Abstract
Toll-like receptor 2 (TLR2) is a pattern recognition receptor that plays an important role in enabling cells of the innate immune system to recognize conserved structural motifs on a wide array of pathogens including gram-positive bacteria. Although microglia have recently been shown to express TLR2, the functional significance of this receptor in mediating microglial activation remains unknown. To ascertain the importance of TLR2 in microglial responses to S. aureus and its cell wall product peptidoglycan (PGN), we evaluated primary microglia from TLR2 knockout (KO) and wild-type (WT) mice. TLR2 was found to play a pivotal role in PGN recognition and subsequent activation in primary microglia, as demonstrated by the attenuated expression of TNF-α, IL-12 p40, MIP-2, and MCP-1 in PGN-treated TLR2 KO microglia compared with WT cells. In contrast, the responses of TLR2 KO and WT microglia to S. aureus were qualitatively similar, indicating that alternative receptors are responsible for recognizing intact bacteria. Microarray analysis confirmed that TLR2 plays a central role in PGN recognition by primary microglia. The expression of MyD88, a central adapter molecule in TLR-dependent signaling, was similar in both TLR2 KO and WT microglia, suggesting that the defect in PGN recognition by the former is not due to alterations in this key signaling intermediate. These findings reveal the complex nature of gram-positive bacterial recognition by microglia, which occurs, in part, through engagement of TLR2.
Keywords: Toll-like receptor 2, microglia, S. aureus, PGN
INTRODUCTION
Microglia are the resident mononuclear phagocytes of the CNS parenchyma and participate in innate immune responses (Aloisi, 2001; Hanisch, 2002). As such, microglia are uniquely poised to provide an initial line of defense against invading microorganisms into the CNS prior to leukocyte infiltration. Gram-positive bacteria are frequent etiologic agents of CNS infectious diseases and are a common pathogen associated with brain abscess (Mathisen and Johnson, 1997; Townsend and Scheld, 1998). Our group has recently reported that exposure of microglia to S. aureus, one of the main etiologic agents of brain abscess in humans, leads to the elaboration of a wide array of proinflammatory mediators and enhanced expression of surface receptors that play a pivotal role in bacterial recognition and antigen presentation (Kielian et al., 2001, 2002, 2004b). Included among the receptors modulated by S. aureus in microglia is the pattern recognition receptor (PRR) Toll-like receptor 2 (TLR2) (Kielian et al., 2002, 2004b).
Toll-like receptors are one group of PRRs expressed by cells of the innate immune system that mediate cellular activation in response to pathogen-associated molecular patterns (PAMP). These PAMPs represent invariant molecular motifs of bacteria, fungi, and viruses that are essential for pathogen survival and are conserved across broad subclasses of pathogens (Medzhitov and Janeway, 2000). In the human and mouse, 11 TLRs have been identified to date, each conferring responsiveness to various infectious agents as well as some endogenous ligands (Kopp and Medzhitov, 2003; Takeda et al., 2003). Of these, TLR2 is capable of recognizing the widest array of PAMPs, including peptidoglycan (PGN), bacterial lipoproteins, lipopolysaccharide (LPS) from Prophyromonas gingivitis and Leptospira interrogans (which differ in structure from the LPS of gram-negative bacteria), glycosylphos-photidylinositol lipid from Trypanosoma cruzi, and yeast zymosan (Qureshi and Medzhitov, 2003; Takeda et al., 2003). With regard to brain abscess, the gram-positive bacterium S. aureus harbors a complex cell wall consisting of multiple layers of two insoluble carbohydrate moieties, PGN and teichoic acid (Morath et al., 2002; Dziarski, 2003; Weber et al., 2003). Because of the immunostimulatory effects of S. aureus and PGN on microglia (Kielian et al., 2001, 2002, 2004b), TLR2 represents a logical candidate receptor for the recognition of these stimuli and subsequent microglial effector functions. Several studies have recently demonstrated TLR2 expression on microglia (Bsibsi et al., 2002; Kielian et al., 2002; Laflamme et al., 2001, 2003; Zekki et al., 2002); however, the functional significance of this receptor in the context of microglial recognition of gram-positive pathogens remains to be directly demonstrated. In the present study, we evaluated the importance of TLR2 in mediating microglial activation in response to S. aureus and PGN using primary microglia isolated from TLR2 knockout (KO) and wild-type (WT) mice. The results presented demonstrate that TLR2 is essential for PGN recognition in primary microglia, whereas alternative receptors are involved in signaling proinflammatory mediator production in response to intact S. aureus.
MATERIALS AND METHODS
Primary Microglia Cell Culture and Reagents
TLR2 knockout mice backcrossed for four generations (C57BL/6 background) were generously provided by Dr. Shizuo Akira (Osaka University, Japan) and distributed in the United States by Dr. Douglas Golenbock (University of Massachusetts, Worcester, MA). Primary microglia were isolated on the same day from age-matched TLR2 KO or WT C57BL/6 pups (Harlan Labs, Indianapolis, IN) as previously described (Kielian et al., 2004b). The purity of microglial cultures was evaluated by immunohistochemical staining, using antibodies against CD11b (BD Pharmingen) and glial fibrillary acidic protein (GFAP; DAKO, Carpenteria, CA) to identify microglia and astrocytes, respectively. The purity of primary microglia cultures was routinely greater than 90%.
Heat-inactivated S. aureus (strain RN6390, kindly provided by Dr. Ambrose Cheung, Dartmouth Medical School) was prepared as previously described (Kielian et al., 2002). PGN derived from S. aureus was obtained from Fluka (Sigma, St. Louis, MO) and Escherichia coli O11:B1 LPS was from List Biological Laboratories (Campbell, CA). All reagents and culture media were verified to have endotoxin levels of <0.03 EU/ml as determined by Limulus amebocyte lysate assay (LAL; Associates of Cape Cod, Falmouth, MA).
ELISA
Comparisons in cytokine and chemokine expression between TLR2 WT and KO primary microglia were performed using standard sandwich enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions (OptEIA mouse tumor necrosis factor-α [TNF-α], interleukin-12 [IL-12] p40, and MCP-1/CCL2, BD Pharmingen; DuoSet mouse MIP-2/CXCL2, R&D Systems, Minneapolis, MN). The level of sensitivity for all ELISAs was 15.6 pg/ml.
Cell Viability Assays
To confirm that the observed changes in proinflammatory mediator expression between TLR2 WT and KO microglia were not due to differences in seeding density or potential confounding proliferative effects, a standard MTT assay based upon the mitochondrial conversionof(3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT) into formazan crystals was performed as previously described (Kielian et al., 2004b).
