Abstract
The objectives of the study are to assess the impact of HIV status on the outcome of patients with non-small-cell lung cancer (NSCLC) in the era of highly active antiretroviral therapy (HAART). Patients diagnosed with HIV-related NSCLC in the HAART era (since January 1996) were identified from a prospective single-centre lung cancer database. The clinicopathological characteristics and outcome of each HIV-positive patient were compared to three age- and stage-matched HIV-negative controls with NSCLC who were diagnosed over the same time period and treated in an identical manner. The results showed that the two groups had similar disease characteristics and received a similar amount of chemotherapy. The median overall survival of the two groups was the same (4 months, log rank P=0.55). None of the HIV-positive patients developed an AIDS defining illness or died of HIV during treatment or follow-up. In conclusion, in this cohort, HIV status does not influence the prognosis of advanced NSCLC. This suggests that the survival of patients with HIV-related NSCLC may have improved since the introduction of HAART, and this may be due to a decrease in HIV-related deaths.
Keywords: HIV, non-small-cell lung cancer, HAART
HIV is associated with a small, but significant risk of developing lung cancer; particularly non-small-cell lung cancer (NSCLC) (Franceschi et al, 1998; Parker et al, 1998; Frisch et al, 2001). Before the introduction of highly active antiretroviral therapy (HAART), patients with HIV-related NSCLC had a worse outcome compared to age-matched HIV-negative controls (Sridhar et al, 1992; Vyzula and Remick, 1996; Tirelli et al, 2000). It has been suggested that this increased mortality was because the patients were younger, and had both more advanced and more aggressive disease compared to HIV-negative patients (Sridhar et al, 1992; Vyzula and Remick, 1996; Whooley et al, 1999; Skarin et al, 2001). Others have speculated that individuals with HIV-related lung cancer were offered suboptimal treatment due to concerns surrounding HIV-related comorbidity (Tirelli et al, 2000).
Since the introduction of HAART, the life expectancy of HIV patients has increased (Palella et al, 1998). This is largely due to a decrease in opportunistic infections; however, the outcome of a number of HIV-related malignancies has also improved (Tam et al, 2002). Currently, there are no controlled data regarding the outcome of patients with HIV-related lung cancer in the HAART era, despite reports that the incidence of HIV-related lung cancer may be rising (Bower et al, 2003).
In this study, we compare the treatment and outcome of HIV-positive and age-matched HIV-negative controls with advanced NSCLC in the HAART era.
PATIENTS AND METHODS
The clinical details of patients with HIV-related NSCLC, diagnosed since the introduction of HAART in January 1996, were retrieved from a prospective single-centre lung cancer database. The HIV-negative control group was derived from the same database. For each HIV-positive case patient, three HIV-negative controls were identified who were of the same gender, had the same stage of lung cancer and were within 6 years of age of the case patient.
All patients included had histologically confirmed lung cancer, which for the cases was diagnosed after testing HIV seropositive. Routine lung cancer staging was undertaken in all patients using the American Joint Committee tumour-node-metastasis (TNM) system. The HIV-positive and control groups were treated in an identical manner using standard chemotherapy regimens (mitomycin, vincristine and cisplatin or gemcitabine and carboplatin, depending on the date of diagnosis). None of the patients had received prior chemotherapy or radiotherapy. The response to treatment was evaluated using the RECIST guidelines (Therasse et al, 2000) and toxicities were recorded according to the National Cancer Institute common toxicity criteria (CTC) version 2.0. Specifically age, date of cancer diagnosis, treatment, toxicity, response to treatment and survival were recorded. Survival was plotted according to the Kaplan–Meier method (Kaplan and Meier, 1958).
RESULTS
Nine HIV-positive and 27 HIV-negative patients with NSCLC were identified between January 1996 and October 2002. The HIV-positive patients and HIV-negative controls had similar characteristics in terms of age, percent with stage IV disease and proportion with adenocarcinoma. However, HIV-positive patients appeared to have a worse performance status at presentation (Tables 1 and 2). The HIV patients were relatively asymptomatic with respect to their HIV disease, with a median CD4 cell count at presentation of 160 mm−3 (range 136–890 mm−3), and a median HIV viral load of 173 copies ml−1 (range <50–200 000 copies ml−1). Three patients had no detectable HIV viraemia and four had been diagnosed with an AIDS defining illness prior to the development of lung cancer.
