Abstract
The frequency of high-risk human papillomavirus (hr-HPV) genotypes in patients with adenocarcinoma in situ (ACIS) with coexisting cervical intraepithelial neoplasia (CIN), ACIS without coexisting CIN, and high-grade CIN (CIN II/III) was studied, in order to gain more insight into the relation between hr-HPV infections and the development of coexisting squamous and glandular lesions. The SPF10 LiPA PCR was used to detect simultaneously 25 different HPV genotypes in biopsies obtained from 90 patients with CIN II/III, 47 patients with ACIS without coexisting CIN, and 49 patients with ACIS and coexisting CIN. hr-HPV was detected in 84 patients (93%) with CIN II/III, 38 patients (81%) with ACIS without CIN, and in 47 patients (96%) with ACIS and coexisting CIN. A total of 13 different hr-HPV genotypes were detected in patients with CIN II/III, and only five in patients with ACIS with/without coexisting CIN. HPV 31, multiple hr-HPV genotypes, and HPV genotypes other than 16, 18, and 45 were significantly more often detected in patients with CIN II/III, while HPV 18 was significantly more often detected in patients with ACIS with/without CIN. There were no significant differences in the frequency of specific hr-HPV genotypes between patients with ACIS with or without coexisting CIN. In conclusion, the frequency of specific hr-HPV genotypes is similar for patients with ACIS without CIN and patients with ACIS and coexisting CIN, but is significantly different for patients with CIN II/III without ACIS. These findings suggest that squamous lesions, coexisting with high-grade glandular lesions, are aetiologically different from squamous lesions without coexisting glandular lesions.
Keywords: ACIS, Coexisting CIN, HPV genotypes
A causal relation between high-risk human papillomavirus (hr-HPV) infections and cervical cancer has been documented in the literature beyond reasonable doubt (Bosch et al, 2002; Wright et al, 2002). High-risk human papillomavirus can be detected in almost 100% of squamous carcinomas and adenocarcinomas of the uterine cervix (Walboomers et al, 1999; Pirog et al, 2000). Numerous studies have suggested that the development of squamous cervical carcinoma is preceded by cervical intraepithelial neoplasia (CIN) (Wright et al, 2002; Bosch et al, 2002).
The frequency of hr-HPV genotypes in CIN lesions has been studied previously and its detection rate rises with increasing severity of the CIN lesion (Bekkers et al, 2002a; Bosch et al, 2002; Wright et al, 2002). Only limited studies are available on the frequency of hr-HPV genotypes in premalignant glandular lesions and the number of patients in most studies is rather low (see Table 1) (Colgan and Lickrish, 1990; Leary et al, 1991; Higgins et al, 1992; Duggan et al, 1995; Anciaux et al, 1997; McLachlin et al, 1997; Pirog et al, 2000; Riethdorf et al, 2000,2002; Madeleine et al, 2001). High-risk human papillomavirus was detected in 66–100% of patients with ACIS (Anciaux et al, 1997; Riethdorf et al, 2000). Approximately 48% of all women diagnosed with ACIS have coexisting squamous lesions (Colgan and Lickrish, 1990; Leary et al, 1991; Higgins et al, 1992; Duggan et al, 1995; Anciaux et al, 1997; McLachlin et al, 1997; Pirog et al, 2000; Riethdorf et al, 2000,2002; Madeleine et al, 2001), but the frequency of specific hr-HPV genotypes in patients with ACIS has not been studied in relation with the presence or absence of coexisting CIN lesions. In this study, the frequency of specific hr-HPV genotypes in patients with ACIS and coexisting CIN is compared with its frequency in patients with ACIS without coexisting CIN, and patients with CIN without ACIS, in order to gain more insight into the relation between hr-HPV infections and the development of coexisting squamous and glandular lesions.