Quantitative Real-Time RT-PCR
Total RNA from TLR2 WT and KO primary microglia was isolated using the TriZol reagent and treated with DNase 1 (both from Invitrogen, Carlsbad, CA) before use in real-time reverse transcription-polymerase chain reaction (RT-PCR) studies. The experimental procedure was performed as previously described with minor modifications (Kielian et al., 2004b). Briefly, TLR2, CD14, and GAPDH primers and TAMRA Taq-Man probes were designed as previously described (Esen et al., 2004) and synthesized by Applied Biosystems (ABI, Foster City, CA). ABI Assays-on-Demand™ Taqman kits were used to examine microglial mannose receptor type I (MRCI) and lectin-like oxidized LDL receptor 1 (LOX-1) expression. The RT reaction was conducted using the iScript cDNA™ synthesis kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. The real-time PCR reactions were performed in a total reaction volume of 25 μl using the iCycler™ kit (Bio-Rad) and analyzed using the iCycler IQ™ multicolor real-time PCR detection system (Bio-Rad). The levels of receptor expression in TLR2 WT and KO primary microglia were calculated after normalizing cycle thresholds against the “housekeeping” gene GADPH and are presented as the fold-induction (2−ΔΔCt) value relative to unstimulated microglia (mean ± SD).
Comparisons in Inflammatory Molecule Expression in TLR2 KO and WT Primary Microglia by Microarray Analysis
Differences in gene expression between S. aureus-and PGN-stimulated TLR2 KO and WT primary microglia were examined by microarray analysis as previously described (Kielian et al., 2004a,b). Briefly, samples were evaluated for changes in inflammatory gene expression using a custom-designed oligonucleotide microarray synthesized by MWG Biotech (Highpoint, NC). This microarray consisted of 280 genes, including apoptotic and neurotrophic factors, pattern recognition receptors, cytokines, chemokines, and their receptors. Microarray printing onto glass slides, probe labeling, hybridization, and data acquisition were performed by the UAMS Microarray Core Facility.
The production of labeled target riboprobes from 5 μg DNA-free total mRNA isolated from primary microglia were generated with the 3DNA Array 350 kit from Genisphere (Hatfield, PA). Hybridization was performed on the Discovery Hybridization Station (Ventana Medical Systems, Tucson, AZ). Slides were removed from the hybridization station, dried, and scanned using a Perkin-Elmer ScanArray 5000 (Perkin Elmer Life and Analytical Sciences, Boston, MA). Quantitation of signal intensities was performed as described below.
Microarray Data Interpretation and Statistical Analysis
Microarray expression data were Lowess transformed in ScanArray Express (Perkin Elmer). The normalized data were log2 transformed, filtered, and subsequently analyzed using customized procedures in Microsoft Excel. Each experimental group (unstimulated, S. aureus, or PGN) consisted of three biological replicates. A biological replicate from each TLR2 KO group was directly compared with one replicate from each TLR2 WT group, resulting in three such comparisons. RNA from each of the pairings was used to make four technical replicates (top and bottom arrays on each slide and a dye swap). From the biological and technical replicates, 12 intensity value pairs were measured per combination of experimental treatments and the ratios of these pairs calculated.
For a given comparison of biological replicates in different experimental groups, a one-sample t-test was performed on the four intensity ratios from the technical replicates with a significance level of 0.01. Genes with a significant P-value had ratios that were statistically different from one (zero in the log domain). The one-sample t-test was repeated for the other biological replicates in the experimental comparison. Using Excel, the three lists of P-values were compared and genes with significant P-values in all three biological replicates were noted. This process was repeated for the remaining comparisons of experimental groups.
Protein Extraction and Western Blotting
Protein extracts were prepared and quantitated from both TLR2 WT and KO primary microglia as previously described (Kielian et al., 2004b). The effects of heat-inactivated S. aureus, PGN, and LPS on microglial TLR2 and MyD88 protein expression were evaluated by Western blot analysis. Microglial protein extracts (10–15 μg of total protein) were electrophoresed on 10% Tris-HCl Ready Gels (Bio-Rad, Hercules, CA) and transferred to a PVDF membrane (Immobilon-P, Millipore, Bedford, MA) using a semi-dry transfer apparatus (Bio-Rad). Blots were probed using rabbit anti-mouse TLR2 or MyD88 antibodies (E-Biosciences, San Diego, CA) followed by a donkey anti-rabbit IgG-HRP conjugate (Jackson ImmunoResearch, West Grove, PA). Duplicative blots were probed with a rabbit anti-actin polyclonal antibody (A-5060; Sigma) for normalization. Blots were developed using the ChemiGlow West substrate (Alpha Innotech, San Leandro, CA) and visualized by exposure to x-ray film (Kodak Biomax, Rochester, NY). For quantitation, nonsaturated autoradiographs were scanned and the pixel intensity for each band was determined using the Image/J program (NIH Image) and normalized to the amount of actin. Results are expressed in arbitrary units as the ratio of TLR2 or MyD88 to actin (mean ± SD).
Statistical Analysis
Significant differences between experimental groups were determined by the Student’s t-test at the 95% confidence interval using Sigma Stat (SPSS Science, Chicago, IL).
RESULTS
S. aureus and PGN augment TLR2 expression in primary microglia
Recent studies have established that microglia constitutively express TLR2 (Bsibsi et al., 2002; Kielian et al., 2002; Zekki et al., 2002; Laflamme et al., 2001, 2003), with elevated receptor expression observed following exposure to the gram-negative cell wall component LPS in vivo (Laflamme et al., 2001, 2003). We previously reported that S. aureus was capable of augmenting TLR2 expression in the N9 microglial cell line (Kielian et al., 2002, 2004b); however, the kinetics of TLR2 induction following bacterial challenge in primary microglia are unknown. Therefore, the effects of S. aureus and its cell wall product PGN on TLR2 mRNA and protein expression in primary microglia were evaluated using quantitative real-time RT-PCR and Western blotting, respectively. As shown in Figure 1, both S. aureus and PGN significantly increased TLR2 mRNA expression in primary microglia with elevated levels persisting up to 24 h post-stimulation, the latest time point examined. To verify that the observed changes in TLR2 mRNA expression correlated with increased protein levels, Western blots were performed. As shown in Figure 2A,B, both S. aureus and PGN significantly enhanced TLR2 protein expression in primary WT microglia. Similar changes were also observed following LPS stimulation. These findings indicate that both S. aureus and PGN are potent inducers of TLR2 expression, which may serve to augment microglial activation in situ during gram-positive CNS infections.
Fig. 1.
