Abstract
Anthrax lethal toxin (LT), a virulence factor secreted by Bacillus anthracis, is selectively toxic to human melanomas with the BRAF V600E activating mutation due to its proteolytic activities toward the mitogen-activated protein kinase kinases (MEKs). To develop LT variants with lower in vivo toxicity and high tumor specificity, and therefore greater potential for clinical use, we generated a mutated LT that requires activation by matrix metalloproteinases (MMPs). This engineered toxin was less toxic than wild-type LT to mice due to the limited expression of MMPs by normal cells. Moreover, the systemically administered toxin produced greater anti-tumor effects than wild-type LT towards human xenografted tumors. This was shown to result from its greater bioavailability, a consequence of the limited uptake and clearance of the modified toxin by normal cells. Furthermore, the MMP-activated LT had very potent anti-tumor activity not only to human melanomas containing the BRAF mutation, but also to other tumor types including lung and colon carcinomas regardless of their BRAF status. Tumor histology and in vivo angiogenesis assays showed that this anti-tumor activity is due largely to the indirect targeting of tumor vasculature and angiogenic processes. Thus, even tumors genetically deficient in anthrax toxin receptors were still susceptible to the toxin therapy in vivo. Moreover, the modified toxin also displayed lower immunogenicity compared to the wild-type toxin. All these properties suggest that this MMP-activated anti-tumor toxin has a potential for use incancer therapy.
The symptoms of many bacterial diseases are due largely to the actions of toxic proteins released by the bacteria. Diphtheria toxin (DT) and Pseudomonas exotoxin A (PE) are two such well-known toxins secreted by the pathogenic bacterium Corynebacterium diphtheriae and the opportunistic pathogen Pseudomonas aeruginosa (1). After binding and entering mammalian cells, DT and PE catalyze the adenosine diphosphate (ADP)-ribosylation and inactivation of elongation factor 2 (EF2), leading to protein synthesis inhibition and cell death (2;3). The powerful lethal action of these toxins has been exploited extensively in the past two decades to target cancer cells by fusing the toxins with antibodies or growth factors that can selectively recognize antigens or receptors on cancer cells. These efforts have resulted in the first FDA-approved “immunotoxin”, DAB389IL2 (denileukin diftitox or Ontak), a fusion of DT catalytic and translocation domains and IL2 (interleukin 2), for treatment of persistent or recurrent T-cell lymphoma (4). With the rapid progress in understanding the structures and functions of anthrax lethal toxin (LT), an important virulence factor secreted by Bacillus anthracis, LT has been identified as a bacterial toxin having a completely different mode of action that can be used for tumor targeting (5).
LT consists of two proteins, the cell binding component protective antigen (PA, 83 kDa) and the enzymatic moiety lethal factor (LF, 89 kDa). To intoxicate mammalian cells, PA binds to the cell surface receptors tumor endothelium marker 8 (TEM8) and capillary morphogenesis gene 2 product (CMG2). PA is proteolytically activated by cell surface furin protease, leaving the carboxyl-terminal 63-kDa fragment (PA63) bound to the cell surface, resulting in the formation of the active PA63 heptamer. The PA63 heptamer then binds and translocates LF into the cytosol of the cell to exert its cytotoxic effects (6). LF is a metalloproteinase, enzymatically cleaves and inactivates the mitogen-activated protein kinase kinases (MEKs) 1-4, 6 and 7 (7-9), and thus efficiently blocks three key mitogen-activated protein kinase (MAPK) pathways, including the ERK, p38, and Jun N-terminus kinase (JNK) pathways (10).
The NCI60 anticancer drug screen revealed that LT is selectively toxic to many human melanoma cell lines, indicating that LT may be a useful therapeutic agent for human melanomas (11). This selective cytotoxicity of LT to human melanomas was later linked to a BRAF-activating mutation occurring in the melanomas (12). BRAF is a serine/threonine kinase immediately upstream of MEK1/2 in the cascade of the ERK MAPK pathway. Davies and colleagues demonstrated that about 70% of human melanomas and a smaller fraction of other human cancer types contain a BRAF Valine600 to glutamic acid mutation (V600E). This mutation involves replacement of a neutral amino acid with a negatively charged one that mimics the phosphorylation of threonine599 and serine602 in the activating loop and thus locks the molecule in the ‘on’ position (13). Human melanomas with the oncogenic BRAF V600E mutation are dependent on the constitutive activation of the ERK pathway for survival. Thus, it was shown that human melanoma cell lines with the BRAF mutation are sensitive to LT, while those without the mutation are generally resistant (14). The anti-melanoma efficacy of LT was further recapitulated in vivo (15). However, LT, a major virulence factor of B. anthracis, has recognized in vivo toxicity, and thus might not be safe to use in human cancer patients (16). Therefore, the development of an attenuated and tumor specific version of LT would be beneficial.
The unique requirement for PA proteolytic activation on the target cell surface provides a way to re-engineer this protein to make its cleavage dependent on proteases that are enriched in tumor tissues. To this end, we previously generated PA mutants that can be activated by matrix metalloproteinases (MMPs) (17). MMPs are overproduced by tumor tissues and implicated in cancer cell growth, angiogenesis, and metastasis (18). However, unlike furin, which is ubiquitously expressed, MMPs are restricted to only a small number of normal cells. Thus, in theory, MMP-activated LT should have higher specificity to tumors. In the current study, we show that the MMP-activated LT not only exhibits much lower toxicity than wild-type LT to mice, but also shows higher toxicity to human tumors in tumor xenograft models. This is attributed, in part, to the unexpected greater bioavailability of MMP-activated PA protein in circulation. Moreover, we unexpectedly found that the MMP-activated LT has potent anti-tumor activity not only to human melanomas with the BRAF V600E mutation, but also to other tumor types, regardless of the BRAF mutation status. We identified that this potent anti-tumor activity is due to the indirect targeting of tumor vasculature and angiogenic processes.
EXPERIMENTAL PROCEDURES
Protein purification
PA, PA-L1, LF, and FP59 were purified as previously described (19).
Cell culture and cytotoxicity assay
All NCI60 human cancer cells and mouse melanoma B16-BL6 and Lewis lung carcinoma LL3 cells were cultured in DMEM with 10% fetal bovine serum (FBS) as described previously (20;21). Human umbilical vascular endothelial cells (HUVEC) and human microvascular endothelial cells (HMVEC) were obtained from Cambrex (Walkersville, MD). HUVEC and HMVEC were cultured in endothelial cell growth medium-2 (EGM-2) plus EGM-2 singleQuots and EGM-2 plus EGM-2 MV singleQuots (Cambrex), respectively. Mouse bone marrow derived macrophages were isolated from C57BL/6, BALB/c, and nude mice as described (22). For cytotoxicity assays, approximately 5,000 cells were seeded into each well in 96-well plates. Then various concentrations of PA proteins, combined with LF (5.5 nM) or FP59 (1.9 nM), were added to the cells. Cell viability was assayed after incubation with the toxins for 72 h using MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide), as described previously (17).
PA proteins binding, LF translocation, and MEKs cleavage analyses
HUVEC and HMVEC cells grown to confluence in 6-well plates were incubated with growth medium containing PA/LF (6 nM/6 nM) or PA-L1/LF (6 nM/6 nM) for 2 h or 4 h at 37°C, then washed five times with Hank’s Balanced Salt Solution (HBSS) (Biofluids, Rockville, MD) to remove unbound toxins. The cells were then lysed and the cell lysates were subjected to SDS-PAGE, followed by Western blotting to detect cell-associated PA proteins, LF, and MEKs cleavages. Anti-PA polyclonal rabbit antiserum (#5308) and anti-LF antiserum (#5309) were made in our laboratory. Anti-MEK1 (Cat No. 07-641) was obtained from Upstate Biotechnology, inc. (Lake Placid, NY), anti-MEK3 (Cat No. sc-961) and anti-MEK4 (Cat No. sc-837) from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Maximum tolerated dose determination
Female C57BL/6J and BALB/c mice (The Jackson Laboratory) between 8-10 weeks of age were used in this study. The mice were housed in a pathogen-free facility certified by the Association for Assessment and Accreditation of Laboratory Animal Care International, and the study was carried out in accordance with protocols approved by the NIH Animal Care and Use Committee. The maximum tolerated doses of PA/LF (3:1 ratio) and PA-L1/LF (3:1 ratio) were determined using a dose escalation protocol aimed at minimizing the number of the mice used. The mice (n = 5) in each group were anesthetized by isoflurane inhalation and injected intraperitoneally with 6 doses of the toxins in 500 μl PBS using the schedule of three times a week for two weeks. The mice were monitored closely for signs of toxicity including inactivity, loss of appetite, inability to groom, ruffling of fur, and shortness of breath, and euthanized by CO2 inhalation at the onset of obvious malaise. The maximum tolerated dose for 6 administrations (MTD6) was determined as the highest dose in which outward disease was not observed in any mice within a 14-day period of observation.