Table 1. Summary of all studies since 1990 with more than 20 ACIS patients in relation with hr-HPV and/or coexisting CIN.
| Author and year | Patients (n) | hr-HPV pos | HPV 16 | HPV 18 | Coexisting CIN |
|---|---|---|---|---|---|
| Riethdorf et al (2002) | 42 | 32 | 22 | 10 | ND |
| (76%) | (53%) | (24%) | |||
| Madeleine et al (2001) | 82 | 71 | 32 | 43 | ND |
| (87%) | (39%) | (52%) | |||
| Riethdorf et al (2000) | 33 | 29 | 21 | 8 | 14 |
| (88%) | (64%) | (24%) | (42%) | ||
| Pirog et al (2000) | 23 | 23 | 10 | 6 | 9 |
| (100%) | (43%) | (26%) | (39%) | ||
| Anciaux et al (1997) | 21 | 16 | 6 | 10 | 9 |
| (76%) | (29%) | (47%) | (43%) | ||
| Duggan et al (1994) | 37 | 23 | 8 | 15 | ND |
| (66%) | (23%) | (43%) | |||
| Higgins et al (1992) | 42 | 39 | 13 | 28 | 22 |
| (95%) | (31%) | (67%) | (52%) | ||
| Leary et al (1991) | 30 | 21 | 10 | 11 | 15 |
| (70%) | (33%) | (37%) | (50%) | ||
| Bekkers et al (present study) | 96 | 85 | 39 | 41 | 49 |
| (89%) | (41%) | (43%) | (51%) |
ACIS=adenocarcinoma in situ; hr-HPV= high-risk human papillomavirus; CIN=cervical intraepithelial neoplasia; HPV=human papillomavirus; ND=not documented.
MATERIAL AND METHODS
Glandular lesions
In the automated databases of the pathology laboratories of the University Medical Center Nijmegen, the Canisius Wilhelmina Hospital Nijmegen, and the Rijnstate Hospital Arnhem, 120 patients diagnosed with ACIS between 1988 and 2000 were identified. No histopathological material was left for further study of nine patients, and six patients had a histopathological diagnosis of invasive carcinoma within 1 month of the diagnosis of ACIS. Of the remaining 105 patients, the histopathological slides of a biopsy-, cone-, or hysterectomy specimen were re-examined independently, by three experienced gynaecopathologists (JB, AWvT, and MM). Complete agreement on the diagnosis of ACIS was reached in 96 patients (91%), according to the criteria of Brown and Wells. The criteria of Brown and Wells take architectural (glandular irregularity and complexity) and cytological criteria (nuclear enlargement, hyperchromasia, pseudostratification of nuclei, increased or abnormal mitoses) into account (Wells and Brown, 1986). The three pathologists reached no consensus on the diagnosis of ACIS in nine patients, and these patients were excluded.
Coexisting squamous intraepithelial lesions were detected and confirmed by consensus of the three pathologists (JB, AWvT, MM) in the slides of 49 of the 96 patients (51%); 35 patients (71%) had coexisting CIN 3 (severe dysplasia, or carcinoma in situ), eight patients (16%) had coexisting CIN 2 (moderate dysplasia), and six patients (12%) had coexisting CIN 1 (mild dysplasia). In 41 of these 49 patients (84%), CIN and ACIS were diagnosed in the same slide/section, and hr-HPV detection was carried out on adjacent sections, containing both the squamous and glandular lesion. In eight patients, CIN was diagnosed in a different slide than in which ACIS was diagnosed and on which hr-HPV detection was performed. Five of these eight patients had coexisting CIN I, two had coexisting CIN II, and one had coexisting CIN III. The mean age of the 96 patients was 38.3 years (28–73) at the time of the diagnosis.
A cervical scrape preceding the diagnosis of ACIS less than 6 months was made in 43 of the 49 patients (88%) with ACIS and coexisting CIN, and in 40 of the 47 patients (85%) with ACIS without coexisting CIN. The cervical scrape indicated a high-grade glandular (moderate atypia–ACIS), a high-grade squamous lesion (moderate dysplasia–CIS), or both in, respectively, 13 (30%), 15 (35%), and 13 (30%) patients with ACIS and coexisting CIN, and in, respectively, 24 (60%), four (10%), and eight (20%) patients with ACIS without coexisting CIN. The remaining six patients had only mild atypia of glandular cells in the cervical scrape.
Squamous lesions
A control group of 90 patients consisted of patients who consulted the colposcopy clinic at the UMC between 1997 and 1999 and who were diagnosed with high-grade CIN in the biopsy specimen of a large-loop excision of the transformation zone. All slides were reviewed by an experienced gynaecopathologist (JB), and the diagnosis of high-grade CIN was confirmed. Of these 90 patients, 18 patients (20%) were diagnosed with moderate dysplasia (CIN II), 36 patients (40%) with severe dysplasia (CIN III), and 36 patients (40%) with carcinoma in situ (CIN III). The mean age at the time of diagnosis was 37.6 years (28–59).