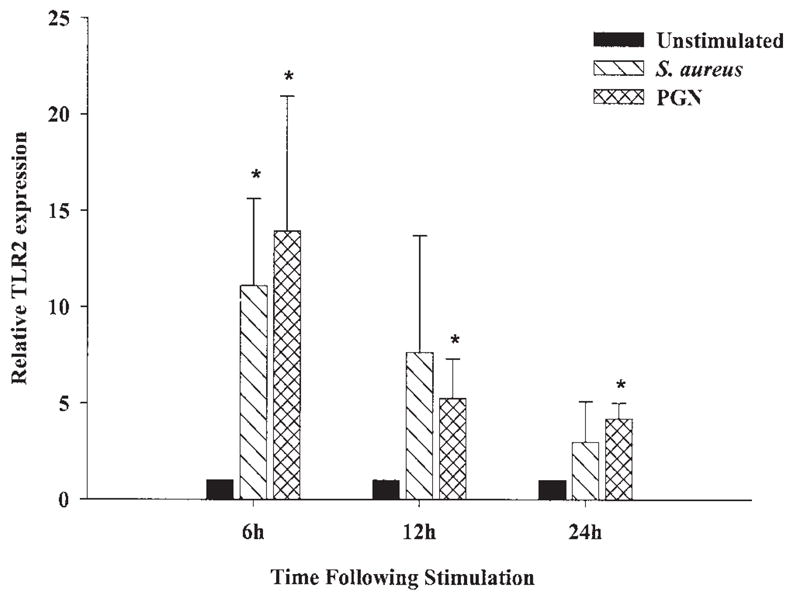
S. aureus and peptidoglycan (PGN) augment TLR2 mRNA expression in primary microglia. The time course profiles of TLR2 mRNA expression following S. aureus or PGN stimulation in primary wild-type microglia were measured by quantitative real-time RT-PCR as described in the Materials and Methods. Each real-time PCR reaction was performed in duplicate for both the target (TLR2) and the “housekeeping” gene GAPDH. The level of gene expression was calculated after normalizing TLR2 signals against GADPH and is presented in relative mRNA expression units (mean ± SD of four independent experiments). Significant differences between untreated versus S. aureus or PGN-stimulated microglia are denoted with asterisks (*P < 0.05).
Fig. 2.
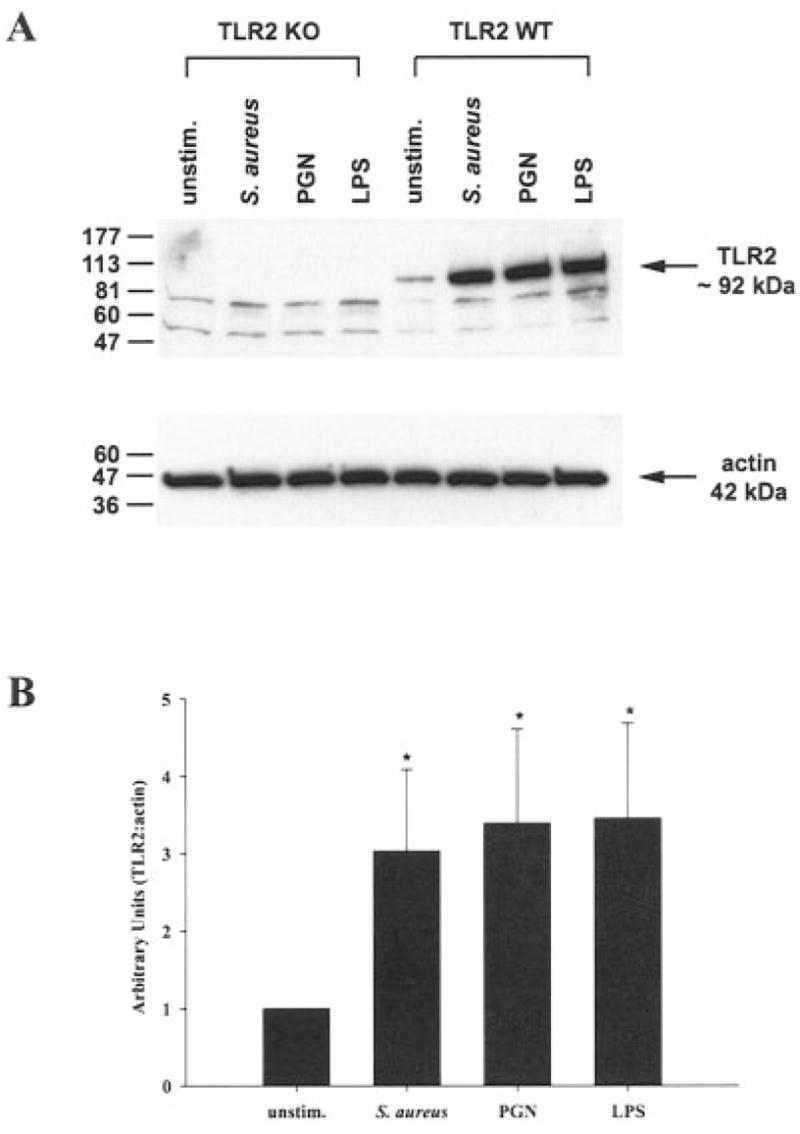
Both S. aureus and peptidoglycan (PGN) enhance TLR2 protein expression in primary microglia. TLR2 knockout (KO) and wild-type (WT) primary microglia were stimulated with either 107 heat-inactivated S. aureus, 10 μg/ml PGN, or 100 ng/ml LPS. Protein extracts from whole cell lysates were prepared 24 h following stimulation and evaluated for TLR2 expression by Western blotting as described in the Materials and Methods. Results are presented as the raw gel data (A) and quantitative analysis of TLR2 expression by densometric scanning (B). For quantitation in B, the pixel intensity of each TLR2 band from WT microglia was normalized to the amount of actin included as a “housekeeping” gene. Results are expressed in arbitrary units as the ratio of TLR2 to actin and represent the mean ± SD of three independent experiments. Significant differences are denoted with asterisks (*P < 0.05).
TLR2 Plays a Pivotal Role in the Recognition of PGN But Not Intact S. aureus in Primary Microglia
In macrophages, TLR2 is important for mediating cellular activation and subsequent proinflammatory mediator production in response to numerous bacterial ligands, including PGN and intact S. aureus (Takeuchi et al., 1999; Underhill et al., 1999). Although microglia have been shown to express TLR2 (Bsibsi, 2002; Kielian et al., 2002; Laflamme et al., 2001, 2003; Zekki et al., 2002), direct functional evidence establishing a role for this receptor in the recognition of gram-positive bacteria by microglia has not yet been reported. To examine the functional importance of TLR2 in mediating S. aureus- and PGN-dependent microglial activation, we compared the responses of primary microglia from TLR2 KO and WT mice to these gram-positive stimuli. The absence of TLR2 expression in KO microglia was confirmed by Western blot analysis (Fig. 2A). As shown in Figure 3, the responses of TLR2 KO microglia to PGN were dramatically attenuated compared with WT cells, with significantly lower levels of the proinflammatory cytokines TNF-α and IL-12 p40 released from KO microglia. In contrast, both TLR2 KO and WT microglia were capable of responding to intact bacteria (Fig. 3). Interestingly, IL-12 p40 production was consistently elevated in TLR2 KO microglia following S. aureus exposure (Fig. 3B). Examination of chemokine expression in TLR2 KO and WT microglia revealed similar differences in the responses of cells to S. aureus versus PGN (Fig. 4). Specifically, TLR2 KO microglia produced significantly lower levels of MIP-2/CXCL2 and MCP-1/CCL2 in response to PGN compared with WT cells, which was not as evident following S. aureus exposure. Although the loss of TLR2 in KO microglia resulted in little residual expression of TNF-α, IL-12 p40, and MIP-2, MCP-1 levels were less affected by the absence of this receptor (Fig. 4B). Importantly, the concentrations of S. aureus and PGN used in this study did not adversely affect microglial cell viability, indicating that the attenuated responses of TLR2 KO microglia to PGN were not due to overt cell death (Figs. 3C and 4C). In addition, the MTT data indicate that the observed differences between TLR2 KO and WT microglia did not result from random differences in cell seeding density or non-specific proliferative effects of the microbial stimuli. Finally, both TLR2 KO and WT microglia were equally responsive to the TLR4 ligand LPS (Figs. 3 and 4), suggesting that the relative inability of TLR2 KO microglia to respond to PGN was not the result of a global activation defect. Collectively, these results demonstrate that TLR2 is a major receptor responsible for mediating microglial activation in response to PGN, whereas alternative receptor(s) are involved in the recognition of intact bacteria.
