Abstract
Bmp7 is expressed in numerous tissues throughout development and is required for morphogenesis of the eye, hindlimb and kidney. In this study we show that the majority if not all of the cis-regulatory sequence governing expression at these anatomical sites during development is present in approximately 20 kilobases surrounding exon 1. In eye, limb and kidney, multiple distinct enhancer elements drive Bmp7 expression within each organ. In the eye, the elements driving expression in the pigmented epithelium and iris are spatially separated. In the kidney, Bmp7 expression in collecting ducts and nephron progenitors is driven by separate enhancer elements. Similarly, limb mesenchyme and apical ectodermal ridge expression are governed by separate elements. Although enhancers for pigmented epithelium, nephrogenic mesenchyme and apical ectodermal ridge are distributed across the approximately 20 kilobase region, an element of approximately 480 base pairs within intron 1 governs expression within the developing iris, collecting duct system of the kidney and limb mesenchyme. This element is remarkably conserved both in sequence and position in the Bmp7 locus between different vertebrates, ranging from Xenopus tropicalis to Homo sapiens, demonstrating that there is strong selective pressure for Bmp7 expression at these tissue sites. Furthermore, we show that the frog enhancer functions appropriately in transgenic mice. Interestingly, the intron 1 element cannot be found in the Bmp7 genes of vertebrates such as Danio rerio and Takifugu rubripes indicating that this modification of the Bmp7 gene might have arisen during the adaptation from aquatic to terrestrial life. Mutational analysis demonstrates that the enhancer activity of the intron 1 element is entirely dependent on the presence of a 10 base pair site within the intron 1 enhancer containing a predicted binding site for the FOXD3 transcription factor.
Keywords: Bone morphogenetic protein, BMP, BMP7, OP1, Kidney development, Eye development, Limb development, Ureteric bud
Introduction
The bone morphogenetic protein (BMP) ligands comprise a large family of growth factors sharing structural homology with the transforming growth factor β proteins. Their expression is essential for key events in early embryonic patterning and development of multiple organ systems (Hogan, 1996). BMPs regulate processes as diverse as cell proliferation, apoptosis and differentiation in a spectrum of tissues. Despite the variety of cellular responses elicited by these growth factors, the signaling pathway downstream of BMPs is relatively simple, with receptor binding leading to phosphorylation and activation of the Smad1, 5 and 8 transcription factors that are translocated to the nucleus together with Smad4 (Massagué, 1998). Differential transcriptional responses to BMPs are largely regulated by association of the phosphorylated Smad complex with cell type specific auxiliary transcription factors that activate distinct genetic programs (Massagué and Wotton, 2000). Our previous work has shown that the developmental functions of BMP7 can be entirely replaced with the closely related BMP6 and largely replaced with the more distantly related BMP4 (Oxburgh et al., 2005). The spatial distribution and overall levels of BMP activities may thus explain their distinct roles in development. Consistent with this idea, exacerbated phenotypes were revealed by compound mutation of the Bmp5, Bmp6 and Bmp7 genes. These BMP family members are expressed in an overlapping fashion in the heart, but their individual inactivation fails to disrupt heart development. However, inactivation of Bmp7 and Bmp5 (Solloway and Robertson, 1999) or Bmp7 and Bmp6 (Kim et al., 2001) leads to retardation in heart development and defects in valve formation and septation. This strongly indicates that at least the 60A subgroup (Bmps 5, 6 and 7) is functionally redundant and that the collective expression of these ligands determines developmental function. In keeping with this conclusion, individual inactivation of these genes reveals phenotypes that closely correspond with their domains of unique expression (Dudley et al., 1995; Dudley and Robertson, 1997; Kingsley et al., 1992; Solloway et al., 1998). Collectively, these studies strongly suggest that developmental roles of BMPs are determined by the cis-regulatory sequences governing their expression rather than via distinct properties of individual ligands. Thus, a mechanistic understanding of BMP function in development will require characterization of the basis of tissue-specific expression of these growth factors. Bmp7 is essential for development of the eye, kidney and hindlimb (Dudley et al., 1999; Dudley et al., 1995; Oxburgh et al., 2004). Here we describe for the first time the cis regulatory sequences controlling Bmp7 expression in these tissues. We have identified an approximately 480 base pair evolutionarily conserved enhancer island within intron 1 of the Bmp7 locus governing expression in all three of these tissues. Surprisingly, we find that this enhancer activity is entirely dependent on the presence of a stretch of 10 base pairs containing a predicted binding site for the transcription factor FOXD3.
Materials and methods
RNA purification, Northern analysis and RACE
RNA was purified from embryonic day 13.5 (E13.5), E17.5 and adult kidneys using the Trizol reagent (Invitrogen) according to the manufacturer’s instructions. 50μg total RNA was separated on a 1% formaldehyde denaturing gel alongside an RNA size ladder (ssRNA ladder, New England Biolabs). The gel was ethidium bromide stained and nucleic acid migration distances calculated. The gel was subsequently blotted to Hybond N nitrocellulose (GE Healthcare) (Sambrook et al., 1989) and hybridized with a 32P-labeled random primed probe (Rediprime, GE Healthcare) representing the entire Bmp7 coding sequence using standard Northern blotting procedures (Sambrook et al., 1989). Autoradiographs were measured and compared to the size standards to determine molecular weights of detected bands. Three prime Rapid Amplification of cDNA ends (RACE) was performed on total RNA using the SMART RACE kit (Clontech) according to the manufaturer’s instructions. 3’ termini of isolated clones were sequenced and aligned with the genomic sequence of Bmp7 (chromosome 2, 172,510,951-172,583,260). Bmp7 cDNA clones isolated from an embryonic kidney library (Stratagene) using the Bmp7 coding sequence probe were sequenced, and their 5’ termini compared to the Bmp7 genomic sequence.