Histopathological analysis
To evaluate the in vivo toxicity of the lethal toxins, C57BL/6 mice were injected with 6 doses of PBS and 45/15 μg of PA-L1/LF. Then the mice were killed by a brief CO2 inhalation. The organs and tissues, including brain, lung, heart, liver, small and large intestines, kidney and adrenal glands, stomach, pancreas, spleen, thyroid, bladder, esophagus, skeletal muscles, thymus, and lymph nodes were fixed for 24 h in 4% paraformaldehyde, embedded in paraffin, sectioned, and stained with hematoxylin/eosin and subjected to microscopic analysis.
In vivo anti-tumor experiments
Various human tumor xenografts were established in nude mice (NCI, Frederick, MD) by subcutaneously injecting 1×107 human tumor cells into the dorsal region of each mouse. The syngeneic mouse B16-BL6 melanoma and LL3 Lewis lung carcinoma were established subcutaneously in C57BL/6 mice by injecting 5×105 cells per mouse. After the human tumor xenografts were well established and the mouse transplanted tumors were visible, the tumor-bearing mice were injected (i.p.) with PA/LF, PA-L1/LF, or PBS in 500 ul PBS for 6 doses (three times per week for two weeks). The longest and shortest tumor diameters were determined with calipers by an investigator unaware of the treatment group, and the tumor weight was calculated using the formula: milligrams = (length in mm ×[width in mm]2)/2. The experiment was terminated when one or more mice in a treatment group presented frank tumor ulceration or the tumor exceeded 10% of body weight. The significance of differences in tumor size was determined by two-tailed Student’s t-test using Microsoft Excel.
Tumor histology and immunohistochemistry
A549/ATCC tumor-bearing nude mice were treated (i.p.) with 30/10 μg of PA-L1/LF or PBS at day 0, 2, 4, and 7. The mice were euthanized 2 h after BrdU injection (i.p.) at day 8. The tumors were dissected and fixed for 24 h in 4% paraformaldehyde, embedded in paraffin, sectioned, and stained with hematoxylin/eosin. The tumor sections were also analyzed using a monoclonal rat anti-mouse CD31 (Research Diagnostics Inc, Concord, MA), or a monoclonal rat anti-human BrdU (Accurate Chemical & Scientific Corporation, Westbury, NY). Images of the histological sections were captured using an Aperio T3 Scanscope (Aperio Technologies, Vista, CA), saved as TIFF files, and were quantified using the Northern Eclipse Image Analysis Software (Empix Imaging, North Tonawanda, NY). For necrosis, the results were expressed as a percentage of necrotic area to total area. Cell proliferation is presented as a percentage of BrdU positive cells among total cells. Tumor vascularization is shown as the number of CD31-positive structures per mm2. All histological evaluation was performed by aninvestigator that was blinded as to the treatment of each mouse.
Angiogenic factor profiling reverse transcription (RT)-PCR analysis
Human cancer A549/ATCC, HT144, HT29, SK-MEL-28 cells were cultured into 6-well plates to 50% confluence and treated with DMEM only or DMEM containing PA-L1/LF (2.4/2.2 nM) overnight. Total RNA was then isolated and subjected for the first-strand cDNA synthesis using the SuperScript II Reverse Transcriptase (Invitrogen). Then, the RT products were used as the templates for the angiogenic factor profiling PCR analysis using the kit purchased from SuperArray Bioscience (PH-065B) (Frederick, MD) following the manufacturer’s instructions.
RT-PCR and transfection
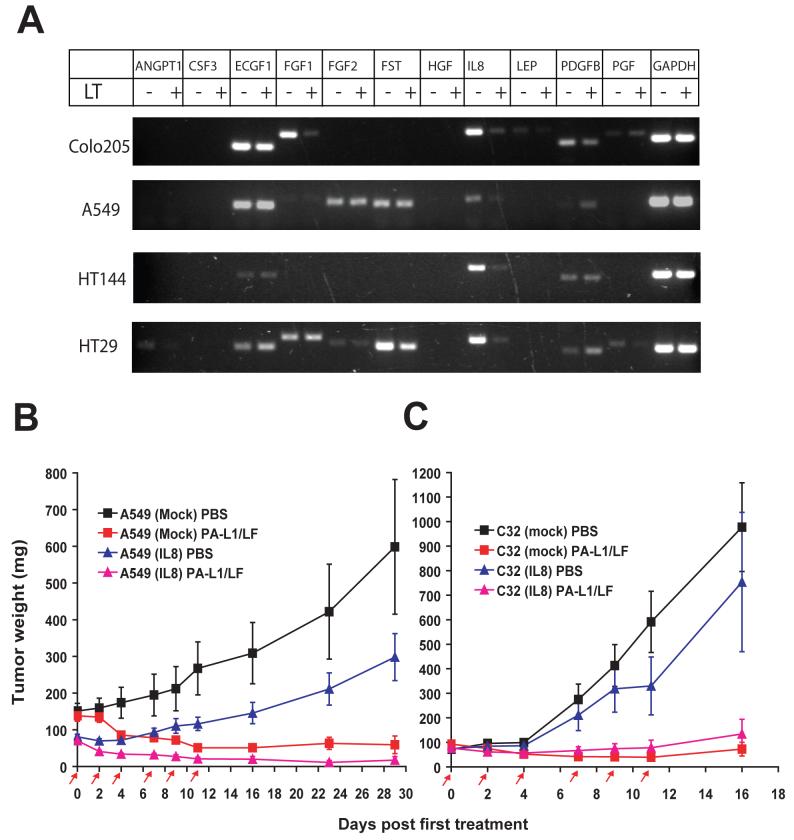
Total RNA isolated from human A594/ATCC cells was subjected to the reverse transcription reaction using the SuperScript II Reverse Transcriptase (Invitrogen). The human IL8 cDNA coding fragment was then amplified using a forward primer AATTCTTAAGCCACCATGACTTCCAAGCTGGCCGTGGCTCTCTT (AflII site is underlined, Kozak sequence in italic, start codon in boldface) and a reverse primer GGAGGATCCTTATGAATTCTCAGCCC TCTTCAAAAACT (BamHI site underlined). The resulting DNA fragment was subcloned into AflII and BamHI sites of pIREShgy2B, a bicistronic mammalian expression vector containing an attenuated version of the internal ribosome entry site of the encephalomyocarditis virus, which allows both the gene of interest and the hygromycin B selection marker to be translated from a single mRNA. The resulting IL8 expression plasmid (confirmed by DNA sequencing) and the empty control vector were transfected into A549/ATCC or C32 cells using Lipofectamine 2000 reagent (Invitrogen). Stably transfected cells were selected by growing them in hygromycin B (500 μg/ml) for two weeks. The colonies expressing the exogenous IL8 were confirmed by RT-PCR using a forward IL8 primer paired with a reverse vector-specific primer. The clones expressing the exogenous IL8 or transfected with an empty vector were pooled separately and used for establishment of tumor xenografts to test their response to PA-L1/LF.
Cell migration assay
A CytoSelect 24-well cell migration assay kit (Cat. CBA-100-C) purchased from Cell Biolabs (San Diego, CA) was used for the assay.HUVEC and HMVEC cells pretreated with or without PA-L1/LF (2.4 nM/2.2 nM) for 2 h, were trypsinized and re-suspended in EGM2 (without MV singleQuots) with or without the same concentration of PA-L1/LF at a density of 1×106 cells/ml. The cells were added into the cell culture inserts (300 ul/well), which were then placed into a 24-well plate containing EGM-2 only or EGM-2 plus MV singleQuots (the complete growth medium containing 5% FBS and angiogenic and growth factors VEGF, FGF2, EGF, and IGF), and incubated for 16 h. Cells which migrated to the other sides of the inserts were stained and measured following the manufacturer’s instructions.