Fig. 3.
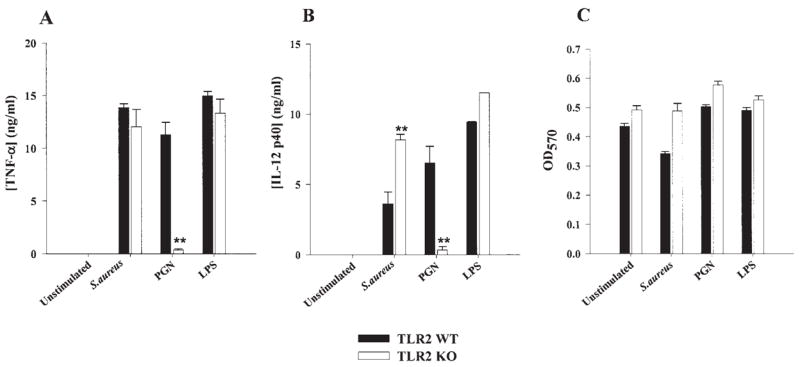
TLR2 plays a pivotal role in peptidoglycan (PGN) recognition by primary microglia and subsequent proinflammatory cytokine production. TLR2 knockout (KO) and wild-type (WT) microglia were seeded at 2 × 105 cells per well in 96-well plates and incubated overnight. The following day, cells were exposed to 107 heat-inactivated S. aureus, 10 μg/ml PGN, or 100 ng/ml LPS for 24 h. Conditioned supernatants were collected and analyzed for TNF-α (A) and IL-12 p40 (B) by ELISA. Results are presented as the amount of cytokine (ng) per ml of culture supernatant (mean ± SD). Microglial cell viability was assessed using a standard MTT assay and the raw OD570 absorbance values are reported (mean ± SD, C). Significant differences between TLR2 WT and KO microglia are denoted with asterisks (**P < 0.001). Results are representative of four independent experiments.
Fig. 4.
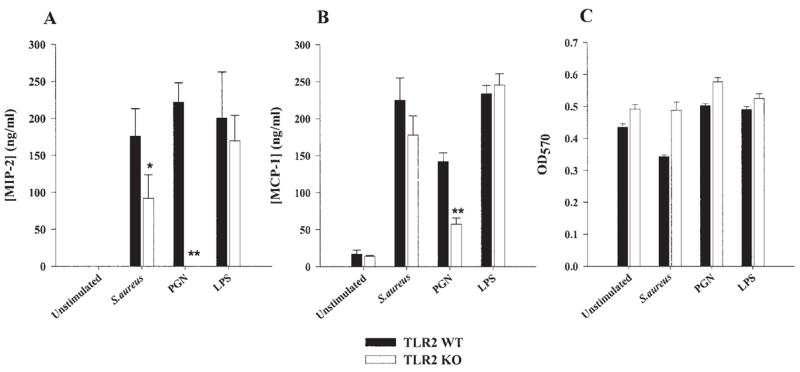
TLR2 KO microglia display defects in chemokine expression following stimulation with either S. aureus or peptidoglycan (PGN). Primary microglia from TLR2 knockout (KO) and wild-type (WT) mice were seeded at 2 × 105 cells per well in 96-well plates and incubated overnight. The following day, cells were exposed to 107 heat-inactivated S. aureus, 10 μg/ml PGN, or 100 ng/ml LPS for 24 h. Conditioned supernatants were collected and analyzed for MIP-2 (A) and MCP-1 (B) by ELISA. Results are presented as the amount of chemokine (ng) per ml of culture supernatant (mean ± SD). Microglial cell viability was assessed using a standard MTT assay and the raw OD570 absorbance values are reported (mean ± SD, C). Significant differences between TLR2 WT and KO microglia are denoted with asterisks (*P < 0.05, **P < 0.001). Results are representative of four independent experiments.
Expanded Analysis of Changes in Gene Expression Influenced by TLR2 in Primary Microglia Using Microarrays
To extend our analysis of inflammatory mediators influenced by TLR2 in primary microglia, a targeted microarray approach was used. The time- and cost-restrictive nature of microarray analysis prevented the evaluation of a wide range of time points; therefore, we chose to examine differences in gene expression between S. aureus and PGN-stimulated TLR2 KO and WT microglia at 6 h to identify immediate changes in inflammatory gene expression. As shown in Table 1, there were no detectable differences in constitutive gene expression between TLR2 KO and WT primary microglia. In contrast, numerous proinflammatory genes were significantly decreased in TLR2 KO microglia following PGN treatment compared with WT cells, the majority of which were chemokines and cellular receptors. In contrast, only a subset of genes were significantly decreased in TLR2 KO microglia in response to intact S. aureus and the relative fold-changes were less compared with those observed following PGN treatment. Collectively, these findings confirm that TLR2 is primarily responsible for regulating microglial inflammatory mediator expression in response to PGN. In addition, a minor role for TLR2 in mediating specific gene expression in response to S. aureus is suggested, although it appears likely that additional PRRs act in concert with TLR2 to induce maximal microglial activation.
TABLE 1.
Analysis of Differential Gene Expression in Primary TLR2 KO Versus WT Microglia
| Gene name | Accession no. | Unstimulated fold change | S. aureus fold changea | PGN fold change |
|---|---|---|---|---|
| Chemokine | ||||
| CXCL2/MIP-2 | NM_009140 | No genes were found | −3.5 | −11.5 |
| CXCL12/SDF-1α | L12029 | −2.7 | −7.7 | |
| CCL3/MIP-α | NM_011337 | −2.3 | −6.1 | |
| CCL4/MIP-1β | NM_013652 | −1.7 | −2.9 | |
| CCL6/C10 | NM_009139 | −3.7 | ||
| CCL12/MCP-5 | NM_011331 | −1.8 | −2.8 | |
| Cytokine | ||||
| IL-1β | NM_008361 | −3.0 | −8.6 | |
| Receptor | ||||
| CXCR2 | NM_009909 | −4.1 | ||
| CCR2 | NM_009915 | −2.9 | −3.3 | |
| CCR4 | NM_009916 | −2.0 | ||
| CCR5 | NM_009917 | −2.9 | ||
| TNFSF4 | NM_011659 | −3.1 | ||
| TNFSF7 | NM_011617 | −2.0 | ||
| IL-18R1 | NM_008365 | −3.6 | ||
| CSF Rβ2 | NM_007781 | −1.8 | ||
| VCAM-1 | NM_011693 | −3.1 | −10.5 | |
| TLR1 | NM_030682 | 1.4 | ||
| TLR3 | AF420279 | −3.4 | ||
| TLR5 | NM_016928 | −2.5 | ||
MIP, macrophage inflammatory protein; SDF-1α, stromal-cell-derived factor-1α; MCP, monocyte chemoattractant protein; IL, interleukin; TNFSF, tumor necrosis factor receptor superfamily; CSF Rβ2, colony-stimulating factor receptor β2; VCAM-1, vascular cell adhesion molecule-1; TLR, Toll-like receptor; WT, wild-type; KO, knockout.