Transgenic reporter constructs
A phage clone spanning approximately 20 kilobases (kb) surrounding the first exon of Bmp7 was isolated from a 129 SVJ genomic library (Stratagene) by screening using a 32P-labeled random primed probe representing the first exon of Bmp7 using standard procedures (Sambrook et al., 1989). The genomic clone was restriction mapped and subcloned into the Hsp68lacZ reporter construct (Sasaki and Hogan, 1996) in five fragments (Fig. 1D). The following restriction enzymes were used to generate fragments: for 142:1, EcoRI-NsiI, for 216:1 NsiI-HindIII, for 216:2 HindIII-NsiI, for 217:1, NsiI-NdeI, for 217:2, NdeI-XbaI. Genomic DNA was digested, separated on 0.8% agarose gels and DNA was purified from bands of appropriate molecular weights using the Geneclean Spin kit (QBioGene) according to the manufacturer’s instructions. Purified DNA was polished using T4 DNA polymerase (New England Biolabs) according to the manufacturer’s instructions, and enzyme was heat inactivated for 20 minutes at 65° C before ligation. The 480 base pair (bp) X. tropicalis Bmp7 intron 1 element was PCR amplified from genomic DNA using Platinum Hi-Fidelity PCR kit (Invitrogen) with 5’ phosphorylated oligonucleotides 5’- GGCTCGGACG TTCTTGGACG TCTCT-3’ and 5’-AGATCCTTAT AATCACAACC AGACA-3’. Hsp68lacZ plasmid was linearized with Smal and dephosphorylated using Calf Intestinal Phosphatase (Both New England Biolabs) according to the manufacturer’s instructions. Linearized plasmid was gel purified using the Geneclean spin kit. Plasmid and insert were ligated using the Takara ligation kit (Takara) according to manufacturer’s instructions and transformed into chemically competent DH5α E. coli prepared according to standard procedures (Sambrook et al., 1989). Ampicillin-resistant colonies were screened by restriction mapping for the presence of genomic DNA and DNA was purified from positive clones using the Qiagen Maxiprep kit (Qiagen) according to manufacturer’s instructions. Purified plasmid DNA was digested with NotI, separated on 0.8% agarose gel, and the anticipated molecular weight corresponding to the transgene was excised and purified using the Qiagen Gel Cleanup Kit (Qiagen). Transgene DNA was verified by agarose gel electrophoresis and suspended to a concentration of 3ng/μl for pronuclear injection in a buffer containing 10 mM PIPES, 5 mM NaCl and 150 mM KCl.
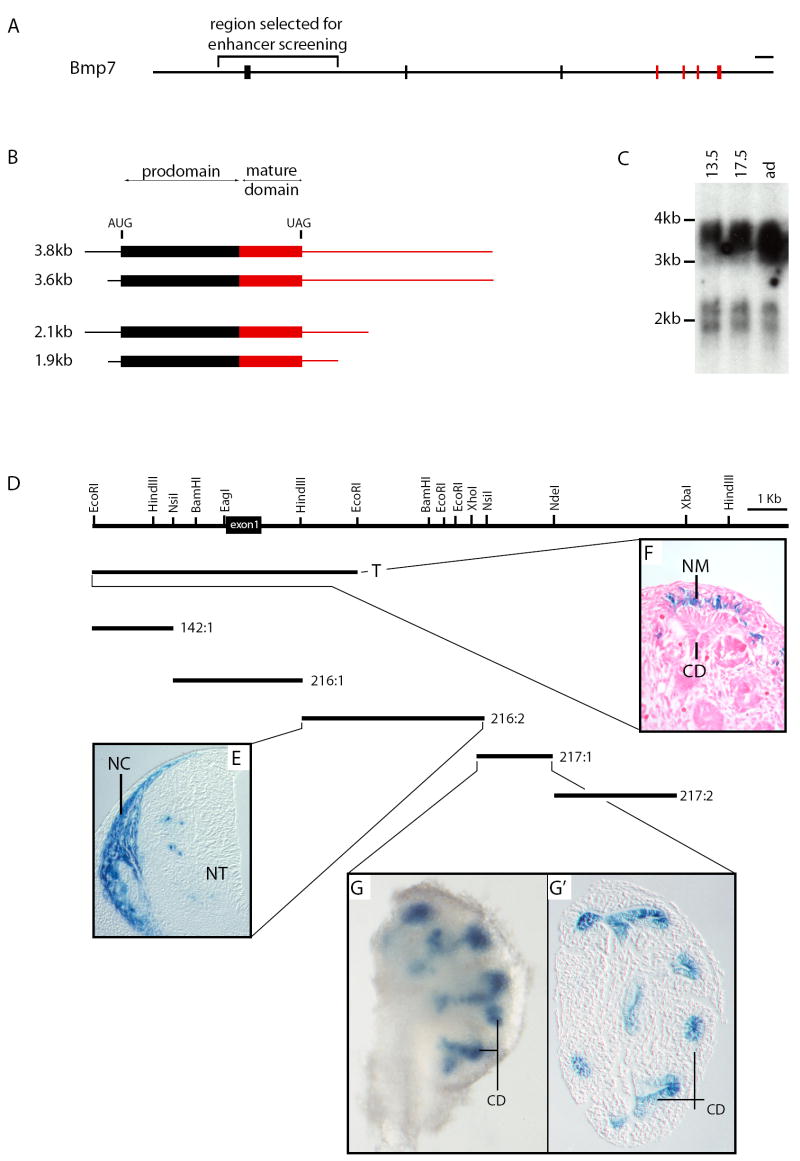
Figure 1. Genomic organization of the murine Bmp7 locus and screening strategy used to identify enhancer elements.
A The Bmp7 gene is comprised of 7 discrete exons distributed over approximately 72 kb on mouse chromosome 2. Exon-intron structure is depicted, with black boxes corresponding to exons comprising the 5’ UTR and encoding mRNA for the prodomain of BMP7 and red boxes corresponding to exons comprising the 3’ UTR and mRNA for the mature domain of BMP7. Bar at top right represents 2.5 kb. B Predicted structures of Bmp7 transcripts based on sequencing of newborn kidney cDNA and 3’ RACE of embryonic kidney mRNA: In addition to the previously published initiation site 102 bp upstream of the first AUG (1.9 and 3.6 kb transcripts), an initiation site was identified 372 bp upstream of the AUG (2.1 and 3.8 kb transcripts). Additionally, two 3’ termini were identified: one 448 bp downstream from the UAG stop codon (1.9 and 2.1 kb transcripts), and another 1887 bp downstream from the UAG (3.6 and 3.8 kb transcripts). Red and black regions correspond to red and black exons in 1A. C Total RNA was isolated from E13.5, E17.5 and adult kidneys (13.5, 17.5 and ad respectively), and subjected to Northern hybridization with a probe corresponding to the entire Bmp7 coding sequence. Four RNA species are detected; 1.9kb, 2.1kb, 3.6kb and 3.8kb. Alternate initiation and termination sites shown in 1B account for these four distinct RNA transcripts. D Strategy used to screen for enhancer elements driving Bmp7 expression in the developing kidney. The genomic region surrounding Bmp7 exon 1 (bracketed in 1A) is shown, and lines below denote sizes of genomic subclones 142:1, 216:1, 216:2, 217:1 and 217:2. The presence of enhancer elements in each of these subclones was assayed using the Hsp68lacZpA reporter in transgenic mice. Reporter constructs drive expression at diverse anatomical sites. E The 216:2 genomic subclone drives expression in neural crest (NC) adjacent to the neural tube (NT). F Expression in nephrogenic mesenchyme (NM) of the kidney is driven by the T fragment. G, G’ Expression in collecting ducts (CD) of the kidney is driven by the 217:1 fragment.