A cervical scrape preceding the diagnosis of CIN II/III less than 6 months was taken of all 90 patients. The cervical scrape indicated a high-grade squamous lesion in 80 patients (89%), a high-grade glandular and squamous lesion in one patient (1%), and low-grade squamous lesions in nine patients (10%).
Taking the preceding cervical scrapes of all patients with ACIS and CIN II/III together, the cervical scrape indicated a high-grade glandular lesion in 37 patients (21%), a high-grade squamous lesion in 99 patients (57%), both in 22 patients (13%), and only low-grade lesions in 15 patients (9%).
HPV detection
A 3-μm section of the histopathological specimens, on which ACIS and/or CIN II/III was diagnosed, was taken for HPV analysis. In 41 of the 49 patients with ACIS and coexisting CIN, HPV detection was carried out on a section containing both the glandular and squamous lesion. HPV detection was performed using a broad-spectrum short-fragment polymerase chain reaction (SPF10 PCR) as previously described (Kleter et al, 1998,1999; Melchers et al, 1999; Quint et al, 2001; Bekkers et al, 2002b). In case of a positive PCR, subsequent genotyping was performed via a reverse hybridisation line probe assay (LiPA), allowing for simultaneous typing of 25 HPV genotypes, including hr-HPV genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68. This SPF10 LiPA PCR HPV detection method is highly sensitive, specific and reproducible and has been clinically validated (Kleter et al, 1998,1999; Melchers et al, 1999; Pirog et al, 2000; Quint et al, 2001). If more than one hr-HPV genotype was detected with the SPF10 LiPA PCR in a single sample of an individual patient, that patient was considered to be infected with multiple hr-HPV genotypes.
Analysis
The frequency of specific hr-HPV genotypes in patients with ACIS without coexisting CIN, ACIS with coexisting CIN, and CIN II/III without ACIS were compared. The frequency of specific hr-HPV genotypes was also investigated in relation with the suspicion of a high-grade glandular and/or high-grade squamous lesion in the preceding cervical scrape. Statistical analysis including χ2 tests and independent t-tests was performed, considering all values of P<0.05 to be significant.
RESULTS
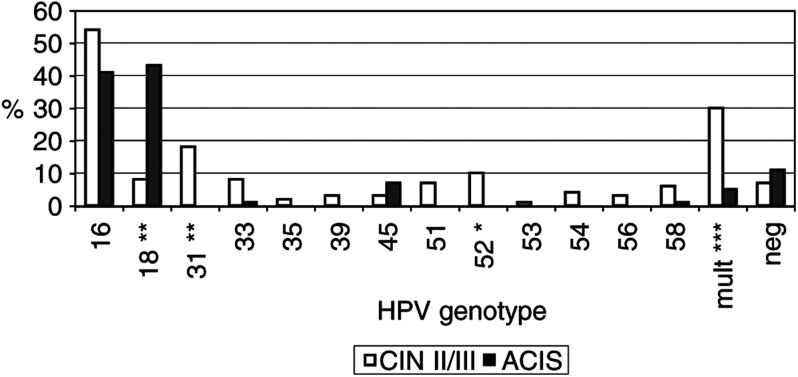
High-risk human papillomavirus genotypes were detected in 93% of the patients with CIN II/III, 81% of the patients with ACIS without coexisting CIN, and in 96% of the patients with ACIS and coexisting CIN. Figure 1 shows that HPV 31 and multiple hr-HPV genotypes were significantly more often detected in patients with CIN II/III, while HPV 18 was significantly more often detected in patients with ACIS (with or without coexisting CIN). Among patients with CIN II/III, 13 different hr-HPV genotypes were detected, compared with only five different hr-HPV genotypes in patients with ACIS with or without coexisting CIN.
Figure 1.
Relative frequency of specific hr-HPV genotypes in patients with CIN II/III (90 patients) and patients with ACIS (96 patients). *P<0.05, **P<0.01, ***P<0.001.
HPV 16 was the most frequently detected HPV genotype in patients with CIN II/III (54%), while both HPV 16 and 18 showed a high frequency of, respectively, 41 and 43% in all patients with ACIS (see Figure 1). HPV 16 or 18 were present in 79% of all patients with ACIS (five patients had a double infection with HPV 16 and 18), and in 61% of the patients with CIN II/III (one patient had a double infection with HPV 16 and 18).