In vivo angiogenesis assay
DIVAA was performed using a DIVAA Starter Kit (Trevigen, Gaithersburg, MD) following the kit manual. Anesthetized 8-week nude mice (NCI, Frederick) were subcutaneously implanted with Trevigen’s basement membrane extract and VEGF and FGF2-containing angioreactors under sterile surgical conditions (day 0). Then the mice were treated with 6 doses of PA-L1/LF or PBS at day 3, 5, 7, 10, 12, and 14. The mice were euthanized by CO2 inhalation at day 16, and the angioreactors were removed. The vascular endothelial cells which had grown into the reactors were quantitated according to the manufacturer’s instructions.
Wound healing experiment
Skin wound healing was performed essentially as described (23). Briefly, C57BL/6J mice (8-10 weeks) were randomly divided into two groups and anesthetized by inhalation of 2% isoflurane before surgical incision. Fifteen mm long full-thickness incisional wounds were made in the shaved middorsal skin. The wounds were neither dressed nor sutured. Starting immediately after wounding, one group was treated with PA-L1/LF (30/10 ug) and the second group was treated with PBS three times per week until all the wounds were healed. The rate of wound healing was determined by daily inspection and the wound was scored as healed when only a minimal residual skin defect was apparent. Surgery and evaluation of the macroscopic progress of wound healing was done by an investigator that was blinded as to the treatment of the mice.
RESULTS
MMP-activated anthrax lethal toxin is cytotoxic to human cancer cells with the BRAF V600E mutation
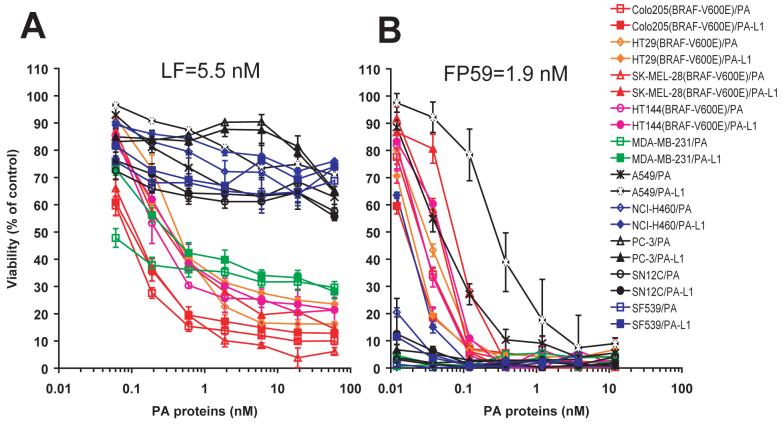
PA-L1 is a mutated PA protein with the furin cleavage site, RKKR, replaced by a MMP-susceptible cleavage sequence, GPLGMLSQ (17). To evaluate the in vitro anti-tumor activity of the MMP-activated LT (PA-L1/LF), cytotoxicity analyses were performed on four BRAF V600E-containing tumor cell lines from the NCI60 cell set (24), Colo205 (colon), HT29 (colon), SK-MEL-28 (melanoma), and HT144 (melanoma), in comparison to six BRAF wild type lines, MDA-MB-231 (breast), A594/ATCC (lung), NCI-H460 (lung), PC-3 (prostate), SN12C (renal), and SF539 (central nervous system). We found that PA-L1/LF was cytotoxic to both melanoma and colon cancer cells having the BRAF mutation at potencies comparable to those of wild-type LT (PA/LF) for these cells (Figure 1A). However, all the tumor cells lacking the BRAF V600E mutation (except MDA-MB-231) were resistant to both PA/LF and PA-L1/LF (Figure 1A). These results demonstrate that not only human melanoma cells but also human colon cancer cells with the BRAF mutation are sensitive to PA/LF and PA-L1/LF.
Fig. 1.
Cytotoxicity of the anthrax lethal toxins to human tumor cells.
(A) Ten different NCI60 cell lines were incubated with various concentrations of PA or PA-L1 in the presence of 5 nM LF for 72 h, and the cell viability was measured as described in Experimental Procedures. Note that all the cells having the BRAF mutation (indicated in red) were sensitive to the lethal toxins, whereas cells without the mutation (except MDA-MB-231 cells) were resistant to the toxins.
(B) The same set of cell lines were also treated with PA or PA-L1 in the presence of 1.9 nM FP59 as described in (A). All the cells were sensitive to the toxins, demonstrating that the cells express MMP activities.
To exclude the possibility that the general insensitivity of the tumor cells without the BRAF mutation to the anthrax lethal toxins is due to a lack of expression of PA receptors on these cells, the tumor cells were also treated with PA/FP59 and PA-L1/FP59. FP59 is a fusion protein of LF amino acids 1-254 and the catalytic domain of Pseudomonas exotoxin A, that kills cells by ADP-ribosylation and, thus, inactivation of EF-2 when it is delivered into the cytosol of the cell in a PA-dependent manner (17). PA/FP59 and PA-L1/FP59 showed a potent and comparable cytotoxicity to all the human cancer cells tested (Figure 1B) regardless of their BRAF status, demonstrating that these tumor cells express PA receptors and MMPs. These findings argue that MMP-activated LT may be a useful reagent for tumor targeting.
Attenuated in vivo toxicity of the MMP-activated anthrax lethal toxin
We next evaluated the toxicity of PA-L1/LF in vivo. Mice were challenged intraperitoneally (i.p.) with 6 doses (three times a week with two-day intervals for two weeks) of PA/LF or PA-L1/LF. A molar ratio of 3:1 of PA protein to LF was used in the challenge experiments based on the fact that each PA heptamer can bind and deliver up to three molecules of LF into cells (25). C57BL/6 mice could tolerate 6 doses of 10/3.3 μg of PA/LF, but could not tolerate doses beyond 15/5 μg of PA/LF. One of 10 mice died after 6 doses of 15/5 μg of PA/LF; and 11 of 11 died after 2 doses of 30/10 μg of PA/LF (Table 1). Several major organ damages associated with vascular collapse had been identified as major lesions in LT-treated mice (16). In contrast, the mice tolerated as many as 6 doses of 45/15 μg of PA-L1/LF. All the mice survived challenge with 6 doses of 30/10 μg and 45/15 μg of PA-L1/LF, respectively, and lacked any outward sign of toxicity (Table 1). Full necropsy analyses of the C57BL/6 mice treated with 6 doses of 45/15 μg of PA-L1/LF did not reveal any gross abnormalities. Further, extensive histological analyses did not uncover damage in major organs and tissues, including brain, lung, heart, liver, small and large intestines, kidney and adrenal gland, stomach, pancreas, spleen, thyroid, bladder, esophagus, skeletal muscle, thymus, and lymph nodes (data not shown). These results demonstrate that the MMP-activated LT has much lower in vivo toxicity than wild-type toxin; the MTD6 (the maximum tolerated 6 doses) for PA-L1/LF is ≥45/15 μg, whereas that of PA/LF is ≥ 10/3.3 and <15/5 μg.
Table 1.
In vivo toxicity of anthrax lethal toxins to mice
| Toxin | Dose | Percent survival for 6 doses | ||
|---|---|---|---|---|
| C57BL/6 | BALB/c | Nude mice | ||
| PA/LF | 10/3.3 μg | 100% (5/5) | - | |
| 15/5 μg | 90% (9/10) | - | 47% (14/30) | |
| 30/10 μg | 0% (0/11) | - | ||
| PA-L1/LF | 15/5 μg | - | - | 100% (10/10) |
| 30/10 μg | 100% (22/22) | - | 100% (42/42) | |
| 45/15 μg | 100% (11/11) | 100% (5/5) | 70% (28/40) | |
-: not done.