Subcloning and mutagenesis
Six individual subclones were generated from the 217:1 genomic fragment by PCR with the Platinum Hi-Fidelity PCR kit (Invitrogen) using the following oligonucleotide combinations: 5’-GAACATTCTT GCCAAACCAT TCAG-3’ and 5’-CGCTATTCTA CGGTGGAAAC AGAC-3’, 5’-TTCCTGTGTT TGGGATGC-3’ and 5’-TTTCTGCTGG TGAGATGAC-3’, 5’-TGTTTCCACC GTAGAATAGC GTC-3’ and 5’-CCATTTTGGT CTCCCAGGTA GTG-3’, 5’-TGTGTGTGTG TGTATTACCC ACCG-3’ and 5’-CAACAGTGAC AATGCTGAGA GACAG-3’, 5’-AGTATTTCTT CCCACCCCTT TCTG-3’ and 5’-AGACACCGCA GGCTGTATGT ATTAC-3’, 5’-CATCAGTAAA GCCTGGTTGG ATTC-3’ and 5’-TTCCGCAATG TCCCGAAAC-3’. An 5’-TTGGCGCGCC-3’ sequence containing an AscI site was added at the 5’ end of each forward amplification oligonucleotide for directional cloning. Similarly, a PCR fragment representing the 480 bp intron 1 enhancer was generated using the oligonucleotide combination 5’-AACATTTGTG TCGGAAGGCA TCGCG-3’ and 5’-AGCCCCCAAC CCCCCACCCC ATAGA-3’. The 217:1 genomic clone was used as template and PCR products were phosphorylated using Polynucleotide Kinase and digested with AscI (both New England Biolabs) according to the manufacturer’s instructions before ligating into AscI and SmaI linearized Hsp68LacZ plasmid. Ampicillin resistant clones were verified by restriction mapping and transgenes were purified.
For deletion mutagenesis, the intron 1 enhancer PCR fragment was polished using T4 polymerase and cloned into an EcoRV linearized pBSIIKS plasmid. Orientation of the insert was verified by restriction mapping, and a clone containing an insert that could be liberated by AscI and SmaI digestion was selected. Six individual mutants of this enhancer element were generated by PCR using the following oligonucleotides: I, 5’-AACCCTGGTG TTCGCAGAGG-3’ and 5’-TCTGGTTCCT GTACCAACAT-3’, II, 5’-CACGTTAAAC ATGTTGGTAC-3’ and 5’-ATTTCCAAAC CGGAGCCGCT-3’, III, 5’-TTGGCCGGCC CTTTGAAATA-3’ and 5’-GGATGCCATT GTTAATTTGT-3’, IV, 5’-TGGCATCCCA AACACAGGAA-3’ and 5’-TTGTTAATTT GTTCCCATGC-3’, V, 5’-TTGGCAGCCC CGGCTCCTGC-3’ and 5’-CAGCCCTCAC TCGTGCTCGG-3’, VI, 5’-CCAGAATTAA CTGCAAAGTG-3’ and 5’-TTCCCTGCGA GGAACGGAAG-3’. This strategy results in deletion of: I, 40 nt, II, 9 nt, III, 40 nt, IV, 10 nt, V, 36 nt and VI, 28 nt. The 480 nt intron 1 enhancer pBSIIKS clone was used as template in each of these reactions. PCR products were phosphorylated, ligated, transformed into competent cells and verified by restriction mapping. The inserts of selected clones were sequenced for confirmation, and AscI/SmaI cloned into the Hsp68LacZ plasmid from which transgenes were purified.
For site directed mutagenesis, The 480 nt intron 1 enhancer pBSIIKS clone was used as template to replace three base pairs within the putative FOXD3 binding sites. Platinum Hi-Fidelity polymerase was used to perform PCR using oligonucleotides 5’-ACCAATTTGT TCCCATGCAG GAG-3’ (mutated bases in bold) and 5’-AATGGCATCC CAAACACAGG AAAGG-3’. PCR products were DpnI digested to degrade template plasmid DNA according to the manufacturer’s instructions (New England Biolabs), and phosphorylated as described above. After gel purification, the PCR product was ligated and transformed into chemically competent E. coli. Mutagenesis of the putative FOXD3 binding site and integrity of the sequence were verified by sequencing. The mutagenized fragment was subcloned into the Hsp68LacZ reporter plasmid as described above.
Generation and analysis of transgenic mice
Transgenes were injected into pronuclei of fertilized mouse oocytes of either F1 CBA/DBA hybrid or SVJ mice using standard procedures (Hogan et al., 1994) and injected oocytes were transferred to oviducts of peudopregnant females of either F1 CBA/DBA hybrid or Swiss Webster strains. For transient transgenic analysis, the day of oviduct transfer was counted as day 0.5 of pregnancy. Transgenic lines were maintained on an ICR background. X-GAL staining of whole embryos was performed as previously described (Oxburgh et al., 2004). For vibratome sectioning, embryos were prefixed in 1% formaldehyde, 0.2% glutaraldehyde for 60 minutes prior to sectioning. 150μm sections were cut and fixed for an additional 30 minutes before X-GAL staining. Stained embryos and vibratome sections were photographed using a stereomicroscope.