Table 2 shows that there were no significant differences in the frequency of HPV 16, 45, and hr-HPV-negative patients between patients with CIN II/III and patients with ACIS and coexisting CIN. The frequency of HPV 18 was significantly lower, and the frequency of HPV 31, multiple hr-HPV, and hr-HPV genotypes other than HPV 16, 18, 31, and 45 was significantly higher in patients with CIN II/III compared with patients with ACIS and coexisting CIN. This means that the frequency of HPV 18 is significantly higher in CIN lesions coexisting with ACIS than in solitary CIN lesions.
Table 2. Frequency of hr-HPV genotypes in patients with CIN II/III compared with patients with ACIS and coexisting CIN.
| hr-HPV genotype | CIN II/III n=90 | ACIS with CIN n=49 | Significance |
|---|---|---|---|
| 16 | 49 (54%) | 19 (39%) | NS |
| 18 | 7 (8%) | 23 (47%) | χ2=26.49, P<0.001 |
| 31 | 15 (17%) | 0 (0%) | χ2=8.38, P<0.01 |
| 45 | 3 (3%) | 5 (10%) | NS |
| Other | 34 (38%) | 2 (4%) | χ2=19.73, P<0.001 |
| Multiple | 26 (29%) | 2 (4%) | χ2=12.48, P<0.001 |
| Total hr-HPV positive | 84 (93%) | 47 (96%) | NS |
| hr-HPV negative | 6 (7%) | 2 (4%) | NS |
ACIS=adenocarcinoma in situ; hr-HPV= high-risk human papillomavirus; CIN=cervical intraepithelial neoplasia; NS=not significant.
Table 3 shows that there were no significant differences in the frequency of HPV 16, 18, 31, 45, multiple hr-HPV, and other hr-HPV genotypes between patients with ACIS without coexisting CIN and patients with ACIS and coexisting CIN. The number of hr-HPV-negative patients was significantly higher in patients with ACIS without coexisting CIN.
Table 3. Frequency of hr-HPV genotypes in patients with ACIS with coexisting CIN compared with patients with ACIS without coexisting CIN.
| hr-HPV genotype | ACIS with CIN n=49 | ACIS without CIN n=47 | Significance |
|---|---|---|---|
| 16 | 19 (39%) | 20 (43%) | NS |
| 18 | 23 (47%) | 18 (38%) | NS |
| 31 | 0 (0%) | 0 (0%) | NS |
| 45 | 5 (10%) | 2 (4%) | NS |
| Other | 2 (4%) | 1 (2%) | NS |
| Multiple | 2 (4%) | 3 (6%) | NS |
| Total hr-HPV positive | 47 (96%) | 38 (81%) | χ2=6.07, P<0.02 |
| hr-HPV negative | 2 (4%) | 9 (19%) | χ2=6.63, P<0.01 |
ACIS=adenocarcinoma in situ; hr-HPV= high-risk human papillomavirus; CIN=cervical intraepithelial neoplasia; NS=not significant.
Among the patients with ACIS and coexisting CIN, there were no significant differences in the frequency of specific hr-HPV genotypes regarding the different degrees of coexisting CIN.
The mean age of the patients with ACIS without CIN was 40.1 years, while the mean age of patients with ACIS and coexisting CIN was 36.3 years (t=2.32, P<0.05). There was no difference between the mean age of the total group of patients with ACIS and the patients with CIN II/III (38.2 and 37.6 years, respectively).
Within the group of patients with CIN II/III, there were no significant differences in the frequency of HPV 16, 18, 31, and 45, multiple hr-HPV, other hr-HPV genotypes, or hr-HPV-negative patients between patients with moderate dysplasia, severe dysplasia, or carcinoma in situ. Multiple hr-HPV genotypes were detected in, respectively, 22, 28, and 33% of these patients.
High-risk human papillomavirus was detected in 33 of the 37 patients (89%) with a cervical scrape that indicated a high-grade glandular lesion, in 92 of the 99 patients (93%) with a cervical scrape that indicated a high-grade squamous lesion, in all 22 patients (100%) with a cervical scrape that indicated both a high-grade glandular and squamous lesion, and in 13 of the 15 patients (87%) with a cervical scrape that indicated only a low-grade lesion. There were no significant differences in the frequency of HPV 16, 18, 31, 45, multiple or other HPV genotypes regarding the presence or absence of a suspicion of a high-grade squamous and/or glandular lesion in the cervical scrapes.