Refers to the ratio of the overall average differential expression level between TLR2 KO and WT primary microglia stimulated with 107 heat-inactivated S. aureus or 10 μg/ml PGN. Negative numbers indicate genes that are significantly decreased in TLR2 KO microglia compared with WT cells, whereas positive numbers indicate genes that are significantly elevated in TLR2 KO microglia versus WT.
Attenuation of Cellular Activation in Response to PGN in TLR2 KO Microglia Is Not Due to Defective MyD88 Expression
Most Toll-like receptors identified to date use a common signal transduction pathway with minor differences that appear to confer specificity in response to distinct ligands (Akira, 2003; Takeda et al., 2003). To determine whether the inability of TLR2 KO microglia to respond to PGN was a result of an additional defect(s) besides the loss of this receptor, we evaluated the expression of the adapter molecule MyD88 in both TLR2 WT and KO microglia. MyD88 is a central player in the signal transduction pathways of the IL-1R, IL-18R, and most TLRs identified to date, providing the bridge between the cytoplasmic domains of these receptors and IL-1R1-associated kinase 1 (IRAK-1) (Akira, 2003; Takeda et al., 2003). The levels of MyD88 protein expression were similar between TLR2 WT and KO microglia, indicating that the defects in PGN recognition in the latter cannot be attributed to alterations in this essential adapter protein (Fig. 5). MyD88 expression was elevated in both TLR2 WT and KO microglia following exposure to either S. aureus, PGN or LPS (Fig. 5B). These findings indicate that the impaired responses of TLR2 KO microglia to PGN result from the absence of this receptor and are not due to alterations in the key signal transduction intermediate MyD88.
Fig. 5.
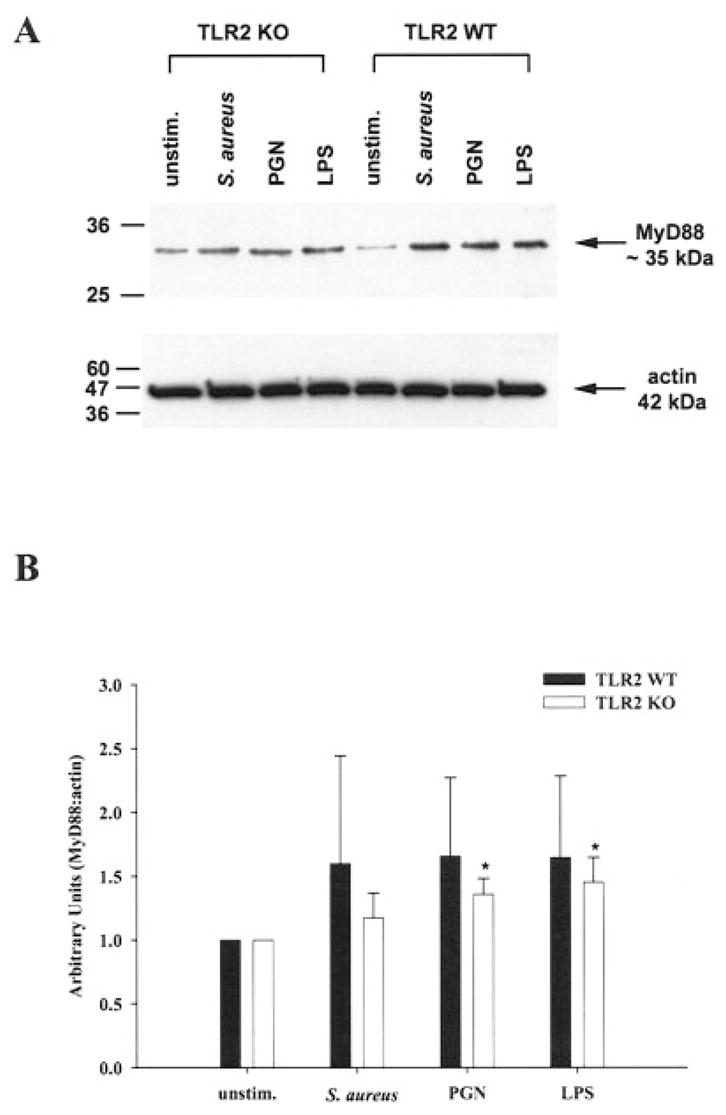
MyD88 expression is increased in primary microglia following S. aureus and peptidoglycan (PGN) exposure. TLR2 knockout (KO) and wild-type (WT) primary microglia were stimulated with either 107 heat-inactivated S. aureus, 10 μg/ml PGN, or 100 ng/ml LPS. Protein extracts from whole cell lysates were prepared 24 h following stimulation and evaluated for MyD88 expression by Western blotting as described in the Materials and Methods. Results are presented as the raw gel data (A) and quantitative analysis of MyD88 expression by densometric scanning (B). For quantitation in B, the pixel intensity of each MyD88 band was normalized to the amount of actin included as a “housekeeping” gene. Results are expressed in arbitrary units as the ratio of MyD88 to actin and represent the mean ± SD of three independent experiments. Significant differences between unstimulated versus S. aureus-, PGN-, or LPS-treated microglia are denoted with asterisks (*P < 0.05).
Microglia Express Numerous PRRs Whose Expression Is Modulated by S. aureus
The finding that TLR2 KO microglia responded similarly to WT cells in response to intact S. aureus indicated that an alternative receptor(s) is responsible for bacterial recognition and subsequent microglial activation. Possible candidates include scavenger and mannose receptors and CD14. CD14 has been shown to mediate macrophage activation in response to PGN and other gram-positive bacteria (Kusunoki et al., 1995; Henneke et al., 2001; Dziarski, 2003). Scavenger receptors represent a large class of cell surface receptors that, reminiscent of TLRs, recognize a wide array of both exogenous and endogenous ligands including PAMPs and modified low-density lipoproteins (Husemann et al., 2002; Peiser et al., 2002). Mannose receptors recognize carbohydrates containing a high degree of mannosylated and fucosylated residues, modifications unique to bacteria, and target organisms for lysosomal degradation (East and Isacke, 2002; Gordon, 2002). To determine the effects of intact S. aureus on specific members of these receptor classes, we evaluated differences in CD14, mannose receptor type I (MRCI), and lectin-like oxidized LDL-receptor 1 (LOX-1) expression in both TLR2 KO and WT microglia, using quantitative real-time RT-PCR. Both CD14 and LOX-1 were significantly increased in TLR2 WT and KO microglia following S. aureus exposure, with maximal receptor expression observed at 6 h following stimulation (Fig. 6A,B). Interestingly, although LOX-1 levels were increased in S. aureus-treated TLR2 KO microglia compared with unstimulated cells, receptor expression was significantly lower compared with WT microglia (Fig. 6B). Similar to previous reports by our group and others, microglial MRCI expression was decreased following cellular activation and was observed in both TLR2 KO and WT microglia (Fig. 6C). These findings suggest that CD14 and/or LOX-1 may participate in the recognition of intact S. aureus by microglia. However, it is highly likely that additional receptors, not examined here, may also contribute to microglial activation in response to this CNS pathogen.