For transient transgenic analyses, embryos were harvested for X-Gal staining, and DNA was purified from yolk-sacs using an Autogen 850 nucleic acid purification instrument according to the manufacturer’s instructions (Autogen Inc). Transgenic embryos were identified by PCR genotyping for the β-galactosidase cDNA. At least seven positive embryos were analyzed for each reporter transgene. In most cases approximately five of these embryos displayed X-Gal staining. A consensus staining pattern was established by comparison of embryos, and representative embryos were photographed. In the case of the reporter transgene containing deletion IV fifteen positive embryos were analyzed. For the transgene generated by site directed mutagenesis, seven positive embryos were analyzed.
For establishment of the transgenic strain, staining patterns of embryos derived from four individual founders were compared. Patterns were identical, but strength of staining differed significantly. The strain displaying the strongest staining was selected for analysis.
Electrophoretic mobility shift assay (EMSA)
Crude nuclear extracts were purified from entire E14.5 kidneys or E16.5 limbs by dissociation of tissue in 10mM HEPES, pH 7.9, 1.5mM MgCl2, 10mM KCl, 0.5mM DTT containing Roche Mini Protease inhibitor (added according to the manufacturer’s instructions) in a Dounce homogenizer on ice. After incubation on ice for 20 minutes, nuclei were sedimented, washed and solubilized in 10mM HEPES pH 7.0, 25% glycerol, 400mM NaCl, 1.5mM MgCl2, 0.2mM EDTA, 0.5mM DTT. 1.5pmol DNA oligonucleotide duplex of sequence: 5’-GGATGCCATT GTTAATTTGT TCCCAT-3’ was end-labeled with 32P by incubation with T4 polynucleotide kinase and γ-32P-ATP according to the manufacturer’s instructions (New England Biolabs). After purification over a Sephadex G-25 spin column, labeled oligonucleotide was brought up to 100μl. 2μl nuclear extract was preincubated for 10 minutes with either: i) water, ii) 1.5 pmol unlabeled non-specific scrambled oligonucleotide duplex, or iii) 1.5pmol unlabeled specific oligonucleotide in Promega binding buffer (Promega Corp) at room temperature. 1μl labeled oligonucleotide was added to each reaction and incubated for a further 20 minutes at room temperature. Gel-loading buffer was added and samples were loaded onto a 4% native polyacrylamide gel and separated at 350V. Gels were subsequently dried and exposed to autoradiography film.
Results
Genomic and transcriptional mapping of the Bmp7 locus
To gain a functional understanding of transcriptional regulation of the Bmp7 locus (Fig. 1A), and specifically to ascertain whether transcription might be initiated from an exon upstream of the first annotated exon in the Ensembl database (Birney et al., 2006), we isolated and sequenced cDNA clones from a newborn kidney library. In addition to the previously published initiation site 102 bp upstream of the first AUG, a second transcriptional initiation site lying a further 270 bp upstream was identified (Fig. 1B). However, no alternate first exon was identified. In addition to the previously published site 255 bp downstream of the stop codon, rapid amplification of cDNA ends (RACE) identified two alternate transcriptional termination sites located 448 bp and 1,887 bp downstream of the stop codon respectively (Fig. 1B). Consistent with this, four discrete mRNA species of 3.8, 3.6, 2.1 and 1.9 kilobases (kb) were detected by Northern blot analysis (Fig. 1C). All transcripts contain the entire coding sequence, and there is a strong bias in favor of transcription of isoforms with 1,887 bp 3’ untranslated regions both in embryonic and adult organs.
Screening for enhancer elements
Comparison of numerous loci indicates that cis acting elements regulating gene expression are generally concentrated around sites of transcriptional initiation and termination (Zhang et al., 2007). To identify elements driving developmental expression of Bmp7, we therefore analyzed the genomic region surrounding the first exon (Fig. 1A, D). Phage clones spanning a 20 kb region approximately 3 kb upstream to 17 kb downstream of exon 1 were isolated from a 129Sv genomic library and subcloned into the Hsp68lacZpA reporter construct (Sasaki and Hogan, 1996). Five reporter constructs spanning the region were generated (Fig. 1D). Additionally, the targeting construct used to generate the Bmp7 reporter mouse by replacement of exon 1 with a β-galactosidase cDNA (Godin et al., 1998) was used as a transgene. The genomic construct contains approximately 4 kb of sequence upstream and 3 kb downstream of exon 1. Notably, this reporter (designated T) contains the endogenous Bmp7 promoter.
Transient transgenic embryos were harvested at E12.5-E13.5, and X-Gal stained. Regulatory sequence surrounding exon 1 of Bmp7 governs developmental expression in neural crest (Fig. 1E), kidney (Fig. 1F, G, G’), eye, central nervous system, and limb (Fig. 2D, F), Two separate domains of Bmp7 expression are seen in the developing kidney, namely in the nephrogenic mesenchyme (Fig. 1F), and collecting ducts (Fig 1G, G’). Cis-regulatory sequence governing nephrogenic mesenchyme expression is located within reporter T but analysis of individual Hsp68lacZpA genomic reporter clones spanning this entire region failed to identify an element driving nephrogenic mesenchyme expression, suggesting that expression in this cell population is dependent on cooperation between two distant enhancer elements represented within T but not smaller subclones. Alternatively, the heterologous Hsp68 promoter may be incompatible with cis-regulatory sequence driving expression in this domain. The 2 kb intron 1 fragment designated 217:1 driving expression in limb, eye and kidney collecting ducts was chosen for further analysis as it regulates expression in the three tissues in which BMP7 signaling is essential for normal development. This 2 kb region was subcloned to generate six reporter constructs each containing approximately 700 to 1,200 bp of overlapping sequence for a further round of transient transgenic analysis (data not shown). A 754 bp fragment driving expression in limb, eye and collecting ducts of the developing kidney was isolated and designated 217XPst.
Figure 2. The intron 1 enhancer element drives expression in a significant subset of Bmp7 expression domains.