MMP-activated anthrax lethal toxin has potent anti-tumor activity in vivo
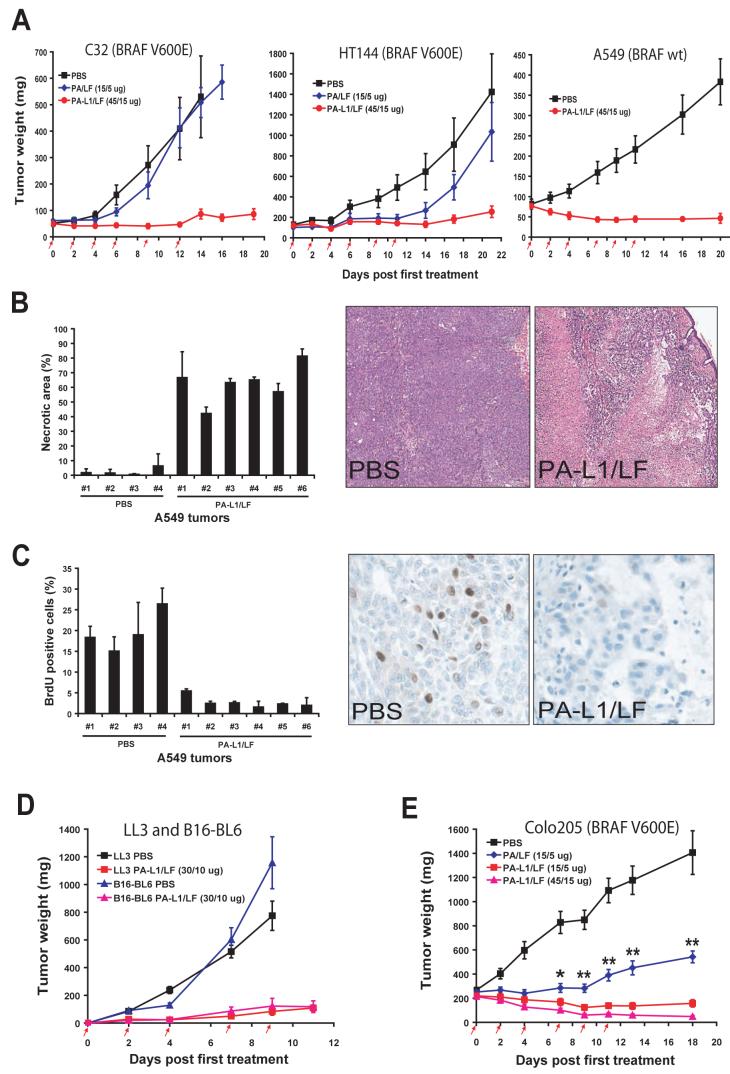
To determine whether the anti-tumor activity of PA-L1/LF in vitro can be recapitulated in vivo, we established human tumor xenografts in nude mice using human melanoma HT144 cells and C32 cells, containing the BRAF V600E mutation, and human non-small cell lung carcinoma A549/ATCC cells, which lack the BRAF mutation. After these tumors were well established, the mice were injected (i.p.) with 6 doses of 45/15 μg of PA-L1/LF (≤MTD6), 6 doses of 15/5 μg of PA/LF (≈MTD6), or PBS. Remarkably, the two human melanomas with the BRAF mutation were very sensitive to PA-L1/LF, with average tumor sizes just 16% and 17%, respectively, of the control tumors treated with PBS at the time when the control mice required euthanasia due to tumor ulceration in compliance with institutional guidelines (Figure 2A). In the case of C32 melanomas, 30% of the tumors achieved complete regression. In contrast, we observed little or no response of these tumors to wild-type LT (Figure 2A). Unexpectedly, PA-L1/LF also exhibited strong toxicity to A549/ATCC carcinomas that do not have the BRAF mutation, resulting in the eradication of 50% of the established tumors (Figure 2A). Histological analyses showed that PA-L1/LF treatment induced extensive tumor necrosis, which did not occur in the PBS-treated tumors (Figure 2B). Furthermore, a bromodeoxyuridine (BrdU) incorporation assay demonstrated that while proliferating cells were evident in the PBS-treated tumors, DNA synthesis in the toxin-treated tumors was greatly inhibited, even in areas with living cancer cells (Figure 2C). These results demonstrate that the MMP-activated LT has potent anti-tumor activity not only to human melanomas with the BRAF mutation, but also to another human tumor type that lacks the BRAF mutation.
Fig. 2.
PA-L1/LF displays broad and potent anti-tumor activity regardless of the BRAF mutation status of the tumor.
(A) Nude mice bearing human C32 melanoma (left), HT144 melanoma (middle), or A549/ATCC lung carcinoma (right) were injected (i.p.) with 6 doses of PBS, PA/LF, or PA-L1/LF as indicated by red arrows (n=10 for each group). Weights of tumors in this and the following experiments are expressed as mean tumor weight ± s.e.m.
(B) PA-L1/LF causes extensive necrosis of A549/ATCC tumors. A549/ATCC tumor-bearing nude mice were treated with 4 doses of 30/10 μg of PA-L1/LF or PBS (at days 0, 2, 4, and 7). Two hours after injection of BrdU, tumors were dissected and subjected to histological analysis. H&E staining shows extensive toxin-dependent necrosis of a representative tumor treated with PA-L1/LF, which is observed in all the toxin-treated A549/ATCC tumors.
(C) BrdU incorporation assay reveals remarkable DNA synthesis cessation in PA-L1/LF-treated but not PBS-treated A549/ATCC tumors. The tumor sections analyzed in (D-E) were stained with an antibody against BrdU 2 h after systemic administration of BrdU. Note, BrdU positive cells are easily detected in PBS-treated tumors, but hardly detected in viable areas of the toxin-treated tumors.
(D) C57BL mice bearing mouse B16-BL6 melanomas or LL3 Lewis lung carcinomas were treated (i.p.) with 5 doses of PBS or PA-L1/LF as indicated (n=10 for each group).
(E) PA-L1/LF displays much stronger anti-tumor activity than PA/LF. Nude mice bearing Colo205 colon carcinoma were treated (i.p.) with 6 doses of PBA, PA/LF, or PA-L1/LF as indicated (n=10 for each group). A significant difference (*, p<0.05; **, p<0.01) is shown between 15/5 μg of PA-L1/LF and 15/5 μg of PA/LF treated tumors.
We further tested the therapeutic efficacy of PA-L1/LF in two mouse syngeneic tumor models, B16-BL6 melanoma and LL3 Lewis lung carcinoma. These two tumors demonstrate a poor response to conventional treatments. C57BL/6 mice bearing B16-BL6 melanomas and LL3 Lewis lung carcinomas were treated (i.p.) with 5 doses of 30/10 μg of PA-L1/LF and PBS (Figure 2D). These tumors were also highly susceptible to the engineered toxin, with the average sizes of B16-BL6 and LL3 tumors treated with the toxin just 10% and 11%, respectively, of those treated with PBS. Because A549/ATCC carcinomas and B16-BL6 melanomas are resistant to PA-L1/LF in the in vitro cytotoxicity assay (Figure 1A and data not shown) but sensitive in vivo, the above data strongly suggest that the potent anti-tumor efficacy of the modified LT might be through targeting of the tumor vasculature.
As shown above, when used at the similar toxic doses (≈MTD6), PA-L1/LF displayed more potent anti-tumor effect than did PA/LF. Next, we directly compared their therapeutic efficacy at the same doses using human colon cancer Colo205 xenografts in nude mice. The Colo205 tumor-bearing mice were treated with 6 doses of 15/5 μg or 45/15 μg of PA-L1/LF, or 15/5 μg of PA/LF. Notably, PA-L1/LF retained remarkable efficacy even when the dose was reduced to 15/5 μg, whereas the same dose of PA/LF only showed a modest anti-tumor effect on Colo205 tumors, which was significantly lower than that of PA-L1/LF (p<0.01) (Figure 2E). Because 6 doses of 15/5 μg of PA/LF showed unacceptable toxicity to nude mice (Table 1), we did not further evaluate the wild-type LT in mice in the following studies with the aim to identify the anti-tumor mechanisms of the MMP-activated LT.