Fig. 6.
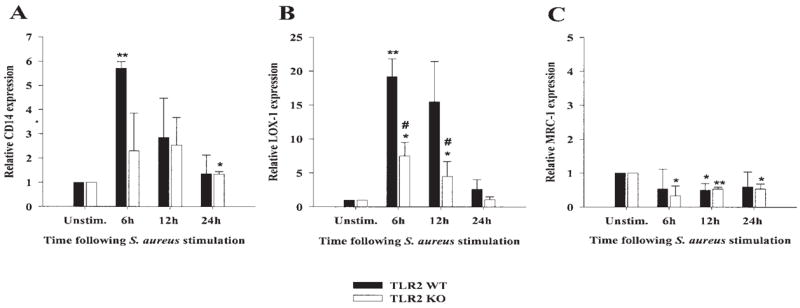
S. aureus increases the expression of additional receptors known to bind bacteria in both TLR2 knockout (KO) and wild-type (WT) microglia. The time course profiles of CD14 (A), LOX-1 (B), and MRC-1 (C) mRNA expression following S. aureus stimulation in both TLR2 KO and WT microglia were measured by quantitative real-time RT-PCR as described in the Materials and Methods. Each real-time PCR reaction was performed in duplicate for both the target (CD14, LOX-1, and MRC-1) and the “housekeeping” gene GAPDH. The level of gene expression was calculated after normalizing target signals against GADPH and is presented in relative mRNA expression units (mean ± SD of three independent experiments). Significant differences between untreated versus S. aureus-stimulated microglia are denoted with asterisks (*P < 0.05, **P < 0.001), whereas significant differences between TLR2 WT and KO microglia are also indicated (# P < 0.05).
DISCUSSION
Gram-positive pathogens remain a common etiologic agent of CNS bacterial infections including brain abscess. The recent emergence of antibiotic-resistant strains of S. aureus has increased the need to understand the mechanism(s) by which this pathogen influences immune activation in the CNS in order to develop future novel therapeutic modalities.
Using primary microglia from TLR2 KO and WT mice, we demonstrated that TLR2 plays an important role in PGN recognition by microglia. Similar results have been reported with TLR2 KO macrophages where cells were nonresponsive to PGN derived from S. aureus (Takeuchi et al., 1999). The finding that microglial responses to intact bacteria were not significantly affected by the loss of TLR2 suggests that microglia use alternative PRRs for S. aureus recognition. Our results are in agreement with recent reports investigating the importance of TLRs in mediating macrophage responses to group B streptococci (GBS) (Henneke et al., 2001, 2002). Specifically, Henneke et al. (2001, 2002) found that macrophage activation and subsequent proinflammatory mediator production following exposure to intact GBS occurred via a TLR2-independent pathway. However, our results differ from earlier studies where TLR2 was shown to play a major role in mediating macrophage activation in response to intact S. aureus (Takeuchi et al., 2000a; Underhill et al., 1999). The reason(s) responsible for these discrepancies are not known but could be attributed to differences in the cell types examined and/or the serotype of S. aureus used. We have previously reported that TLR2 plays a critical role in the recognition of both S. aureus and PGN in primary astrocytes (Esen et al., 2004). It is intriguing that this relationship was not observed in primary microglia, where cells used TLR2 primarily for PGN recognition but not for intact bacteria. This finding may be explained by the fact that, unlike astrocytes, microglia are considered to be potent bactericidal effector cells and likely require a larger receptor repertoire to respond to infectious pathogens.
A direct comparison of gene expression profiles between TLR2 KO and WT microglia by microarray analysis confirmed some of our findings by ELISA and revealed additional factors influenced by TLR2. For example, the expression of both MIP-2 and IL-1β were significantly attenuated in TLR2 KO microglia in response to PGN, which was also observed at the protein level by ELISA (Figure 4A and data not shown). In addition, numerous CC chemokines, which serve as potent monocyte and lymphocyte chemoattractants, were also significantly decreased in PGN-stimulated TLR2 KO microglia suggesting that the loss of TLR2 may dictate the influx of these cell types into the inflamed CNS. Likewise, the expression of several chemokine receptors was reduced in TLR2 KO microglia following PGN exposure, indicating that TLR2-dependent signaling may influence the subsequent migration of activated microglia at sites of CNS infection.
Although our studies with TLR2 KO primary microglia revealed an important role for this receptor in mediating PGN-dependent activation, it is clear that alternative receptor(s) are involved in microglial responses to intact bacteria. Microarray analysis revealed that TLR2 is capable of modestly influencing a subset of genes in response to S. aureus, suggesting that TLR2 may operate in concert with multiple PRRs to acquire maximum ligand specificity and sensitivity in microglia. There are several candidate receptors that may participate in the recognition of intact S. aureus by microglia, including TLR1, TLR6, TLR9, CD14, and members of the scavenger and mannose receptor family. All these receptors are expressed on microglia (Marzolo et al., 1999; Nadeau and Rivest, 2000; Dalpke et al., 2002; Husemann et al., 2002; Kielian et al., 2002) and have been shown to mediate recognition of gram-positive PAMPs by macrophages (Weidemann et al., 1994; Cleveland et al., 1996; Ozinsky et al., 2000; Peiser et al., 2000; Thomas et al., 2000). To begin to investigate the potential involvement of alternative receptors in microglial responses to S. aureus, we examined the expression of CD14, the scavenger receptor LOX-1, and mannose receptor type I in primary microglia. The expression of both CD14 and LOX-1 were increased significantly following S. aureus exposure, suggesting that they may participate in bacterial recognition by microglia. We are currently investigating the functional importance of these receptors in the context of S. aureus-dependent microglial activation using both knockout mice and/or pharmacological inhibitors of these receptors. Future studies in our laboratory will address the potential involvement of additional TLRs in mediating microglial responses to intact S. aureus by using primary cells isolated from MyD88 KO mice, since the majority of TLRs identified to date require this adapter molecule for cell signaling and subsequent activation (Akira, 2003; Takeda et al., 2003). Evidence to support a critical role for MyD88 in gram-positive bacterial recognition is provided by reports that MyD88 KO mice are highly susceptible to gram-positive bacterial infections and macrophages from these animals do not respond to gram-positive bacterial products (Henneke et al., 2001; Takeuchi et al., 2000a,b). It is important to acknowledge that gram-positive bacteria present the host with a wide array of potential immunostimulatory antigens during the course of infection including PGN, lipoteichoic acid (LTA), CpG DNA, and secreted toxins (Dziarski, 2003; Weber et al., 2003). Furthermore, not only are the types of bacterial antigens diverse within a given organism, but the molecular structure of essential cell wall components such as PGN and LTA are highly heterogeneous, adding another level of complexity to the system (Weber et al., 2003). This extremely intricate milieu of bacterial antigens necessitates the use of multiple PRRs to ensure the establishment of effective anti-bacterial immunity in the CNS and other organs.