Comparison of Bmp7 expression driven from the endogenous Bmp7 locus with expression driven from the intron 1 enhancer element. Heterozygous Bmp7lacZ reporter mice (Bmp7+/lacZ) and Bmp7Pst4 embryos were dissected at E8, E9.5 and E10.5 and whole-mount X-GAL stained. A At E8, endogenous Bmp7 expression is seen in head-folds (HF), heart (HT) and notochord (NT), with an expanded domain in the caudal notochord corresponding to the position of the node (ND). B The intron 1 enhancer element drives expression mainly in a diffuse region of the tail bud (TB) surrounding the node and also weakly in the heart. C At E9.5, endogenous Bmp7 expression is seen in heart, dorsal forebrain (DFB), isthmus or midbrain-hindbrain junction (IST), optic placode (OPT), otic placode (OTC), Rathke’s pouch (RP), limb bud (LB) and Wolffian duct (WD). D The intron 1 enhancer element drives expression in subset of these domains: isthmus, limb bud and Wolffian duct. Weak expression can be seen in the heart and ectopic expression is seen in the ventral forebrain (VFB). E At E10.5, additional domains of endogenous Bmp7 expression are apparent in mesonephric tubules (MN) and ureteric bud (UB). F These domains are represented in the intron 1 enhancer, where an additional ectopic domain of expression can be seen in the dorsal root ganglion (DRG).
Developmental expression regulated by the intron 1 enhancer 217XPst
Four individual lines of transgenic mice were generated using the 217XPst:Hsp68lacZpA reporter transgene. When assayed for β-galactosidase expression at E10 and E12.5, embryos from each of the lines display similar staining patterns, with the exception of variability in signal intensity (data not shown). Representative data is shown. To make accurate comparisons between expression domains governed by the intron 1 enhancer and those governed by the endogenous Bmp7 gene, comparable X-gal stained embryos from the Bmp7lacZ reporter mouse were analyzed. Interestingly, the 754 bp intron 1 regulatory island appears to determine a significant proportion of endogenous Bmp7 expression. At E8, expression is localized diffusely in an area of the tailbud surrounding the Bmp7 expressing node (Fig. 2A, B). At E9.5, comparison of 217XPst:Hsp68lacZpA with Bmp7 reveals expression in the midbrain hindbrain junction or isthmus, Wolffian duct and limb mesenchyme (Fig. 2C, D). Weak staining can also be seen in the myocardium of the developing heart (Fig. 2C, D). Although strong expression can also be seen in the forebrain of reporter mice, it is localized to the ventral rather than the Bmp7 expressing dorsal aspect (Fig. 2C, D). This pattern is seen consistently in separate lines of 217XPst:Hsp68lacZpA reporter mice, and indicates that the Bmp7 forebrain enhancer may be only partially represented in fragment 217XPst. At E12.5, transgene expression can be seen in the collecting duct system (Fig. 3A, B), the iris (Fig. 3C, D) and the limb mesenchyme (Fig. 3E, F). Interestingly, comparison with expression of Bmp7 shows that the intron 1 enhancer drives expression in discrete sub-domains within these developing organs.
Figure 3. The intron 1 enhancer element drives expression in specific domains of the eye, limb and kidney.

A Bmp7 is expressed in both the collecting duct system (CD) and nephrogenic mesenchyme (NM) of the developing kidney B The intron 1 enhancer drives expression only in collecting ducts C In the eye, Bmp7 is expressed in the pigmented epithelium (PE) and nascent iris (IR). D The intron 1 enhancer drives expression solely in the nascent iris of the eye. E Bmp7 is expressed in the apical ectodermal ridge (AER), limb mesenchyme (LM) and precartilage condensates (PC) of the developing limb. F Intron 1 enhancer driven expression is limited to limb mesenchyme.
Identification of a 480 base pair conserved enhancer element in intron 1
It is surprising that expression of Bmp7 at so many distinct anatomical sites and phases in development is determined by a single enhancer element. To enquire whether all expression driven by this regulatory element might be determined by a single key transcription factor binding site, or whether different transcription factor binding sites govern expression at distinct locations, we next analyzed the primary sequence of the 217XPst regulatory region. Sequence comparison between 217XPst fragment and Bmp7 loci from chick, human, Xenopus tropicalis, Takifugu rubripres and Danio rerio identified a 480 bp sequence with extensive homology between mouse, chick, human and frog (Fig. 4). In contrast, this sequence was absent in fish (data not shown). Sequence homology searches of entire fish genomes with the Bmp7 intronic island also failed to reveal the presence of any significant conservation (data not shown). Thus, this regulatory island appears first in the amphibian Bmp7 homolog and is strongly conserved through evolution of vertebrates. Predicted transcription factor binding sites for OCT-1, AGL-3 and FOXD3 are located within this conserved region.
Figure 4. The Bmp7 intron 1 enhancer is strongly conserved from human to frog genomes, but is not represented in the fish.
A Alignment of homo sapiens (hsap), mus musculus (mmus), gallus gallus (ggal) and xenopus tropicalis (xtrop) Bmp7 loci identifies a highly homologous 480 bp region located within the murine intron 1 enhancer. This region is not represented in either zebrafish or fugu genomes. Three putative transcription factor binding sites conserved across all species were predicted using the Transfac database: OCT1, AGL3 and FOXD3. B The 480 bp region from mouse recapitulates expression in eye (E), limb (L) and Wolffian duct (WD) seen in 217XPst:Hsp68lacZpA at E10.5. C The 480 base pair region from X. tropicalis intron 1 drives Hsp68 reporter gene expression in embryonic eye (E) and limb (L), but not Wolffian duct (WD) at E11.5.
The identification of this conserved 480 bp island within 217XPst raises the question of whether it accounts for the β-galactosidase expression pattern seen using the 217XPst:Hsp68lacZpA reporter, and if so to what degree this regulatory island displays functional conservation through evolution. To answer these questions we generated two reporter transgenes containing either the conserved 480 bp region from the mouse or the X. tropicalis Bmp7 locus. The mouse reporter transgene directed expression in the embryonic eye, limb, Wolffian duct and forebrain comparably with 217XPst:Hsp68lacZpA, but did not direct expression in the heart (Fig. 4B). Interestingly, the X. tropicalis reporter transgene also directed expression in the same sites with the exception of the Wolffian duct (Fig. 4C). The exact localization of expression within limb mesenchyme differs between xenopus and mouse, and we thus conclude that the 480 bp element located in intron 1 is a partially conserved enhancer driving Bmp7 expression at key sites such as eye and limb mesenchyme.