Higher bioavailability and decreased immunogenicity of the MMP-activated protective antigen
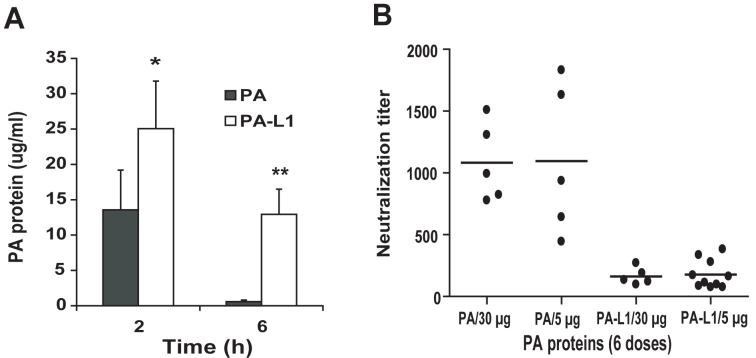
The above results showing that PA-L1/LF has higher in vivo anti-tumor activity than PA/LF (Figure 2E) were at first surprising, because PA/LF showed similar or higher in vitro toxicity than PA-L1/LF in all the cancer cells tested (Figure 1A). We previously reported that the proteolytic processing and the subsequent oligomerization of PA63 on cell surfaces are essential for the cellular uptake and eventual degradation of PA in the endocytic pathway (26). Given that fewer cell types express MMPs than furin, we assumed that PA-L1 might be cleared from plasma more slowly than PA. To test this hypothesis, 100 μg of PA or PA-L1 was intravenously injected into mice, and the plasma clearance of the PA proteins was measured (Figure 3A). We demonstrated that PA-L1 remained in circulation much longer than PA did; 6 h after the injection, when PA was hardly detected (0.57 ± 0.23 μg /ml), there was still a significant amount of PA-L1 in the plasma (12.9 ± 3.6 μg /ml), indicating that PA-L1 has a better bioavailability in vivo than PA, which may contribute to its higher in vivo anti-tumor activity.
Fig. 3.
Increased plasma half-life and decreased immunogenicity of the MMP-activated protective antigen.
(A) PA-L1 has a longer plasma half-life than PA. Mice were injected (i.v.) with 100 μg of PA or PA-L1, euthanized at 2 h or 6 h, blood samples were collected, and PA protein concentrations were measured using ELISA. There is a significant difference (*, p<0.05; **, p<0.01) between PA and PA-L1. (B) C57BL/6 mice were injected i.p. with 6 doses of 5 or 15 μg of wild-type PA or PA-L1, respectively within a period of two weeks. Ten days later, the mice were bled, and the titers of the serum neutralizing antibodies against PA measured in a cytotoxicity assay using mouse macrophage RAW264.7 cells challenged with LT (75 ng/ml each of PA and LF). The titers of the PA neutralizing antibodies were expressed as mean of fold dilution ± s.e.m of the sera that could protect 50% of RAW264.7 cells from LT treatment. Note that the neutralizing activities from the mice treated with wild-type PA were approximately 6-fold higher that those from PA-L1 treated mice: PA vs. PA-L1 (6×5 μg): 1097 ± 272 vs. 178 ± 36, p=0.0002; PA vs. PA-L1 (6×30 μg): 1081 ± 142 vs. 162 ± 31, p=0.0004.
PA has a well-known immunogenic activity and is a major component of the only licensed anthrax vaccine (Anthrax Vaccine Absorbed) currently used in USA. This raises a practical concern that repeat uses of PA proteins in therapy may induce neutralizing antibodies that may interfere with their later uses. The fact that PA-L1 can not be internalized and degraded in the endocytic pathway as efficiently as wild-type PA by most normal cell types due to the limit expression of MMPs suggested that antigen presenting cells (such as dendritic cells and macrophages) may not efficiently present PA-L1 peptides via MHC class II pathway to induce humoral immune response. To test this possibility, we administered (i.p.) 6 doses of PA or PA-L1 into C57BL/6 mice using the same schedule as in the tumor treatment studies. Ten days later the mice were bled, and the PA neutralizing antibody activities measured. Significantly, we found that the PA neutralizing antibody titers from wild-type PA treated mice were much higher (∼6 fold) than those treated with PA-L1 (Figure 3B). These results indicated that the MMP-activated toxin has much lower immunogenicity compared to the wild-type toxin, suggesting that the engineered toxin might be used for several cycles of treatment without compromising its therapeutic activity.
The potent anti-tumor activity of the MMP-activated anthrax lethal toxin is not solely dependent on its inhibitory effect on IL8
In tumor tissues, cancer cells usually induce tumor angiogenesis by communicating with tumor stromal cells (such as fibroblasts, macrophages, endothelial cells, etc.) by either direct interactions or through secretion of various growth factors and angiogenic factors (27-29). To determine whether LT can affect expression of angiogenic factors by cancer cells, we performed human angiogenic factor profiling analyses with four human cancer cells A549/ATCC, HT144, Colo205, and HT29 cells using the MultiGene-12 RT-PCR Profiling Kit (SuperArray Bioscience Corporation). The effects of LT treatment on expression of 11 well-characterized angiogenic factors were evaluated using these cancer cells (Figure 4A). We showed that interleukin-8 (IL8) was the only factor down-regulated by LT treatment in all four cell lines (Figure 4A). Further analysis revealed that the expression of vascular endothelial growth factor (VEGF) by these cancer cells was not affected by LT treatment (data not shown). These findings, together with the results from a previous study showing that LT can down-regulate IL8 expression in HUVEC (30), suggest that many cell types may share a common LT-susceptible pathway for regulating IL8.
Fig. 4.
The potent anti-tumor activity of PA-L1/LF is not solely dependent on its inhibitory effects on IL8.
(A) Angiogenic factor profiling RT-PCR analysis reveals that the expression of IL8 by tumor cells is down-regulated by anthrax lethal toxin. Colo205, A549/ATCC, HT144, and HT29 cells were treated with or without PA/LF (10/3.3 nM) for 8 h, then the total RNA was isolated, and subjected to the angiogenic factor RT-PCR profiling analyses following the recommendations of the manufacturer. Note that IL8 is consistently down-regulated by PA/LF in all four cancer cell lines. ANGP1, angiopoietin 1; CSF3, colony stimulating factor 3; ECGF1, endothelial cell growth factor 1; FGF1 and FGF2, fibroblast growth factor 1 and 2; FST, follistatin; HGF, hepatocyte growth factor; LEP, leptin; PDGFB, platelet derived growth factor B; PGF, placental growth factor.
(B-C) Both A549/ATCC carcinomas (B) and C32 melanomas (C) transfected with lethal LT ‘resistant’ IL8 retain susceptibility to PA-L1/LF. Nude mice bearing tumors transfected with IL8 or the empty vector were treated with 6 doses of 30/10 μg of PA-L1/LF or PBS. PA- L1/LF shows potent anti-tumor activity against the tumors transfected with either IL8 or the empty vector.
It is well established that IL8 plays an important role in tumor angiogenesis, and that IL8 has been demonstrated as an effective target in tumor therapy in animal models (27;28). We therefore asked whether the inhibitory effect of LT on IL8 could account for the potent anti-tumor activity of PA-L1/LF. To do so, we cloned a human IL8 cDNA fragment lacking the 3’untranslated region which contains an AU-rich element through which LT regulates IL8 mRNA stability (30). This LT ‘resistant’ IL8 coding sequence was subcloned into a mammalian expression vector, pIRESHgy2b, under the control of the CMV promoter, and transfected into A549/ATCC and C32 cells. Stable cell clones expressing the exogenous IL8 were isolated and expression of the exogenous IL8 was confirmed to be unaffected by PA/LF treatment (data not shown). These IL8-transfected cells and the empty vector-transfected cells were pooled separately, and used to establish tumor xenografts in nude mice. The tumor-bearing mice were treated with 6 doses of PBS or 30/10 μg of PA-L1/LF. The results showed that the strong anti-tumor efficacy of PA-L1/LF was not compromised in either A549/ATCC or C32 tumors with “resistant” IL8 (Figure 4B) and 4C. These results demonstrate that the potent anti-tumor activity of PA-L1/LF is not solely dependent on its inhibitory effect on IL8. In both cases, we observed that the tumors over-expressing IL8 grew slower than the tumors transfected with the empty vector (Figure 4B and 4C). The reason for this phenomenon is unclear; one possibility is that the over-expressed IL8 may trigger acute innate immune responses due to its chemotactic activities for neutrophils and macrophages, providing an unfavorable microenvironment for tumor growth.