Table 1. Comparison of clinicopathological properties of cases (HIV+) and controls (HIV−).
| HIV positive | HIV negative | |
| n | 9 | 27 |
| Median age | 45 years | 48 years |
| (range 32–58) | (range 30–55) | |
| Adenocarcinoma | 66% | 70% |
| Stage IV disease | 66% | 70% |
| ECOG performance status <2 at presentation | 22% | 52% |
| (range 1–4) | (range 1–4) | |
| Treated with chemotherapy | 88% | 66% |
| Median number of cycles of chemotherapy | 3.5 | 4 |
| (range 1–6) | (range 1–6) | |
| Median overall survival | 4 months | 4 months |
| (range 2–15+) | (range 1–24+) | |
| Cancer-related deaths | 77% | 74% |
Table 2. HIV-positive patients with NSCLC.
| Age (Years) | ECOG performance status | Histology | Stage | Site of metastasis | Treatment | Response | Survival (months) | |
| 1 | 50 | 3 | Squamous | IV | Bone | Chemotherapy | PD | 2 |
| 2 | 58 | 2 | Adeno | IV | Bone | Palliative | 2 | |
| 3 | 44 | 1 | Squamous | IIIB | Chemotherapy | PR | 5 | |
| 4 | 48 | 4 | Squamous | IV | Brain | Chemotherapy | PR | 5 |
| 5 | 57 | 4 | Adeno | IIIB | Chemotherapy | PR | 3 | |
| 6 | 45 | 1 | Adeno | IIIB | Chemotherapy | PR | 15+ | |
| 7 | 43 | 3 | Adeno | IV | Bone | Chemotherapy | PD | 2 |
| 8 | 32 | 2 | Adeno | IIIB | Chemotherapy | SD | 8 | |
| 9 | 43 | 2 | Adeno | IV | Bone | Chemotherapy | SD | 7 |
The median overall survival in both groups was 4 months (range 2–15+ months for HIV positives vs 1–24+ months for HIV negatives). The 1-year survival for the HIV-positive patients was 11% compared to 22% for the HIV-negative group. The two groups received a similar number of cycles of chemotherapy (Table 1). Stable disease or a partial response was achieved in 50% of the patients treated with chemotherapy in both the groups of patients.
Chemotherapy was stopped early in three HIV-positive patients, two due to progression of disease and one due to chemotherapy toxicity. In all, 50% of HIV-positive patients treated with chemotherapy, developed grade 3 or 4 haematological toxicity; however, there were no chemotherapy-related deaths. Seven patients were on HAART at the start of treatment, two stopped due to concern surrounding interaction of chemotherapy and HAART. None of the HIV-positive patients developed an AIDS defining diagnosis or died of HIV-related disease during treatment or follow-up.
DISCUSSION
Non-small-cell lung cancer occurs more frequently in the HIV population compared to HIV negatives (relative risk 4.5, 95% Confidence Interval: 4.2–4.8) (Frisch et al, 2001). Moreover, it has been reported that the incidence of NSCLC may be rising in people with HIV (Bower et al, 2003). The reason for the link between these two diseases is not clear; unlike most HIV-associated malignancies, no viral oncogene has been implicated in NSCLC. It has been speculated that the relationship is due to increased tobacco exposure in the HIV-positive population (Parker et al, 1998). In addition, pulmonary opportunistic infections and chronic immune suppression have both been implicated in the disease process; however, there is no clear consensus (Tirelli et al, 2000; Bower et al, 2003).
