Abstract
Because host–parasite interactions are often specific to the host and parasite genotype, it may be important whether a host reproduces by selfing or outcrossing. The latter is associated with higher genetic diversity among the offspring and may reduce parasite success. Here, we test whether outbred offspring of Daphnia magna have an advantage over selfed offspring in the presence of a parasite transmitted from mothers to offspring. Using outdoor mesocosms, we set up monoclonal and polyclonal host populations of D. magna infected with a prevalence of 100% with the horizontally and vertically transmitted microsporidian parasite Octosporea bayeri. These populations diapaused after sexual reproduction and hatchlings were screened for signs of O. bayeri. Parasite prevalence was 98.9% for hatchlings from the monoclonal treatment, but only 85.2% among the hatchlings from the polyclonal populations, indicating a short-term benefit for outbreeding. This benefit occurs, we hypothesize, not owing to inbreeding depression, but because the vertically transmitted parasite is less able to establish itself in the relatively new genetic environment of the outbred offspring, as compared to the more stable environment when transmitted to selfed offspring. To quantify the fitness consequences of this 14% prevalence difference, we studied the within-season epidemiology of O. bayeri, using an epidemiological model. We then examined whether descendants of outbred offspring produce more resting eggs than the descendants of selfed offspring. The data and our model show that Daphnia which are uninfected at the beginning of the growth season have a large advantage when the entire season is considered. Our data support the Red Queen hypothesis which states that in the presence of coevolving parasites, outbreeding is favoured in the host.
Keywords: sexual reproduction, selfing, outbreeding, vertical transmission, Red Queen, coevolution
1. Introduction
The success of a parasite genotype often depends on the genotype of its host. Reciprocal cross-infection experiments with parasites and hosts from different populations often reveal strong host–parasite interactions (Parker 1985; Ebert 1994; Kaltz et al. 1999; Lively et al. 2004). Within-population host–parasite interactions may also be specific, down to the particular genotypes of hosts and parasites (Carius et al. 2001; Schulenburg & Ewbank 2004). This has implications for the spread of infectious diseases and for the evolution of both antagonists. Among the more prominent ideas is the suggestion that sexual reproduction may be beneficial owing to its role in the generation of new host and parasite variants (Jaenike 1978; Hamilton 1980; Hamilton et al. 1990). Increased genetic variation among outbred hosts may help hosts cope with ever-coevolving parasites. Empirical work was generally supportive of this hypothesis, but only few examples exist for direct short-term advantages of genetic diversity in relation to parasites (Shykoff & Schmid-Hempel 1991; Baer & Schmid-Hempel 1999; Lively & Dybdahl 2000).
In subdivided populations with local extinctions and colonizations, genetic bottlenecks can be frequent, leading to low local genetic diversity and inbreeding of entire populations. This may have consequences for host–parasite interactions in two forms. First, inbreeding depression may negatively affect the function of genes that help provide an immune response against the parasite. Inbreeding has been associated with increased disease incidence or virulence (O'Brien & Evermann 1988; Keller et al. 1994; Coltman et al. 1999; Hedrick et al. 2001). Second, parasites have been reported to adapt to the local host gene pool (Lively 1989; Ebert 1994; Mopper et al. 1995; Morand et al. 1996), an effect that is stronger in populations with low genetic diversity (Gandon & Michalakis 2002) and may be even stronger in host monocultures (Barrett 1981; Holguin & Bashan 1992). This effect may be most extreme in cases of host genotype-specific adaptation by parasites with vertical transmission, as these parasites form the most intimate associations with their hosts. Such specific adaptation may be broken when the parasite comes in contact with novel host genotypes (Ebert 1998).
In the D. magna metapopulation described here, the local populations are often highly inbred, although occasional immigrants give rise to outbred offspring. Here, we study the effect of out- and inbreeding on the success of offspring in relation to a vertically and horizontally transmitted microsporidian parasite.
1.1 The study system
We study a metapopulation of the freshwater crustacean D. magna Strauss from rock pool habitats in southwest Finland. Daphnia magna is the largest European Daphnia species, with an adult size up to 5 mm (length at maturity approximately 1.8–2.2 mm, generation time 8–16 days at 20°C). It typically inhabits small- to medium-sized rock pools along the Baltic Sea. Besides their patchy distribution, the rock pool habitat is characterized by an inherent instability: they frequently dry up, and are then suddenly invaded by brackish water from the Baltic Sea or rainwater. Rock pools freeze solid in winter such that no Daphnia can survive, except in form of resting eggs. Daphnia reproduce asexually during most of the warmer season, whereas sexual reproduction produces resting stages to endure the harsh winter conditions. The resting stages also serve for short- and long-distance dispersal. The length of an asexual generation (hatching to first reproduction) is 10–20 days, and there are typically about 8–12 generations during the five-month season in our study area.