DISCUSSION
The number of patients with ACIS and coexisting CIN lesions among all patients with ACIS in this study is similar to the numbers reported in the literature (Table 1). No significant differences in the frequency of different hr-HPV genotypes were observed between patients with ACIS with coexisting CIN, and patients with ACIS without coexisting CIN, as has been reported previously (Anciaux et al, 1997; Riethdorf et al, 2000; Zaino, 2002). However, significant differences in the frequency of HPV 18, HPV 31, multiple hr-HPV genotypes, and hr-HPV genotypes other than HPV 16, 18, 31, and 45 were observed between patients with CIN II/III and patients with ACIS, either with or without coexisting CIN. One may hypothesise that coexisting glandular and squamous lesions share a common aetiology, different from solitary squamous lesions, under the influence of specific hr-HPV genotypes, as has been previously described for squamous lesions (Burghardt and Ostor, 1983; Park et al, 1998; Bekkers et al, 2002b). Indeed, in 41 of the 49 patients (84%) in the present study the ACIS lesion was adjacent to the coexisting squamous lesion. Furthermore, Colgan and Lickrish (1990) found no differences in the linear extend, circumferential extend, multifocality, or the co-presence of invasive adenocarcinoma between patients with ACIS without CIN and ACIS with coexisting CIN lesions. These observations seem to support the hypothesis that ACIS and coexisting CIN lesions share a common aetiology.
Only five different hr-HPV genotypes (HPV 16, 18, 33, 45, 58) were detected in patients with ACIS (of which HPV 33 and 58 in <2% of the patients), compared with 13 different hr-HPV genotypes in patients with CIN II/III. Other authors also found a limited number of hr-HPV genotypes (three to seven) among patients with ACIS (Pirog et al, 2000; Madeleine et al, 2001). This may suggest that only certain hr-HPV genotypes are able to infect and/or induce lesions in the glandular mucosa. This fact may be the reason for the significantly lower detection rate of multiple hr-HPV genotypes, and the significantly higher detection rate of HPV 18 in the cervix of patients with ACIS. However, higher rates (13–22%) of multiple hr-HPV infections among patients with ACIS have been described in the literature (Pirog et al, 2000; Madeleine et al, 2001). The lower rate of multiple hr-HPV genotypes in the present study is probably the result of the very restrictive diagnostic criteria, and the consensus agreement by three pathologists, that was used to diagnose ACIS.
The high frequency of a single hr-HPV genotype in patients with ACIS compared with patients with CIN II/III may reflect the monoclonal aspect of the ACIS lesion, as is often the case in invasive carcinomas (Walboomers et al, 1999). Patients with (squamous) CIS in this study showed a higher prevalence (33%) of multiple hr-HPV genotypes, as well as a higher number of different hr-HPV genotypes (12), which may be regarded as another indication that ACIS lesions may have a biologic behaviour and/or aetiology that is different from squamous in situ lesions (Schoolland et al, 2002).
The number of hr-HPV-negative patients with ACIS without CIN in this study was significantly higher than in patients with ACIS and coexisting CIN. ACIS may be a precursor of different subtypes of invasive adenocarcinoma (like small cell or endometrioid adenocarcinoma) that have a lower or no prevalence of hr-HPV (Lee, 1999; Pirog et al, 2000; Schoolland et al, 2002). These subtypes are rare, and distinction on a biopsy with only ACIS proved to be difficult (Lee, 1999; Schoolland et al, 2002). It is possible that these hr-HPV-negative subtypes of ACIS are less often associated with coexisting CIN, resulting in a higher rate of hr-HPV-negative patients among patients with ACIS without CIN.
The significant difference in age between patients with ACIS without CIN and patients with ACIS with coexisting CIN, in which the latter are younger, has been described previously (Colgan and Lickrish, 1990; Pirog et al, 2000). Different explanations for this age difference are possible. Firstly, the lesions in patients with ACIS and coexisting CIN may show a faster progression, leading to detection at a younger age. This has been described previously for HPV 18-related lesions (Barnes et al, 1988), but we did not find a difference in HPV 18 prevalence between patients with ACIS alone or ACIS and coexisting CIN. Secondly, the involvement of the squamous epithelium may be the reason for the detection of ACIS with coexisting CIN at a younger age, since ecto-cervical lesions are more easily detected. Thirdly, it may be a coincidental finding especially since we did not find a difference in mean age between patients with CIN II/III and the total group of patients with ACIS.