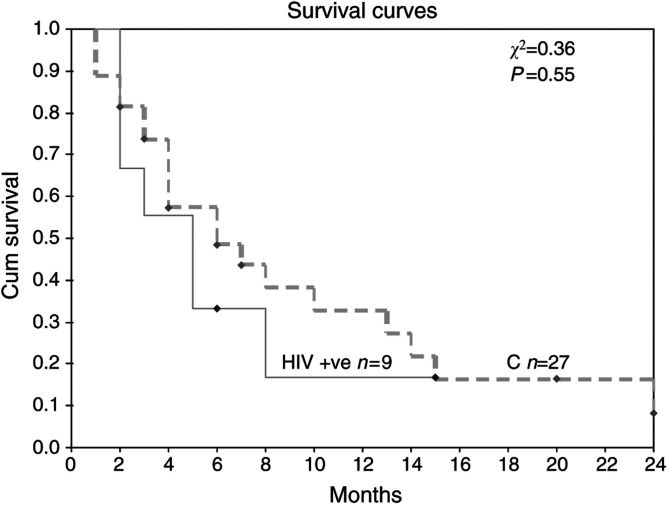
Before the introduction of HAART, HIV-related lung cancer patients had a worse outcome than HIV-negative age-matched controls (Sridhar et al, 1992; Vyzula and Remick, 1996; Tirelli et al, 2000) (Table 2). It was uncertain whether this was due to more aggressive lung cancer (Sridhar et al, 1992; Vyzula and Remick, 1996) or diagnostic delays and suboptimal treatment compared to HIV-negative controls (Tirelli et al, 2000). This controlled study compares the outcome of these patients since the introduction of HAART. It shows that the survival of the HIV-positive and HIV-negative groups is now the same, suggesting that the outcome of HIV-associated NSCLC has improved (Table 2, Figure 1). Since in this study the groups were matched for NSCLC stage and the treatment protocols used were the same in both the groups, the results support the hypothesis that NSCLC is not more aggressive in the immunocompromised. The prior reports of worse survival in the HIV-positive patients may therefore have reflected excess deaths due to immunodeficiency rather than NSCLC. Indeed, there were fewer HIV-related deaths in this study compared to those in the pre-HAART era (0 vs 16–54%, respectively) (Hoffman et al, 2000; Tirelli et al, 2000) (Table 3 ). Moreover, in this study, HIV-positive and -negative patients received equivalent amounts of chemotherapy. This does not appear to be the case for patients in the pre-HAART era, where dose modifications were common (Sridhar et al, 1992; Hoffman et al, 2000). These two factors may help explain the improved outcome of patients in this study.
Figure 1.
Kaplan–Meier overall survival duration curve from diagnosis of lung cancer according to HIV serostatus.
Table 3. Comparison with other studies in the pre-HAART era.
|
Vyzula and Remick (1996) |
Tirelli et al (2000) |
Sridhar et al (1992) |
Our study |
|||||
| Reference | HIV +ve | HIV −ve control | HIV +ve | HIV −ve control | HIV +ve | HIV −ve control | HIV +ve | HIV −ve control |
| Number | 11 | 49 | 36 | 102 | 16 | 32 | 9 | 28 |
| NSCLC (%) | 100 | 100 | 86 | 81 | 94 | 97 | 100 | 100 |
| Stage IIIB/IV (%) | 75 | NA | 84 | 81 | 50 | 50 | 100 | 100 |
| Median age (years) | 44 | <55 | 38 | 53 | 47 | 46 | 42 | 48 |
| Median survival (months) | 7.5 | 11 | 5 | 10 | 3 | 10 | 4 | 4 |
| P-value for survival | 0.003 | 0.0001 | 0.001 | 0.57 | ||||
NA=not applicable.
Three of the four studies in the pre-HAART era were not well controlled. Two had a significantly older HIV-negative cohort (Hoffman et al, 2000; Tirelli et al, 2000), while the other was not matched for stage of disease (Vyzula and Remick, 1996). It may be that these confounding factors biased the results in favour of HIV negatives; however, there was a high degree of significance in all four studies (see Table 2). It has also been speculated that a smaller proportion of HIV patients with operable disease are offered surgery, resulting in worse outcome (Tirelli et al, 2000). This issue remains unresolved, as all of the patients in this study had advanced disease. The HIV-positive and -negative groups present in this study were remarkably similar, with a similar median age, proportion with adenocarcinoma and a similar amount of chemotherapy given. The HIV-positive patients did appear to have a poorer performance status than the negative group, which is associated with a worse outcome (Schiller et al, 2002).
Advanced HIV-related NSCLC is still associated with a poor outcome in the HAART era (median survival 4 months). However, this may reflect the advanced and aggressive nature of the disease in young people as a whole, rather than exclusively in people with HIV. It has been speculated that the high proportion of adenocarcinomas in the young, which is associated with early metastasis, may be responsible for this poor survival (Hoffman et al, 2000). There are no specific data on the median survival of patients with advanced NSCLC pre-HAART era; however, despite being incomplete, pooled data give a figure of approximately 2 months (n=23) (Sridhar et al, 1992; Vyzula and Remick, 1996; Alshafie et al, 1997).