Exposure of primary microglia to either S. aureus or PGN led to a significant induction in TLR2 expression. The mechanism(s) responsible for augmented TLR2 levels following microglial exposure to these gram-positive stimuli is unknown but could be envisioned to occur by one of the following pathways. First, receptor ligation via both TLR2-dependent and -independent pathways likely induces the expression of transcription factors that lead to the direct activation of TLR2 gene expression, such as NF-κB. Second, we have established that both S. aureus and PGN are potent inducers of proinflammatory cytokine expression in activated microglia (Kielian et al., 2002, 2004b). It is possible that these cytokines (i.e., TNF-α, IL-1β) act in an autocrine/paracrine manner to augment TLR2 expression. The rapid kinetics of TLR2 mRNA upregulation in response to both S. aureus and PGN observed in this study suggest that these microbial stimuli directly lead to changes in receptor expression. However, it is important to consider that these two possibilities are probably not mutually exclusive, and it is likely that both are contributing to elevated TLR2 levels on microglia subsequent to S. aureus or PGN exposure.
Comparisons of proinflammatory mediator expression between TLR2 KO versus WT microglia revealed a couple of interesting differences. For example, although TLR2 was shown to play an important role in the activation and subsequent release of the neutrophil chemoattractant MIP-2 by PGN-stimulated microglia, it did not dramatically influence the production of the CC chemokine MCP-1. The reason(s) responsible for this TLR2-independent production of MCP-1 following microglial exposure to PGN is not known. Another interesting observation in the present study was that IL-12 p40 expression was significantly increased in TLR2 KO microglia compared with WT cells following S. aureus exposure. This finding reveals a TLR2-independent mechanism for the induction of IL-12 p40 and further supports our observations that alternative receptors are involved in mediating microglial activation in response to intact bacteria. Potential explanations to account for the elevated levels of IL-12 p40 produced from S. aureus-stimulated TLR2 KO microglia include the augmented expression of a receptor that is critical for dictating IL-12 production or alterations in a signal transduction pathway(s) that is responsible for quelling IL-12 production by activated cells. The involvement of TLR2 in dictating microglial responses to S. aureus versus PGN is depicted schematically in Figure 7.
Fig. 7.
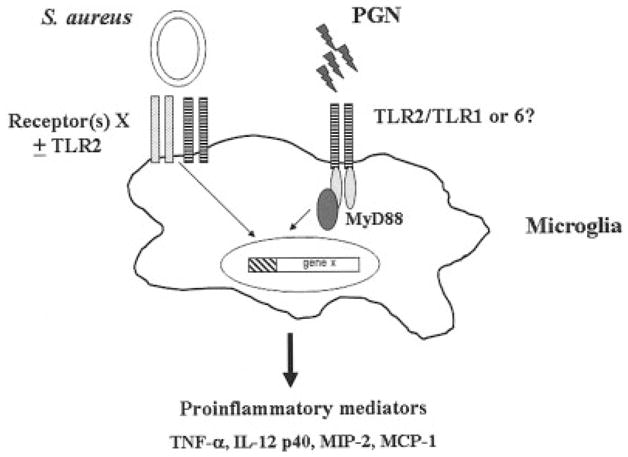
Pathways for recognition of intact S. aureus versus peptidoglycan (PGN) by microglia. Microglia primarily use TLR2 (hatched bars) for PGN recognition and subsequent proinflammatory mediator expression. It is currently unknown whether TLR2 heterodimerizes with TLR1 or TLR6 to facilitate PGN recognition by microglia. In contrast, alternative receptor(s) (denoted here as receptor(s) X) are responsible for mediating microglial activation in response to intact bacteria. Under certain circumstances, these receptor(s) may signal through cooperative interactions with TLR2. It is likely that intact S. aureus engages multiple cell surface receptors on microglia to ensure efficient innate immune responses within the CNS parenchyma.
In summary, this study demonstrates that TLR2 plays an important role in PGN-dependent microglial activation and subsequent proinflammatory mediator expression. In contrast, microglia use alternative receptors to recognize intact S. aureus. The functional receptor redundancy allowing microglia to respond to S. aureus is not surprising, since bacterial pathogens have the potential for devastating consequences in a tissue that has limited regenerative capacity such as the CNS.
Acknowledgments
The authors thank Dr. Shizuo Akira for generously providing the TLR2 KO mice, Dr. Marjorie Beggs in the UAMS Microarray Core Facility for her expertise with microarray design and execution, and Patrick Mayes and Anessa Haney for excellent technical assistance. This work was supported by the NIH National Institute of Mental Health (RO1 MH65297) (to T.K.). The UAMS Microarray Core facility is supported, in part, by the BRIN program of the National Center for Research Resources (P20 RR-16460).
Grant sponsor: NIH National Institute of Mental Health; Grant number: RO1 MH65297l; Grant sponsor: National Center for Research Resources (BRIN program); Grant number: P20 RR-16460.