Homology predictions within the conserved intronic enhancer element
To begin to ascertain the basis of transcriptional regulation by this enhancer, we identified putative transcription factor binding sites by sequence comparison. C. elegans has only a single Bmp (Savage-Dunn, 2001) in contrast to the human, with over ten closely related ligands (Reddi, 2005). Our working hypothesis is that Bmps have undergone duplication events, retaining a conserved signaling function but acquiring additional novel domains of expression. Considering that other Bmp family members are co-expressed with Bmp7 in the collecting duct system of the kidney (Bmp3), the eye (Bmp4) and limb mesenchyme (Bmp5) an interesting possibility is that the Bmp7 intronic enhancer might be partially conserved from an ancestral Bmp gene. To explore this idea we undertook exhaustive sequence alignments between the Bmp7 intronic enhancer and the entire loci of all annotated mouse Bmp genes as well as members of the growth and differentiation factor (Gdf) family that function via activation of the Smad 1, 5 and 8 effector pathway. Four regions of 90-100% homology, each spanning approximately 25 bp were identified (Fig. 5A). We also included the three putative transcription factor binding sites identified by homology searching using stringent criteria in the Transfac database. Using this rationale, we identified 6 potential transcription factor binding sites, designated I-VI.
Figure 5. Deletion mutagenesis to localize transcription factor binding site(s) driving Bmp7 expression in limb mesenchyme (LM), Wolffian Duct (WD) and isthmus (IST).
A Schematic of Bmp7 intron 1 enhancer element showing the two different strategies used to identify putative transcription factor binding sites: i) Regions of strong homology with intronic sequence in other TGFβ superfamily ligand genes (red bars), ii) Transfac-predicted binding sites conserved in all species (black marks). Reporter genes I-VI containing deletions spanning single regions were used to generate transgenic animals which were analyzed at E10.5 for expression in isthmus (IST), limb mesenchyme (LM) and Wolffian duct (WD). B – G Only deletion IV spanning the predicted FOXD3 binding site significantly affects expression in the three domains assayed.
Analysis of selected deletions within the intron 1 enhancer
To ascertain whether any one of the 6 potential transcription factor binding sites identified in silico is essential for developmental expression governed by the 480 bp Bmp7 intron 1 enhancer, we generated a series of deletion constructs in which each one of these regions was removed in isolation (Fig. 5A). Our panel of 6 Hsp68lacZ reporter deletion constructs was then tested in transgenic embryos. Embryos were dissected at E10.5 and stained for β-galactosidase expression. E10.5 was chosen for this analysis because the majority of domains of Bmp7 expression relevant to this study are evident at this time-point and whole-mount X-Gal staining is robust. Since Bmp7 expression in the eye is only initiated at or around this time-point, we focused our analysis on Wolffian duct, ureteric bud, limb and isthmus. While some variability can be seen in amplitude of expression and degree of ectopic expression between the different constructs, only the 10 bp deletion spanning the core of the predicted FOXD3 binding site abrogates expression (Fig. 5E). Thus, we conclude that the putative FOXD3 binding site within the conserved 480 bp enhancer is absolutely necessary for expression in the limb, isthmus and Wolffian duct.
Site-directed mutagenesis of the putative FOXD3 binding site
To verify that the loss of expression caused by deletion of the putative FOXD3 binding site from the intron 1 enhancer was due to abrogated transcription factor binding at this specific site rather than loss of transcription factor binding secondary to alterations in DNA structure or changes in spacing between enhancer elements, we performed site directed mutagenesis of three base pairs within the core of the putative FOXD3 binding site (Fig. 6A). Embryos transgenic for a Hsp68lacZ reporter containing the 480 bp mutated Bmp7 intron 1 sequence did not show any X-Gal staining in limb, isthmus or Wolffian duct (Fig. 6B), demonstrating that the putative FOXD3 binding site is indeed required for Bmp7 intron 1 driven expression in these tissues.
Figure 6. Site directed mutagenesis of the putative FOXD3 binding site in the intron 1 enhancer and electrophoretic mobility shift assay to verify protein binding to the site in embryonic kidney and limb nuclear lysates.
A The putative FOXD3 binding site of the Bmp7 intron 1 enhancer element (wild type) was altered by site directed mutagenesis to incorporate three mismatched base pairs (mutation). The mutant 480 bp intron 1 enhancer element was subsequently cloned into the Hsp68lacZ reporter. B Transgenic embryos generated using the mutagenized Bmp7 intron 1 reporter gene described in A show complete lack of expression in limb, isthmus and Wolffian duct. C A radiolabeled oligonucleotide duplex spanning the predicted FOXD3 binding site was used as a probe for a gel-shift assay with extracts of E16.5 limb and E14.5 kidney. Nucleotides removed in reporter IV (Fig. 5) of the mutational analysis are shown in bold. D Retardation of migration of the oligonucleotide by addition of crude nuclear extracts of embryonic limb or kidney was assayed on a 4% native gel in: i) the absence of cold competitor oligonucleotides (-), ii) the presence of a cold non-specific scrambled oligonucleotide (scr), and iii) the presence of a cold oligonucleotide identical in sequence to the probe (spec). The arrow marks probe retarded in the gel by the binding of protein from the extracts, and the arrowhead marks free probe.
Nuclear factor binding to the predicted FOXD3 binding site
To conclusively determine that nuclear proteins in the developing limb and kidney indeed do associate with the putative FOXD3 binding site in the conserved enhancer, we performed an electrophoretic mobility shift assay. A radiolabeled oligonucleotide spanning the predicted FOXD3 binding site in intron 1 (Fig. 6C) was used to assay whether crude nuclear extracts of E14.5 kidney and E16.5 limb could retard migration of this probe in a native polyacrylamide gel. To ensure specificity of nuclear factor binding, extracts were preincubated either with non-specific unlabeled oligonucleotide, or unlabeled oligonucleotide identical to the probe. As shown in Figure 6D, the probe is retarded by extracts from both embryonic kidney and limb, and this is competed by addition of unlabeled probe oligonucleotide, demonstrating that this interaction is specific. Thus, nuclear factors expressed in embryonic limb and kidney bind to the predicted FOXD3 binding site required for developmental expression of Bmp7 in these tissues. We conclude that the putative FOXD3 binding site within the conserved intron 1 enhancer element of Bmp7 directly binds one or multiple transcription factors that enhance transcription specifically in the limb and Wolffian duct of the developing embryo.