MMP-activated anthrax lethal toxin impairs the function of primary human endothelial cells
We next attempted to determine the underlying mechanism of the potent anti-tumor activity of systemically administered PA-L1/LF. To investigate whether the functions of endothelial cells could be directly impacted by PA-L1/LF, two human primary endothelial cells, HMVEC and HUVEC, were used. As expected, these cells could efficiently bind and proteolytically process PA or PA-L1 to the active PA63 form (Figure 5A), demonstrating that these two highly proliferating endothelial cells cultured in growth factor- and angiogenic factor- enriched medium (mimicking tumor microenvironments) express furin as well as MMP activities. Further, these primary endothelial cells could bind and translocate LF into the cytosol of the cells, resulting in MEK1, MEK3, and MEK4 cleavage in a PA protein-dependent manner (Figure 5A). Consistent with the evidence that these cells express MMP activities in test culture conditions, these endothelial cells were highly sensitive to PA-L1/FP59 (Figure 5B). Moreover, the growth of these cells was modestly inhibited by PA-L1/LF, with 50% inhibition observed after 72 h incubation with toxin (Figure 5C). Of note, migration of both these endothelial cells toward a gradient of serum and angiogenic factors (such as FGFb and VEGF) was significantly perturbed (Figure 5D). These results strongly suggest that PA-L1/LF can inhibit endothelial cell proliferation and migration, which both play critical roles in tumor angiogenesis.
Fig. 5.
PA-L1/LF impairs the function of primary human endothelial cells.
(A) PA protein-dependent translocation of LF into the cytosol of HMVEC and HUVEC cells. HUVEC and HMVEC cells were incubated with either PA-L1/LF (6 nM/6 nM) or PA/LF (6 nM/6 nM) for 2 or 4 h. The binding and proteolytic processing of PA proteins, the binding and translocation of LF, and the MEKs cleavages were detected by Western blotting using the corresponding antibodies. The non-specific bands, indicated by the arrow heads left of images, served as protein loading controls in these experiments.
(B-C) Cytotoxicity of PA-L1/FP59 (B) and PA-L1/LF (C) to human primary vascular endothelial cells. HUVEC and HMVEC were treated with the indicated toxins as described in Fig. 1. The expression of MMPs by the endothelial cells was evidenced by their high sensitivity to PA-L1/FP59.
(D) PA-L1/LF can efficiently inhibit the migration of vascular endothelial cells toward angiogenic factors-containing endothelial cell growth medium (GM). The experiments were performed as described in the Materials and Methods section. SFM, serum and angiogenic factors free medium.
MMP-activated anthrax lethal toxin demonstrates potent anti-tumor vasculature activity
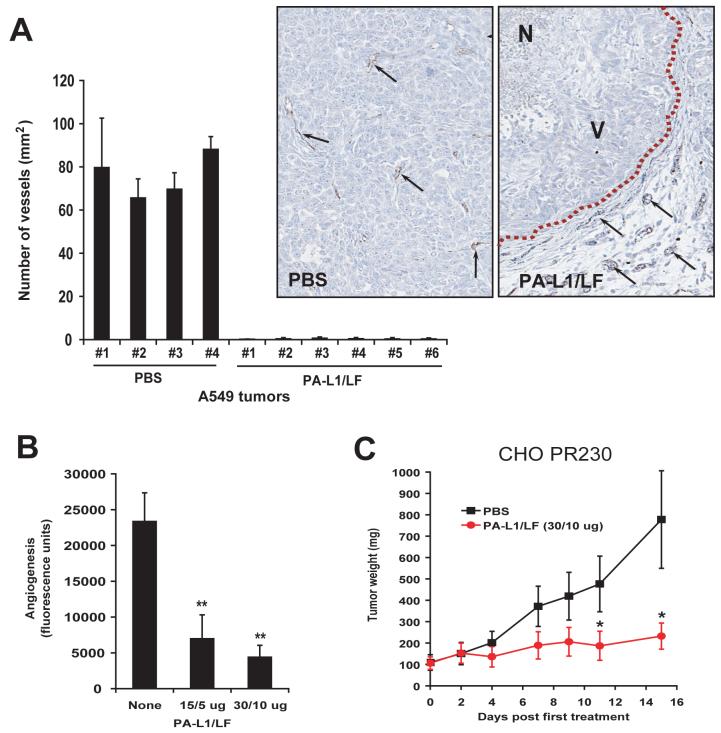
To directly investigate the effects of PA-L1/LF on tumor vasculature, we stained A549/ATCC tumors isolated from mice treated with either PBS or PA-L1/LF using an antibody against the endothelial cell surface marker CD31. Notably, microvascular structures were easily detected in the PBS-treated tumors, but hardly detected in the toxin-treated tumors, even within the viable tumor areas (Figure 6A). Importantly, the endothelial cells in the normal surrounding tissues of the toxin-treated tumors remained intact (Figure 6A, inserts), suggesting that the anti-vasculature activity of PA-L1/LF is tumor-specific. This is likely due to the fact that the endothelial cells in normal tissues are relatively quiescent, and lack expression of MMPs, whereas those in tumor tissues enriched with angiogenic factors and growth factors are highly proliferative, express MMPs, and are MEK-dependent.
Fig. 6.
PA-L1/LF demonstrates potent anti-tumor vasculature and angiogenesis activities.
(A) Sections of A549/ATCC tumors treated with PBS or PA-L1/LF, as described inFig. 2Bwere stained with an antibody against the endothelial cell marker CD31. CD31-positive structures were quantified using the Northern Eclipse Image Analysis Software (Empix Imaging, North Tonawanda, NY). In inserts, black arrows point to the examples of CD31 positive endothelial cells; dash line, the boundary between the tumor and its surrounding normal tissues. N, necrotic area; V, area with viable cancer cells.
(B) Directed in vivo angiogenesis analysis demonstrates that PA-L1/LF can inhibit tumor cell independent in vivo angiogenesis. There is a significant difference (**, p<0.01) between the angioreactors treated with PBS (n=8) and treated with PA-L1/LF (15/5 μg, n=8; 30/10 μg, n=10).
(C) Anthrax toxin receptors-deficient CHO tumors are susceptible to PA-L1/LF. CHO PR230 tumor-bearing nude mice were injected (i.p.) with 6 doses of 30/10 μg of PA-L1/LF as indicated (n=6 for each group). There is a significant difference (*, p<0.05) between the tumors treated with PA and PA-L1.
To further evaluate the effect of PA-L1/LF on angiogenesis in vivo, we performed the directed in vivo angiogenesis assay (DIVAA) (31) by subcutaneously implanting nude mice with “angioreactors” containing basement membrane extracts, VEGF, and FGF2. Then the mice were treated (i.p.) with 6 doses of PBS or PA-L1/LF. Significantly, both the 15/5 μg and 30/10 μg doses of PA-L1/LF efficiently decreased in vivo angiogenesis (Figure 6B). These results, together with those described above, suggested that the potent anti-tumor activity of the MMP-activated LT is due largely to the indirect targeting of tumor vasculature and angiogenic processes.
To directly test this hypothesis, we next used tumor cells that were rendered deficient in anthrax toxin receptors (26).Thus, the anthrax toxin receptor-deficient Chinese hamster ovary (CHO) cell line, PR230, which cannot bind PA proteins (26) was xenografted to mice, and the mice were treated with PA-L1/LF or PBS. Consistent with our hypothesis, the anthrax toxin receptor-ablated CHO cell tumors remained highly sensitive to PA-L1/LF treatment (Figure 6C).
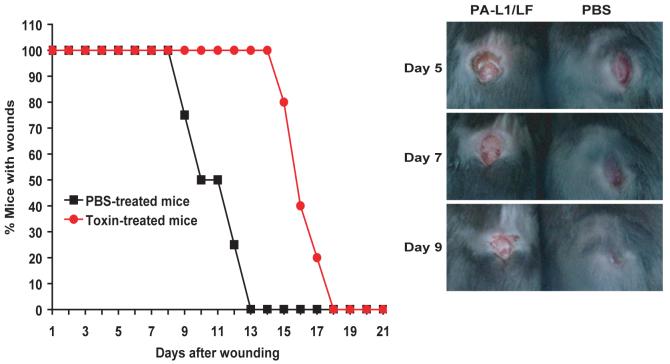
MMP-activated anthrax lethal toxin delays, but does not abrogate, skin wound healing
Many post-developmental tissue repair and tissue remodeling processes are dependent on angiogenesis. Furthermore, tumor angiogenesis is believed to recapitulate important aspects of physiological angiogenesis (32). Skin wound healing is one such physiological tissue remodeling process that is associated with extensive neo-angiogenesis (33). To test the effects of PA-L1/LF on physiological angiogenesis, full-thickness incisional skin wounds were made in C57BL/6 mice. The mice were then treated (three times per week) with either PA-L1/LF (30/10 μg) or PBS, and the wound healing time was determined (Figure 7). No overt qualitative macroscopic differences were observed in healing wounds from toxin-treated and mock-treated mice (Figure 7). However, toxin-treated mice displayed a fifty percent delay in the average healing time, showing that systemic PA-L1/LF treatment moderately impairs, but does not abrogate, a physiological tissue repair process (Figure 7).