It is of note that two of the patients in this study stopped HAART during or at the time of chemotherapy. The continuation of HAART is thought to reduce opportunistic infections and preserve immune parameters (Powles et al, 2002; Bower et al, 2003). However, if interactions between chemotherapy and HAART do occur, it may be worth considering a modification of the antiretroviral regimen rather than stopping the chemotherapy, as long-term prevention of HIV progression may be less of a priority in view of the poor prognosis of these patients. Prophylaxis against opportunistic infection is therefore crucial in this setting.
The data presented here suggest that the introduction of HAART may have improved outcome in advanced NSCLC in HIV-positive individuals. This may be due to decreased HIV-related mortality and an increased ability to tolerate treatment. These individuals may now have a similar outcome to HIV-negative people with the disease, provided that they are treated in a similar manner.
References
- Alshafie MT, Donaldson B, Oluwole SF (1997) Human immunodeficiency virus and lung cancer. Br J Surg 84: 1068–1071 [PubMed] [Google Scholar]
- Bower M, Powles T, Nelson M, Shah P, Cox S, Mandelia S, Gazzard B (2003) HIV-related lung cancer in the era of highly active antiretroviral therapy. AIDS 17: 371–375 [DOI] [PubMed] [Google Scholar]
- Franceschi S, Dal Maso L, Arniani S, Crosignani P, Vercelli M, Simonato L, Falcini F, Zanetti R, Barchielli A, Serraino D, Rezza G (1998) Risk of cancer other than Kaposi's sarcoma and non-Hodgkin's lymphoma in persons with AIDS in Italy. Cancer and AIDS Registry Linkage Study. Br J Cancer 78: 966–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch M, Biggar RJ, Engels EA, Goedert JJ (2001) Association of cancer with AIDS-related immunosuppression in adults. JAMA 285: 1736–1745 [DOI] [PubMed] [Google Scholar]
- Hoffman PC, Mauer AM, Vokes EE (2000) Lung cancer. Lancet 355: 479–485 [DOI] [PubMed] [Google Scholar]
- Kaplan E, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53: 457–481. 1-1-0058. Ref Type: Abstract [Google Scholar]
- Palella Jr FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD (1998) Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 338: 853–860 [DOI] [PubMed] [Google Scholar]
- Parker MS, Leveno DM, Campbell TJ, Worrell JA, Carozza SE (1998) AIDS-related bronchogenic carcinoma: fact or fiction? Chest 113: 154–161 [DOI] [PubMed] [Google Scholar]
- Powles T, Imami N, Nelson M, Gazzard BG, Bower M (2002) Effects of combination chemotherapy and highly active antiretroviral therapy on immune parameters in HIV-1 associated lymphoma. AIDS 16: 531–536 [DOI] [PubMed] [Google Scholar]
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346: 92–98 [DOI] [PubMed] [Google Scholar]
- Skarin AT, Herbst RS, Leong TL, Bailey A, Sugarbaker D (2001) Lung cancer in patients under age 40. Lung Cancer 32: 255–264 [DOI] [PubMed] [Google Scholar]
- Sridhar KS, Flores MR, Raub Jr WA, Saldana M (1992) Lung cancer in patients with human immunodeficiency virus infection compared with historic control subjects. Chest 102: 1704–1708 [DOI] [PubMed] [Google Scholar]
- Tam HK, Zhang ZF, Jacobson LP, Margolick JB, Chmiel JS, Rinaldo C, Detels R (2002) Effect of highly active antiretroviral therapy on survival among HIV-infected men with Kaposi sarcoma or non-Hodgkin's lymphoma. Int J Cancer 98: 916–922 [DOI] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, Van Oostrom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors (RECIST guidelines). J Natl Cancer Inst 92: 205–216 [DOI] [PubMed] [Google Scholar]
- Tirelli U, Spina M, Sandri S, Serraino D, Gobitti C, Fasan M, Sinicco A, Garavelli P, Ridolfo AL, Vaccher E (2000) Lung carcinoma in 36 patients with human immunodeficiency virus infection. The Italian Cooperative Group on AIDS and Tumors. Cancer 88: 563–569 [DOI] [PubMed] [Google Scholar]
- Vyzula R, Remick SC (1996) Lung cancer in patients with HIV-infection. Lung Cancer 15: 325–339 [DOI] [PubMed] [Google Scholar]
- Whooley BP, Urschel JD, Antkowiak JG, Takita H (1999) Bronchogenic carcinoma in young patients. J Surg Oncol 71: 29–31 [DOI] [PubMed] [Google Scholar]