References
- Akira S. Toll-like receptor signaling. J Biol Chem. 2003;278:38105–38108. doi: 10.1074/jbc.R300028200. [DOI] [PubMed] [Google Scholar]
- Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- Cleveland MG, Gorham JD, Murphy TL, Tuomanen E, Murphy KM. Lipoteichoic acid preparations of gram-positive bacteria induce interleukin-12 through a CD14-dependent pathway. Infect Immun. 1996;64:1906–1912. doi: 10.1128/iai.64.6.1906-1912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalpke AH, Schafer MK, Frey M, Zimmermann S, Tebbe J, Weihe E, Heeg K. Immunostimulatory CpG-DNA activates murine microglia. J Immunol. 2002;168:4854–4863. doi: 10.4049/jimmunol.168.10.4854. [DOI] [PubMed] [Google Scholar]
- Dziarski R. Recognition of bacterial peptidoglycan by the innate immune system. Cell Mol Life Sci. 2003;60:1793–1804. doi: 10.1007/s00018-003-3019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East L, Isacke CM. The mannose receptor family. Biochim Biophys Acta. 2002;1572:364–386. doi: 10.1016/s0304-4165(02)00319-7. [DOI] [PubMed] [Google Scholar]
- Esen N, Tanga FY, DeLeo JA, Kielian T. Toll-like receptor 2 (TLR2) mediates astrocyte activation in response to the Gram-positive bacterium Staphylococcus aureus. J Neurochem. 2004;88:746–758. doi: 10.1046/j.1471-4159.2003.02202.x. [DOI] [PubMed] [Google Scholar]
- Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927–930. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- Henneke P, Takeuchi O, van Strijp JA, Guttormsen HK, Smith JA, Schromm AB, Espevik TA, Akira S, Nizet V, Kasper DL, Golenbock DT. Novel engagement of CD14 and multiple toll-like receptors by group B streptococci. J Immunol. 2001;167:7069–7076. doi: 10.4049/jimmunol.167.12.7069. [DOI] [PubMed] [Google Scholar]
- Henneke P, Takeuchi O, Malley R, Lien E, Ingalls RR, Freeman MW, Mayadas T, Nizet V, Akira S, Kasper DL, Golenbock DT. Cellular activation, phagocytosis, and bactericidal activity against group B streptococcus involve parallel myeloid differentiation factor 88-dependent and independent signaling pathways. J Immunol. 2002;169:3970–3977. doi: 10.4049/jimmunol.169.7.3970. [DOI] [PubMed] [Google Scholar]
- Husemann J, Loike JD, Anankov R, Febbraio M, Silverstein SC. Scavenger receptors in neurobiology and neuropathology: their role on microglia and other cells of the nervous system. Glia. 2002;40:195–205. doi: 10.1002/glia.10148. [DOI] [PubMed] [Google Scholar]
- Kielian T, Barry B, Hickey WF. CXC chemokine receptor-2 ligands are required for neutrophil-mediated host defense in experimental brain abscesses. J Immunol. 2001;166:4634–4643. doi: 10.4049/jimmunol.166.7.4634. [DOI] [PubMed] [Google Scholar]
- Kielian T, Mayes P, Kielian M. Characterization of microglial responses to Staphylococcus aureus: effects on cytokine, costimulatory molecule, and Toll-like receptor expression. J Neuroimmunol. 2002;130:86–99. doi: 10.1016/s0165-5728(02)00216-3. [DOI] [PubMed] [Google Scholar]
- Kielian T, Bearden ED, Baldwin AC, Esen N. IL-1 and TNF-alpha play a pivotal role in the host immune response in a mouse model of Staphylococcus aureus-induced experimental brain abscess. J Neuropathol Exp Neurol. 2004a;63:381–396. doi: 10.1093/jnen/63.4.381. [DOI] [PubMed] [Google Scholar]
- Kielian T, McMahon M, Bearden ED, Baldwin AC, Drew PD, Esen N. S. aureus-dependent microglial activation is selectively attenuated by the cyclopentenone prostaglandin 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) J Neurochem. 2004b;90:1163–1172. doi: 10.1111/j.1471-4159.2004.02579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp E, Medzhitov R. Recognition of microbial infection by Toll-like receptors. Curr Opin Immunol. 2003;15:396–401. doi: 10.1016/s0952-7915(03)00080-3. [DOI] [PubMed] [Google Scholar]
- Kusunoki T, Hailman E, Juan TS, Lichenstein HS, Wright SD. Molecules from Staphylococcus aureus that bind CD14 and stimulate innate immune responses. J Exp Med. 1995;182:1673–1682. doi: 10.1084/jem.182.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme N, Soucy G, Rivest S. Circulating cell wall components derived from gram-negative, not gram-positive, bacteria cause a profound induction of the gene-encoding Toll-like receptor 2 in the CNS. J Neurochem. 2001;79:648–657. doi: 10.1046/j.1471-4159.2001.00603.x. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Echchannaoui H, Landmann R, Rivest S. Cooperation between toll-like receptor 2 and 4 in the brain of mice challenged with cell wall components derived from gram-negative and gram-positive bacteria. Eur J Immunol. 2003;33:1127–1138. doi: 10.1002/eji.200323821. [DOI] [PubMed] [Google Scholar]
- Marzolo MP, von Bernhardi R, Inestrosa NC. Mannose receptor is present in a functional state in rat microglial cells. J Neurosci Res. 1999;58:387–395. [PubMed] [Google Scholar]
- Mathisen GE, Johnson JP. Brain abscess. Clin Infect Dis. 1997;25:763–779. doi: 10.1086/515541. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- Morath S, Stadelmaier A, Geyer A, Schmidt RR, Hartung T. Synthetic lipoteichoic acid from Staphylococcus aureus is a potent stimulus of cytokine release. J Exp Med. 2002;195:1635–1640. doi: 10.1084/jem.20020322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau S, Rivest S. Role of microglial-derived tumor necrosis factor in mediating CD14 transcription and nuclear factor kappa B activity in the brain during endotoxemia. J Neurosci. 2000;20:3456–3468. doi: 10.1523/JNEUROSCI.20-09-03456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiser L, Gough PJ, Kodama T, Gordon S. Macrophage class A scavenger receptor-mediated phagocytosis of Escherichia coli: role of cell heterogeneity, microbial strain, and culture conditions in vitro. Infect Immun. 2000;68:1953–1963. doi: 10.1128/iai.68.4.1953-1963.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiser L, Mukhopadhyay S, Gordon S. Scavenger receptors in innate immunity. Curr Opin Immunol. 2002;14:123–128. doi: 10.1016/s0952-7915(01)00307-7. [DOI] [PubMed] [Google Scholar]
- Qureshi ST, Medzhitov R. Toll-like receptors and their role in experimental models of microbial infection. Genes Immun. 2003;4:87–94. doi: 10.1038/sj.gene.6363937. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000a;165:5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Takeda K, Hoshino K, Adachi O, Ogawa T, Akira S. Cellular responses to bacterial cell wall components are mediated through MyD88-dependent signaling cascades. Int Immunol. 2000b;12:113–117. doi: 10.1093/intimm/12.1.113. [DOI] [PubMed] [Google Scholar]
- Thomas CA, Li Y, Kodama T, Suzuki H, Silverstein SC, El Khoury J. Protection from lethal gram-positive infection by macrophage scavenger receptor-dependent phagocytosis. J Exp Med. 2000;191:147–156. doi: 10.1084/jem.191.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend GC, Scheld WM. Infections of the central nervous system. Adv Intern Med. 1998;43:403–447. [PubMed] [Google Scholar]
- Underhill D, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- Weber JR, Moreillon P, Tuomanen EI. Innate sensors for Gram-positive bacteria. Curr Opin Immunol. 2003;15:408–415. doi: 10.1016/s0952-7915(03)00078-5. [DOI] [PubMed] [Google Scholar]
- Weidemann B, Brade H, Rietschel ET, Dziarski R, Bazil V, Kusumoto S, Flad HD, Ulmer AJ. Soluble peptidoglycan-induced monokine production can be blocked by anti-CD14 monoclonal antibodies and by lipid A partial structures. Infect Immun. 1994;62:4709–4715. doi: 10.1128/iai.62.11.4709-4715.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zekki H, Feinstein DL, Rivest S. The clinical course of experimental autoimmune encephalomyelitis is associated with a profound and sustained transcriptional activation of the genes encoding toll-like receptor 2 and CD14 in the mouse CNS. Brain Pathol. 2002;12:308–319. doi: 10.1111/j.1750-3639.2002.tb00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