Discussion
Bmp7 is expressed in numerous tissues throughout development and is required for morphogenesis of the eye, hindlimb and kidney (Dudley et al., 1995; Godin et al., 1998). In this study we have shown that the majority if not all of the cis-regulatory sequence regulating Bmp7 expression at these anatomical sites is contained within approximately 20 kb of genomic sequence surrounding exon 1. In each of these organs, multiple distinct enhancer elements drive Bmp7 expression. In the eye, we show that elements driving expression in the pigmented epithelium and iris are spatially separated. In the kidney, Bmp7 expression in collecting ducts and nephron progenitors is driven by separate enhancer elements. Similarly in the limb, mesenchyme and apical ectodermal ridge (AER) expression are governed by separate elements. Although enhancers for pigmented epithelium, nephrogenic mesenchyme and AER are distributed across approximately 20 kb, an element of approximately 480 bp within intron 1 governs expression within the developing iris, kidney collecting duct system and limb mesenchyme.
Partial functional conservation can be demonstrated between the X. tropicalis and M. musculus sequences, with the X. tropicalis enhancer driving expression in limb and eye only, but not Wolffian duct. This cis-acting element is conserved in sequence and position within Bmp7 loci of vertebrates from amphibian to human. Thus, there is strong selective pressure for Bmp7 expression at one or several of these tissue sites. Interestingly, this element is not present in the Bmp7 genes of more ancestral vertebrates such as Danio rerio and Takifugu rubripes. This new feature of the Bmp7 gene probably arose prior to divergence of amphibians and amniotes, possibly during the adaptation from aquatic to terrestrial life.
In the mouse eye, Bmp7 regulates lens placode formation (Wawersik et al., 1999) and optic fissure development (Morcillo et al., 2006). Transgenic overexpression studies of the BMP antagonist noggin demonstrate that BMP signaling plays a profound role in development of the ciliary body at the iridocorneal junction (Zhao et al., 2002). The role of specific BMPs in this aspect of development is currently unknown, but considering the expression of Bmp7 in the developing iris, and the potential role of this growth factor in iris smooth muscle development (Jensen, 2005), Bmp7 expression driven by the intron 1 enhancer may be involved in ciliary body development. However, the anatomical similarities between the teleost and mammalian eye (McMahon et al., 2004), suggest that addition of a Bmp7 expression domain in the developing iris are unlikely to account for selective pressure underlying addition of the intron 1 enhancer to the Bmp7 gene.
Development of the vertebrate kidney requires a series of reciprocal inductive interactions between collecting ducts and nephrogenic mesenchyme, or nephron progenitors. Inactivation of Bmp7 results in depletion of nephron progenitor cells, and causes premature arrest of kidney development (Dudley et al., 1999; Dudley et al., 1995; Oxburgh et al., 2004). Additionally, in vitro studies have shown that BMPs act to limit growth and branching of the collecting duct system (Bush et al., 2004). Bmp7 is expressed in both nephrogenic mesenchyme and collecting ducts (Dudley and Robertson, 1997) although the relative importance of these distinct cell populations as sources of BMP7 is presently unclear. Interestingly, in this analysis we show that the evolutionarily most ancient intron 1 enhancer derived from Xenopus tropicalis does not drive expression in the Wolffian duct, the epithelial tubule in the intermediate mesoderm that gives rise to the permanent kidney. Thus, addition of the intron 1 enhancer cannot simply be explained by selective pressures modifying Bmp7 expression domains in the kidney collecting duct.
Polydactyly is seen in approximately 65% of Bmp7 null embryos (Dudley et al., 1995). Studies of compound mutants demonstrate that Bmp7 cooperates with Bmp4 to regulate digit patterning (Katagiri et al., 1998). Incomplete penetrance of the polydactyly phenotype is probably due to genetic redundancy. Conditional inactivation of Bmp4 in limb mesenchyme similarly results in polydactyly due to delayed induction of the apical ectodermal ridge (AER) and increased Sonic Hedgehog (Shh) signaling from the zone of polarizing activity (ZPA) (Selever et al., 2004). BMP signals from limb mesenchyme thus act indirectly to restrict the range of Shh secreted from the ZPA and limit the formation of anterior digits. We show here that the Bmp7 intron 1 enhancer element drives expression strongly in limb mesenchyme, and thus is likely to regulate the digit patterning activity of Bmp7 essential to normal development. Interestingly, molecular and morphological studies fail to identify a structure homologous to the tetrapod autopod in the teleost (Ahlberg and Milner, 1994; Sordino et al., 1995). The fossil record shows that digit patterning was a feature of early tetrapods such as Acanthostega and Icthyostega, but that the number of digits per autopod was eight (Ahlberg and Milner, 1994). The digit number of the autopod was subsequently reduced to five in the common ancestor of amphibians and amniotes. An intriguing possibility is that adaptation of the autopod from an octa- to pentadactyl morphology more suitable for terrestrial life selected for a novel Bmp7 expression domain in limb mesenchyme driven by the intron 1 enhancer.
The transcriptional network driving Bmp7 expression is poorly understood. Through mutational analysis we have identified a FOXD3 transcription factor binding site essential for expression in kidney collecting duct and limb mesenchyme. The likelihood that FOXD3 is responsible for activating Bmp7 in these developing tissues is small since it is expressed exclusively in the neural crest (Dottori et al., 2001). At present we have not identified alternate candidate factors. However, comparison of expression patterns governed by intron 1 enhancer elements in different species may yield important clues in answering this question. For example, in this study we found that the X. tropicalis enhancer drives expression only in forebrain and limb whereas the mouse enhancer drives expression in the Wolffian duct in addition to these locations. Paradoxically, the latter expression patterns appear to be driven by a single FOXD3 transcription factor binding site posing the question as to how the FOXD3 binding site derived from the X. tropicalis enhancer differs in transcriptional activation from the mouse enhancer when placed into the context of the mouse genome. Sequence comparison in this region shows that these species differ by two base pairs indicating that transcription factor binding may differ between them. Close examination of the pattern of limb mesenchyme expression driven by the X. tropicalis enhancer reveals that the expression domain only partially overlaps with that of the mouse. Thus the differences in expression between mouse and xenopus intron 1 enhancers may be explained by the fact that multiple FOXD-like transcription factors with subtly different binding specificities associate with this cis-regulatory sequence in limb mesenchyme and Wolffian duct. Future comparative studies of these Bmp7 enhancer elements will address the identities of these factors, expanding our understanding of the transcriptional circuitry regulating developmental expression of Bmp7.