Daphnia rock pool populations form a metapopulation system with discrete habitat patches and frequent extinctions and colonizations (Hanski & Ranta 1983; Pajunen 1986; Ebert et al. 2001). In our study area, the average proportion of pools containing D. magna populations in a given year is approximately 20%, and the average yearly extinction probability is approximately 17%. The number of extinctions is on average balanced by the number of new colonizations (Pajunen 1986; Pajunen & Pajunen 2003). The dynamic nature of this metapopulation has strong consequences for the genetic population structure. Pools are usually colonized by one or few founder females, followed by clonal expansion. When there is one founder clone, resting egg production requires selfing, leading to an inbreeding coefficient F of 0.5, i.e. 50% of the previously heterozygous loci will be homozygote in the selfed offspring. Therefore, most rock pool populations are inbred, and the outbred offspring of rare immigrants enjoy a strong selective advantage (equal to hybrid vigour; Ebert et al. 2002; Haag et al. 2005).
Daphnia species in rock pools are often parasitized with a number of different endoparasites (e.g. bacteria, microsporidia and protozoa) and epibionts (e.g. fungi, algae and peritrich ciliates; Green 1957, 1974; Bengtsson & Ebert 1998; Ebert et al. 2001), most of which are Daphnia specific or even species specific. Many of these parasites cause a considerable reduction in host fecundity and survival (Stirnadel & Ebert 1997; Decaestecker et al. 2005). Of particular interest in our metapopulation is the microsporidian parasite Octosporea bayeri, which occurs in approximately 45% of all populations, or 80%, if one excludes newly founded (young) populations (Ebert et al. 2001). Octosporea is specific to D. magna. It is vertically (transovarial) transmitted to the parthenogenetic offspring with 100% fidelity, but can also be horizontally transmitted through water-borne spores released from decaying dead hosts (Vizoso et al. 2005). This possibility for horizontal transmission may explain why the parasite can persist, despite reducing host fecundity by 10–40% and reducing host survival (Vizoso et al. 2005). In spring, its prevalence varies widely across populations, while in autumn its prevalence is often close to 100% in all populations owing to horizontal transmission (Lass & Ebert 2006). Transovarial transmission also occurs through the resting egg, but transmission fidelity is reduced in these cases (Vizoso et al. 2005). In this paper, we explore whether imperfect transmission of Octosporea to sexual offspring leads to an outbreeding advantage in highly inbred D. magna populations. We quantify the fitness advantage of reduced transmission through outbred eggs with the help of an epidemiological model.
2. Material and methods
2.1 Vertical transmission during asexual reproduction
To quantify how efficiently O. bayeri vertically transmits to asexual offspring of D. magna, we collected infected hosts from 19 rock pools and cloned them in the laboratory (isofemale lines). From each clone, we took four female offspring shortly after birth, totalling 76 females, and kept them individually in jars filled with 90 ml Daphnia medium (modified after Klüttgen et al. 1994). Animals were kept at 20°C and fed three times a week with five million cells of the green algae Scenedesmus sp. All offspring from the first three clutches of each of these females (19 clones×4 replicates) were kept separately from their mother but under the same conditions. Thus, horizontal transmission was excluded. At an age of 10 days, these offspring were checked for infections using squash preparation and phase contrast microscopy (400× magnification). The spores of O. bayeri are easily visible in infected hosts. The infection status of the mothers was also confirmed in this way.
2.2 Vertical transmission during sexual reproduction
In spring 2004, we collected 55 naturally infected D. magna females from 21 natural rock pool populations. Females were cloned (isofemale lines) in the laboratory and then propagated by placing approximately 50 individuals in buckets in the field. Each bucket contained 6 l rock pool water. All these clones were infected, and therefore each bucket population had a prevalence of 100%. Daphnia from these bucket cultures were then used to set up 22 populations in 40 l mesocosms filled with 30 l filtered (30 μm) rock pool water. We further added 0.5 l of water from the Baltic Sea to compensate for dilution by incoming rainwater. In 11 of these containers, we set up monoclonal treatments consisting of 200 females of a single clone, with a different clone in each container. In the other 11 containers, we put 50 females from four clones each (polyclonal treatment, 11 times four clones in total). Within each treatment group all clones were different, but clones used in the monoclonal treatment were also used in the polyclonal treatment. Within each polyclonal population, all four clones originated from different rock pool populations. While in the monoclonal populations, all sexual eggs were the result of selfing; in the polyclonal populations, most sexual eggs resulted from outcrossing. At this stage, all individuals in all mesocosms were infected with O. bayeri (prevalence 100%), and none carried a resting egg. All containers were randomized and blind coded. In early October, we lowered the water level to 10 cm without removing the resting eggs, covered the containers (to keep them from filling with snow) and kept them outdoors to outlive the Finnish winter. On 29 April 2005, we opened all containers and allowed the Daphnia to hatch naturally from the resting eggs. There were no surviving Daphnia from the previous summer. Every 2–4 days, we collected all hatchlings and brought them to the laboratory where we kept them at room temperature for at least 10 days in fresh medium under good conditions to avoid mortality and gave them plenty of food (green algae Scenedesmus sp.). We collected more than 50 hatchlings in all but three replicates. We then tested the first 50 hatchlings from each replicate population for infections. The spores of the parasite can usually be seen as early as 6 days after hatching using squash preparation and phase contrast microscopy (200–400× magnification). Since hatchlings were only briefly exposed to the parasite, their infection resulted from vertical transmission from the infected mother to the resting egg with near certainty, rather than from horizontal transmission after hatching. For each mesocosm, we calculated the percentage of uninfected hatchlings among all hatchlings tested for infection.