Fig. 7.
PA-L1/LF delays, but does not prevent, incisional skin wound healing.
C57BL/6 mice with the incisional skin wounds were treated with either PA-L1/LF (30/10 μg) (n=7) or PBS (n=8) three times per week until all the wounds were healed. The average wound healing time was delayed for the toxin-treated mice compared to the mock-treated group (14.5 days vs. 10 days, p<0.001, Mann-Whitney U-test, two-tailed). Inserts, representative examples of the appearance of skin wounds from mice treated with PA-L1/LF (left) or PBS (right) at days 5-9.
DISCUSSION
The Sanger Institute’s Cancer Genome Project and subsequent studies conducted by other investigators identified the BRAF V600E mutation as occurring in approximately 70% of human melanomas and less frequently in other cancer types, such as colon, ovarian, and papillary thyroid cancer, representing about 8% of total human cancers (12;34). BRAF is immediately downstream of RAS in the kinase cascade and there is a trend showing that the BRAF mutation is present in cancer types with activating RAS mutations (12;34). However, the RAS and the BRAF mutations typically demonstrate mutual exclusivity, suggesting that either mutation is sufficient to deregulate the common downstream MEK-ERK kinase cascade, upon which the tumors with these mutations are dependent for survival.
Recently, based on their NCI60 anticancer drug screen, Rosen and colleagues demonstrated that tumor cells with the BRAF, but not the RAS mutation, are sensitive to MEK inhibition (35). It is not surprising that tumors with activating RAS mutations are less sensitive to MEK inhibition, because RAS can also activate the PI3K pathway to support tumor survival (36). Therefore, molecular targeting of the BRAF-MEK-ERK pathway would be selective to tumors with the BRAF mutation. We reported recently that LT, which can inactivate MEK1/2 and other MEKs by enzymatic cleavage, is selectively toxic to human melanoma cell lines having the BRAF mutation, but not to those with the RAS mutations (14). This LT selective toxicity to human melanomas with BRAF V600E was verified in an experimental therapy of SK-MEL-28 melanoma xenografts in athymic mice (15). However, the fact that anthrax LT is an important virulence factor in anthrax pathogenesis and has recognized toxicity to mice (16) means that wild-type LT might not be accepted for use in human patients.
To achieve the goal of decreasing in vivo toxicity of LT while retaining its anti-tumor activity, we developed an attenuated version of the toxin (PA-L1/LF), which cannot be cleaved by the ubiquitously expressed protease furin, but is instead activated by MMPs, including MMP-2, MMP-9, and MT1-MMP (membrane type 1 MMP). MMPs are involved in tumor survival, angiogenesis, invasive growth, and metastasis (17;37). We showed that all the cancer cells tested express MMPs and thus, are highly sensitive to PA-L1/FP59. Furthermore, the cancer cells with the BRAF mutation are susceptible to both PA/LF and PA-L1/LF to comparable degrees, whereas the cancer cells without BRAF V600E are generally resistant to the toxins. Moreover, in addition to melanoma cells, colon cancer cells with the BRAF mutation are also sensitive to the toxins, indicating that the addiction to the activating BRAF mutation is not cell lineage-specific. As expected, PA-L1/LF has much lower toxicity than wild-type toxin in the mice; C57BL/6 mice easily tolerate 6 doses of 45/15 μg of PA-L1/LF given systemically, while they can only tolerate doses close to 15/5 μg of PA/LF, and cannot tolerate even 2 doses of 30/10 μg of PA/LF (Table 1). These results indicate that most of the normal tissues lack expression of MMPs and that PA-L1/LF is much safer than PA/LF when used in vivo.
The first surprising finding in this work came from the in vivo anti-tumor efficacy study. We found that PA-L1/LF has a potent anti-tumor activity not only against human melanomas with BRAF V600E, but also against other human tumor types, including colon and lung carcinomas, and mouse tumors, regardless of their BRAF status. We further observed that this potent anti-tumor activity is due largely to targeting of tumor vasculature and angiogenic processes. We showed that: (a) PA-L1/LF strongly inhibits the migration of human primary endothelial cells towards a gradient of serum and angiogenic factors, an essential step for tumor angiogenesis; (b), PA-L1/LF can efficiently block angiogenesis in vivo; (c) tumor blood vessels are largely absent in A549/ATCC tumors treated with PA-L1/LF in comparison with those treated with PBS; and (d) anthrax toxin-receptor-deficient CHO tumor xenografts are susceptible to PA-L1/LF.
Recently, Sparmann and Bar-Sagi showed that activation of RAS in human cancer cells results in up-regulation of IL8, leading to recruitment of mouse neutrophils and macrophages, which in turn produce growth factors and angiogenic factors to promote tumor angiogenesis and growth (27). They further showed that inhibition of IL8 by a neutralizing antibody or ablation of macrophages can significantly inhibit the growth of tumor xenografts (27), attesting to the importance of IL8 and macrophages in tumorigenesis. To determine whether the anti-tumor efficacy of PA-L1/LF was solely due to its ability to down-regulate expression of IL8, we transfected IL8 lacking 3’ UTR into A549/ATCC and C32 cells; we found these tumor xenografts with ’resistant’ IL8 are still very susceptible to PA-L1/LF.
It has been noted for two decades that the macrophages from certain inbred mice and rats are uniquely sensitive to LT in that these macrophages can be lysed by the toxin in just 90 minutes (38). Recently, the genetic trait of the sensitivity has been assigned to the Nalp1b locus, encoding a polymorphic protein existing in the inflammasome complex (39). How Nalp1b is linked to macrophage sensitivity to LT is still unclear. We ruled out the possibility that the potent anti-tumor efficacy of PA-L1/LF is due to the unique toxicity of the toxin to tumor associated macrophages because macrophages isolated from the bone marrow of mice used for tumor xenografts are ‘resistant’ to LT. While macrophages derived from BALB/c mice are lysed by PA/LF (LT) within 4 h, macrophages from C57BL6 and nude mice cannot be killed even after 24 h (Figure S1).
Another interesting finding in this study is that PA-L1/LF not only displays much lower in vivo toxicity but also shows higher anti-tumor efficacy than does the wild-type toxin. This is due in part to the unexpected greater bioavailability and longer half-life of PA-L1 in circulation as compared to PA. We previously showed that following binding to its cellular receptors, PA must be proteolytically cleaved on cell surfaces for formation and internalization of the PA heptamer into the endocytic pathway (26). Thus, the rates of processing on cell surfaces are believed to largely determine the clearance of PA proteins from circulation (40). The fact that furin protease is widely expressed whereas MMPs are restricted to a small number of normal cells explains why PA-L1 has a longer plasma half-life.
Rosen and colleagues demonstrated that PD0325901, a potent small molecule MEK inhibitor, is efficient in inhibition of the growth of human tumor xenografts containing the BRAF V600E, but has limited efficacy against tumors without the BRAF mutation (35), indicating that the action of the compound is through direct targeting of the cancer cells. In addition to targeting the MEK-ERK pathway, LT also has activity against the other major MAPK pathways via enzymatic cleavage of MEK3 and 6 (p38 pathway) and MEK4 and 7 (JNK pathway) (10), providing an explanation for our observations that PA-L1/LF has broader anti-tumor activity than PD0325901. In addition to the tumors with the BRAF mutation, we demonstrate that the tumors without the mutation, including those from human as well as mouse origins and even those derived from the toxin-receptor-deficient CHO cells, are all susceptible to PA-L1/LF. As presented in this study, LF has an additional advantage over small molecule inhibitors in that it can be specifically delivered to cancer cells using tumor-selective PA proteins (20;21;37). Because of its catalytic nature, LF might be more potent than small molecule MEK inhibitors in targeting the MEK-ERK pathway.