Supplementary Material
Acknowledgments
Many thanks to the Mouse Transgenic and MRI Core Facility at Maine Medical Center Research Institute for pronuclear injections and to the Bioinformatics and Genomics Core for DNA purification. Thanks to Dr. Andrew T. Dudley for help in isolating the phage clone used in this study and Dr. Elizabeth Bikoff for helpful comments on the manuscript. The project described was supported by Grant Number P20 RR18789 to L. O. from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and R01 HD34435 to E. J. R. from the NIH. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlberg PE, Milner AR. The origin and early diversification of tetrapods. Nature. 1994;368:507–514. [Google Scholar]
- Birney E, et al. Ensembl 2006. Nucleic Acids Res. 2006;34:D556–61. doi: 10.1093/nar/gkj133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush KT, et al. TGF-[beta] superfamily members modulate growth, branching, shaping, and patterning of the ureteric bud. Developmental Biology. 2004;266:285–298. doi: 10.1016/j.ydbio.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Dottori M, et al. The winged-helix transcription factor Foxd3 suppresses interneuron differentiation and promotes neural crest cell fate. Development. 2001;128:4127–38. doi: 10.1242/dev.128.21.4127. [DOI] [PubMed] [Google Scholar]
- Dudley AT, et al. Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme. Genes Dev. 1999;13:1601–13. doi: 10.1101/gad.13.12.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley AT, et al. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–2807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Robertson EJ. Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev Dyn. 1997;208:349–362. doi: 10.1002/(SICI)1097-0177(199703)208:3<349::AID-AJA6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Godin RE, et al. Regulation of BMP7 expression during kidney development. Development. 1998;125:3473–82. doi: 10.1242/dev.125.17.3473. [DOI] [PubMed] [Google Scholar]
- Hogan BL, et al. Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1994. [Google Scholar]
- Hogan BLM. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Jensen AM. Potential roles for BMP and Pax genes in the development of iris smooth muscle. Dev Dyn. 2005;232:385–92. doi: 10.1002/dvdy.20224. [DOI] [PubMed] [Google Scholar]
- Katagiri T, et al. Skeletal abnormalities in doubly heterozygous Bmp4 and Bmp7 mice. Dev Genet. 1998;22:340–8. doi: 10.1002/(SICI)1520-6408(1998)22:4<340::AID-DVG4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Kim RY, et al. Bmp6 and Bmp7 are required for cushion formation and septation in the developing mouse heart. Dev Biol. 2001;235:449–66. doi: 10.1006/dbio.2001.0284. [DOI] [PubMed] [Google Scholar]
- Kingsley DM, et al. The mouse short ear skeletal morphogenesis locus is associated with defects in a bone morphogenetic member of the TGFβ Superfamily. Cell. 1992;71:399–410. doi: 10.1016/0092-8674(92)90510-j. [DOI] [PubMed] [Google Scholar]
- Massagué J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–91. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Massagué J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. Embo J. 2000;19:1745–54. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon C, et al. Using zebrafish to study the complex genetics of glaucoma. Comp Biochem Physiol C Toxicol Pharmacol. 2004;138:343–50. doi: 10.1016/j.cca.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Morcillo J, et al. Proper patterning of the optic fissure requires the sequential activity of BMP7 and SHH. Development. 2006;133:3179–90. doi: 10.1242/dev.02493. [DOI] [PubMed] [Google Scholar]
- Oxburgh L, et al. TGFb superfamily signals are required for morphogenesis of the kidney mesenchyme progenitor population. Development. 2004;131:4593–4605. doi: 10.1242/dev.01324. [DOI] [PubMed] [Google Scholar]
- Oxburgh L, et al. BMP4 substitutes for loss of BMP7 during kidney development. Dev Biol. 2005;286:637–46. doi: 10.1016/j.ydbio.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Reddi AH. BMPs: from bone morphogenetic proteins to body morphogenetic proteins. Cytokine Growth Factor Rev. 2005;16:249–50. doi: 10.1016/j.cytogfr.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Sambrook J, et al. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sasaki H, Hogan BL. Enhancer analysis of the mouse HNF-3 beta gene regulatory elements for node/notochord and floor plate are independent and consist of multiple sub-elements. Genes Cells. 1996;1:59–72. doi: 10.1046/j.1365-2443.1996.04004.x. [DOI] [PubMed] [Google Scholar]
- Savage-Dunn C. Targets of TGF beta-related signaling in Caenorhabditis elegans. Cytokine Growth Factor Rev. 2001;12:305–12. doi: 10.1016/s1359-6101(01)00015-6. [DOI] [PubMed] [Google Scholar]
- Selever J, et al. Bmp4 in limb bud mesoderm regulates digit pattern by controlling AER development. Dev Biol. 2004;276:268–79. doi: 10.1016/j.ydbio.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Solloway MJ, et al. Mice lacking Bmp6 function. Dev Genet. 1998;22:321–39. doi: 10.1002/(SICI)1520-6408(1998)22:4<321::AID-DVG3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Solloway MJ, Robertson EJ. Early embryonic lethality in Bmp5;Bmp7 double mutant mice suggests functional redundancy within the 60A subgroup. Development. 1999;126:1753–68. doi: 10.1242/dev.126.8.1753. [DOI] [PubMed] [Google Scholar]
- Sordino P, et al. Hox gene expression in teleost fins and the origin of vertebrate digits. Nature. 1995;375:678–81. doi: 10.1038/375678a0. [DOI] [PubMed] [Google Scholar]
- Wawersik S, et al. BMP7 acts in murine lens placode development. Dev Biol. 1999;207:176–88. doi: 10.1006/dbio.1998.9153. [DOI] [PubMed] [Google Scholar]
- Zhang ZD, et al. Statistical analysis of the genomic distribution and correlation of regulatory elements in the ENCODE regions. Genome Res. 2007;17:787–97. doi: 10.1101/gr.5573107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, et al. BMP signaling is required for development of the ciliary body. Development. 2002;129:4435–42. doi: 10.1242/dev.129.19.4435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.