-cellulose column chromatography, and Sephadex G-200 gel filtration. Optimum enzyme activity was recorded at pH 5.0 to 6.0 and at 30 to 40°C. The enzyme was stable up to 30°C and within the pH range of 4.0 to 6.5. The Km value was found to be 0.48 × 10−4 M when tannic acid was used as the substrate. Metal salts at 20 mM inhibited the enzyme to different levels.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