In summary, PA-L1/LF has unanticipated anti-tumor activity exceeding the wild-type toxin with respect to both safety and efficacy, due to its direct inactivation of the MEKs, indirect targeting tumor vasculature and angiogenesis, lower non-specific targeting of normal tissues that lack MMPs, and extended plasma half-life compared to wild-type toxin. The modified protective antigen also shows a decreased immunogenicity. We therefore propose clinical trials of the MMP-activated anthrax lethal toxin in cancer patients. In theory, many tumor types are expected to respond to PA-L1/LF therapy, but patients with tumors containing the BRAF mutation may derive additional benefits due to the direct toxicity of the toxin to the cancer cells.
Supplementary Material
Acknowledgments
This research was supported by the intramural research programs of the National Institute of Allergy and Infectious Diseases and the National Institute of Dental and Craniofacial Research, National Institutes of Health. We thank Rasem Fattah for protein purification, and Jason Wiggins, Dr. Kuang-HuaChen, and Dr. Karin List for technical assistance.
REFERENCES
- 1.Collier RJ. Toxicon. 2001;39:1793–1803. doi: 10.1016/s0041-0101(01)00165-9. [DOI] [PubMed] [Google Scholar]
- 2.Liu S, Leppla SH. Mol. Cell. 2003;12:603–613. doi: 10.1016/j.molcel.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Liu S, Milne GT, Kuremsky JG, Fink GR, Leppla SH. Mol. Cell Biol. 2004;24:9487–9497. doi: 10.1128/MCB.24.21.9487-9497.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olsen E, Duvic M, Frankel A, Kim Y, Martin A, Vonderheid E, Jegasothy B, Wood G, Gordon M, Heald P, Oseroff A, Pinter-Brown L, Bowen G, Kuzel T, Fivenson D, Foss F, Glode M, Molina A, Knobler E, Stewart S, Cooper K, Stevens S, Craig F, Reuben J, Bacha P, Nichols J. J Clin. Oncol. 2001;19:376–388. doi: 10.1200/JCO.2001.19.2.376. [DOI] [PubMed] [Google Scholar]
- 5.Liu S, Schubert RL, Bugge TH, Leppla SH. Expert Opin. Biol. Ther. 2003;3:843–853. doi: 10.1517/14712598.3.5.843. [DOI] [PubMed] [Google Scholar]
- 6.Leppla SH. Bacillus anthracis toxins. In: Alouf JE, Popoff MR, editors. The Comprehensive Sourcebook of Bacterial Protein Toxins. Academic Press; Burlington, MA: 2006. [Google Scholar]
- 7.Duesbery NS, Webb CP, Leppla SH, Gordon VM, Klimpel KR, Copeland TD, Ahn NG, Oskarsson MK, Fukasawa K, Paull KD, Vande Woude GF. Science. 1998;280:734–737. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- 8.Vitale G, Pellizzari R, Recchi C, Napolitani G, Mock M, Montecucco C. Biochem. Biophys. Res. Commun. 1998;248:706–711. doi: 10.1006/bbrc.1998.9040. [DOI] [PubMed] [Google Scholar]
- 9.Vitale G, Bernardi L, Napolitani G, Mock M, Montecucco C. Biochem. J. 2000;352(Pt 3):739–745. [PMC free article] [PubMed] [Google Scholar]
- 10.Baldari CT, Tonello F, Paccani SR, Montecucco C. Trends Immunol. 2006;27:434–440. doi: 10.1016/j.it.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Koo HM, VanBrocklin M, McWilliams MJ, Leppla SH, Duesbery NS, Woude GF. Proc. Natl. Acad. Sci. U. S. A. 2002;99:3052–3057. doi: 10.1073/pnas.052707699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 13.Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, Jones CM, Marshall CJ, Springer CJ, Barford D, Marais R. Cell. 2004;116:855–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 14.Abi-Habib RJ, Urieto JO, Liu S, Leppla SH, Duesbery NS, Frankel AE. Mol. Cancer Ther. 2005;4:1303–1310. doi: 10.1158/1535-7163.MCT-05-0145. [DOI] [PubMed] [Google Scholar]
- 15.Abi-Habib RJ, Singh R, Leppla SH, Greene JJ, Ding Y, Berghuis B, Duesbery NS, Frankel AE. Clin. Cancer Res. 2006;12:7437–7443. doi: 10.1158/1078-0432.CCR-06-2019. [DOI] [PubMed] [Google Scholar]
- 16.Moayeri M, Haines D, Young HA, Leppla SH. J. Clin.Invest. 2003;112:670–682. doi: 10.1172/JCI17991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu S, Netzel-Arnett S, Birkedal-Hansen H, Leppla SH. Cancer Res. 2000;60:6061–6067. [PubMed] [Google Scholar]
- 18.Egeblad M, Werb Z. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Leung HJ, Leppla SH. Cell. Microb. 2007;9:977–987. doi: 10.1111/j.1462-5822.2006.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S, Bugge TH, Leppla SH. J. Biol. Chem. 2001;276:17976–17984. doi: 10.1074/jbc.M011085200. [DOI] [PubMed] [Google Scholar]
- 21.Liu S, Aaronson H, Mitola DJ, Leppla SH, Bugge TH. Proc. Natl. Acad. Sci. U. S. A. 2003;100:657–662. doi: 10.1073/pnas.0236849100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swanson MS, Isberg RR. Infect. Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bugge TH, Kombrinck KW, Flick MJ, Daugherty CC, Danton MJ, Degen JL. Cell. 1996;87:709–719. doi: 10.1016/s0092-8674(00)81390-2. [DOI] [PubMed] [Google Scholar]
- 24.Shoemaker RH. Nat Rev. Cancer. 2006;6:813–823. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 25.Mogridge J, Cunningham K, Lacy DB, Mourez M, Collier RJ. Proc. Natl. Acad. Sci. U. S. A. 2002;99:7045–7048. doi: 10.1073/pnas.052160199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu S, Leppla SH. J. Biol. Chem. 2003;278:5227–5234. doi: 10.1074/jbc.M210321200. [DOI] [PubMed] [Google Scholar]
- 27.Sparmann A, Bar-Sagi D. Cancer Cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 28.Mizukami Y, Jo WS, Duerr EM, Gala M, Li J, Zhang X, Zimmer MA, Iliopoulos O, Zukerberg LR, Kohgo Y, Lynch MP, Rueda BR, Chung DC. Nat. Med. 2005;11:992–997. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- 29.Zeng Q, Li S, Chepeha DB, Giordano TJ, Li J, Zhang H, Polverini PJ, Nor J, Kitajewski J, Wang CY. Cancer Cell. 2005;8:13–23. doi: 10.1016/j.ccr.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Batty S, Chow EM, Kassam A, Der SD, Mogridge J. Cell Microbiol. 2006;8:130–138. doi: 10.1111/j.1462-5822.2005.00606.x. [DOI] [PubMed] [Google Scholar]
- 31.Guedez L, Rivera AM, Salloum R, Miller ML, Diegmueller JJ, Bungay PM, Stetler-Stevenson WG. Am. J Pathol. 2003;162:1431–1439. doi: 10.1016/S0002-9440(10)64276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dvorak HF. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 33.Singer AJ, Clark RA. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 34.Sebolt-Leopold JS, Herrera R. Nat Rev Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 35.Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, Ye Q, Lobo JM, She Y, Osman I, Golub TR, Sebolt-Leopold J, Sellers WR, Rosen N. Nature. 2006;439:358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Brocker EB, LeBoit PE, Pinkel D, Bastian BC. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 37.Liu S, Redeye V, Kuremsky JG, Kuhnen M, Molinolo A, Bugge TH, Leppla SH. Nat. Biotechnol. 2005;23:725–730. doi: 10.1038/nbt1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedlander AM. J. Biol. Chem. 1986;261:7123–7126. [PubMed] [Google Scholar]
- 39.Boyden ED, Dietrich WF. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 40.Moayeri M, Wiggins JF, Leppla SH. Infect. Immun. 2007;75:5175–5184. doi: 10.1128/IAI.00719-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.