There was no significant difference in the overall sensitivity of the preceding cervical scrape for the detection of either ACIS or CIN II/III in the present study (respectively 93 and 90%). The observed sensitivity to detect ACIS was higher than reported in the literature (Lee, 1999; Schoolland et al, 2002). However, these studies used cervical scrapes from a cervical cancer screening programme, while in the present study several patients had more than one preceding cervical scrape taken, which may have alerted the pathologist, leading to the higher sensitivity.
We did not find a relation between the presence of certain hr-HPV genotypes in the biopsy, and the sensitivity of the cervical scrape in detecting ACIS and/or CIN II/III lesions. This indicates that the detection of a lesion in the cervical scrape is not influenced by the genotype of hr-HPV infecting that lesion.
In conclusion, patients with ACIS have significantly more often HPV 18 infections, while patients with CIN II/III have significantly more often infections with HPV 31, HPV genotypes other than 16, 18, 31, and 45, and multiple hr-HPV genotypes. The detection of high-grade glandular and/or squamous lesion by cervical scrapes is not influenced by the hr-HPV genotype associated with the lesion. Among patients with ACIS, patients with coexisting CIN lesions tend to be younger, but they have a similar frequency of different hr-HPV genotypes than patients with ACIS without CIN, while the frequency of specific hr-HPV genotypes differs significantly from those of patients with CIN II/III without ACIS. These findings suggest that squamous lesions, coexisting with high-grade glandular lesions, are aetiologically different from squamous lesions without coexisting glandular lesions. The clinical implication of these findings needs further study.
References
- Anciaux D, Lawrence WD, Gregoire L (1997) Glandular lesions of the uterine cervix: prognostic implications of human papillomavirus status. Int J Gynecol Pathol 16: 103–110 [DOI] [PubMed] [Google Scholar]
- Barnes W, Delgado G, Kurman RJ, Petrilli ES, Smith DM, Ahmed S, Lorincz AT, Temple GF, Jenson AB, Lancaster WD (1988) Possible prognostic significance of human papillomavirus type in cervical cancer. Gynecol Oncol 29: 267–273 [DOI] [PubMed] [Google Scholar]
- Bekkers RL, Melchers WJ, Bakkers JM, Hanselaar AG, Quint WG, Boonstra H, Massuger LF (2002a) The role of genotype-specific human papillomavirus detection in diagnosing residual cervical intraepithelial neoplasia. Int J Cancer 102: 148–151 [DOI] [PubMed] [Google Scholar]
- Bekkers RL, Melchers WJ, Bulten J, Boonstra H, Quint WG, Hanselaar AG, Massuger LF (2002b) Localized distribution of human papillomavirus genotypes in the uterine cervix. Eur J Gynaecol Oncol 23: 203–206 [PubMed] [Google Scholar]
- Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV (2002) The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 55: 244–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt E, Ostor AG (1983) Site and origin of squamous cervical cancer: a histomorphologic study. Obstet Gynecol. 62: 117–127 [PubMed] [Google Scholar]
- Colgan TJ, Lickrish GM. (1990) The topography and invasive potential of cervical adenocarcinoma in situ, with and without associated squamous dysplasia. Gynecol Oncol 36: 246–249 [DOI] [PubMed] [Google Scholar]
- Duggan MA, McGregor SE, Benoit JL, Inoue M, Nation JG, Stuart GC (1995) The human papillomavirus status of invasive cervical adenocarcinoma: a clinicopathological and outcome analysis. Hum Pathol 26: 319–325 [DOI] [PubMed] [Google Scholar]
- Higgins GD, Phillips GE, Smith LA, Uzelin DM, Burrell CJ (1992) High prevalence of human papillomavirus transcripts in all grades of cervical intraepithelial glandular neoplasia. Cancer 70: 136–146 [DOI] [PubMed] [Google Scholar]
- Kleter B, van Doorn LJ, ter Schegget J, Schrauwen L, van Krimpen K, Burger M, ter Harmsel B, Quint W (1998) Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am J Pathol 153: 1731–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleter B, van Doorn LJ, Schrauwen L, Molijn A, Sastrowijoto S, ter Schegget J, Lindeman J, ter Harmsel B, Burger M, Quint W (1999) Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol 37: 2508–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary J, Jaworski R, Houghton R (1991) In-situ hybridization using biotinylated DNA probes to human papillomavirus in adenocarcinoma-in-situ and endocervical glandular dysplasia of the uterine cervix. Pathology 23: 85–89 [DOI] [PubMed] [Google Scholar]
- Lee KR (1999) Adenocarcinoma in situ with a small cell (endometrioid) pattern in cervical smears: a test of the distinction from benign mimics using specific criteria. Cancer 87: 254–258 [PubMed] [Google Scholar]
- Madeleine MM, Daling JR, Schwartz SM, Shera K, McKnight B, Carter JJ, Wipf GC, Critchlow CW, McDougall JK, Porter P, Galloway DA (2001) Human papillomavirus and long-term oral contraceptive use increase the risk of adenocarcinoma in situ of the cervix. Cancer Epidemiol Biomarkers Prev 10: 171–177 [PubMed] [Google Scholar]
- McLachlin CM, Shen LH, Sheets EE, Kozakewich H, Perlman SE, Tate JE, Crum CP (1997) Disparities in mean age and histologic grade between human papillomavirus type specific early cervical neoplasms. Hum Pathol 28: 1226–1229 [DOI] [PubMed] [Google Scholar]
- Melchers WJ, Bakkers JM, Wang J, de Wilde PC, Boonstra H, Quint WG, Hanselaar AG (1999) Short fragment polymerase chain reaction reverse hybridization line probe assay to detect and genotype a broad spectrum of human papillomavirus types. Clinical evaluation and follow-up. Am J Pathol 155: 1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Sun D, Genest DR, Trivijitsilp P, Suh I, Crum CP (1998) Coexistence of low and high grade squamous intraepithelial lesions of the cervix: morphologic progression or multiple papillomaviruses? Gynecol Oncol 70: 386–391 [DOI] [PubMed] [Google Scholar]
- Pirog EC, Kleter B, Olgac S, Bobkiewicz P, Lindeman J, Quint WG, Richart RM, Isacson C (2000) Prevalence of human papillomavirus DNA in different histological subtypes of cervical adenocarcinoma. Am J Pathol 157: 1055–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quint WG, Scholte G, van Doorn LJ, Kleter B, Smits PH, Lindeman J (2001) Comparative analysis of human papillomavirus infections in cervical scrapes and biopsy specimens by general SPF(10) PCR and HPV genotyping. J Pathol 194: 51–58 [DOI] [PubMed] [Google Scholar]
- Riethdorf L, Riethdorf S, Lee KR, Cviko A, Loening T, Crum CP (2002) Human papillomaviruses, expression of p16INK4, and endocervical glandular neoplasia. Hum Pathol 33: 899–904 [DOI] [PubMed] [Google Scholar]
- Riethdorf S, Riethdorf L, Milde-Langosch K, Park TW, Loning T (2000) Differences in HPV 16- and HPV 18 E6/E7 oncogene expression between in situ and invasive adenocarcinomas of the cervix uteri. Virchows Arch 437: 491–500 [DOI] [PubMed] [Google Scholar]
- Schoolland M, Segal A, Allpress S, Miranda A, Frost FA, Sterrett GF (2002) Adenocarcinoma in situ of the cervix. Cancer 96: 330–337 [DOI] [PubMed] [Google Scholar]
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N (1999) Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189: 12–19 [DOI] [PubMed] [Google Scholar]
- Wells M, Brown LJ (1986) Glandular lesions of the uterine cervix: the present state of our knowledge. Histopathology 10: 777–792 [DOI] [PubMed] [Google Scholar]
- Wright Jr TC, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ (2002) 2001 Consensus Guidelines for the management of women with cervical cytological abnormalities. JAMA 287: 2120–2129 [DOI] [PubMed] [Google Scholar]
- Zaino RJ (2002) Symposium part I: adenocarcinoma in situ, glandular dysplasia, and early invasive adenocarcinoma of the uterine cervix. Int J Gynecol Pathol 21: 314–326 [DOI] [PubMed] [Google Scholar]