2.3 Within-season infection dynamics
Following the protocol of Lass & Ebert (2006), we monitored the infection dynamics in five natural rock pool populations from the moment of first hatching in late April 2005 to the end of the summer season. These pools had been part of an earlier epidemiological study reported in that study. In this case, we took monthly samples of approximately 50 D. magna from these five populations, brought them to the laboratory and kept them under good conditions at room temperature, feeding them plenty of food (green algae Scenedesmus- sp.) for at least 8 days before checking each individual for spores of O. bayeri. Animals that died earlier were immediately removed to prevent horizontal transmission and tested for spores. We calculated prevalence as the percentage of infected hosts in the sample for each sampling date and pool.
3. Results
3.1 Vertical transmission during asexual reproduction
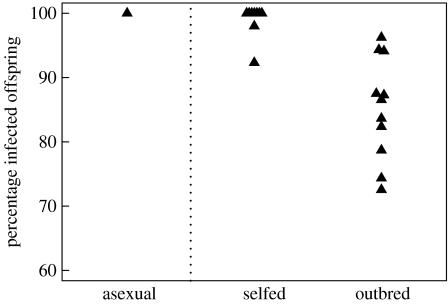
Each of the 336 asexual offspring from infected females from 19 D. magna clones was infected (figure 1), confirming an earlier smaller experiment on vertical transmission of O. bayeri into asexual host offspring (Vizoso et al. 2005).
Figure 1.
Percentage of D. magna offspring infected with the microsporidian parasite O. bayeri from infected mothers who reproduced by parthenogenesis (asexual), selfing (monoclonal populations) or mostly outcrossing (polyclonal populations). Note, the selfing and outbreeding treatment are part of the same mesocosm field experiment, while the asexual treatment was done under different conditions in the laboratory.
3.2 Vertical transmission during sexual reproduction
The number of hatchlings recovered in the mesocosms was between 0 and 299. In one monoclonal mesocosm, no hatchling was found and in three further monoclonal mesocosms, only 6, 13 and 19 individuals were caught. In all other populations, a minimum of 45 females were analysed. We found more hatchlings in the outbreeding (polyclonal) than in the selfing (monoclonal) treatment (2819 overall versus 1786), but this difference was not significant (Wilcoxon rank sum test: p=0.22). The average parasite prevalence among the selfed hatchlings (monoclonal mesocosm) was 98.9% (in total, 5 uninfected out of 364 tested individuals), with only two populations having less than 100% prevalence (92.3 and 98%). In contrast, uninfected hatchlings were found in all 11, mostly outbreeding populations (146 uninfected among 1006 tested individuals; the larger number of animals results from two populations in which all hatchlings were tested). The average proportion of infected offspring in polyclonal mesocosms was significantly lower than in the monoclonal populations (85.2 versus 98.9%; Kruskal–Wallis test: Χ12=13.03, p<0.0003; figure 1).
The fitness consequences of this reduced prevalence for the mostly outbred offspring are difficult to assess, therefore we will explore the epidemiological consequences below with the help of observational data and an epidemiological model.
3.3 Within-season infection dynamics
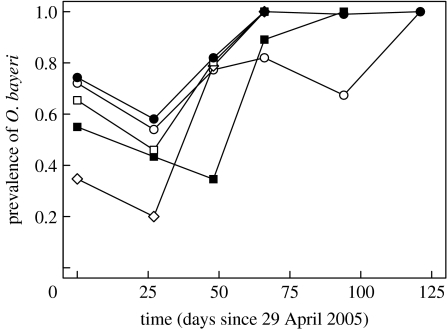
The five rock pool populations varied in their spring prevalence, ranging from 35 to 75%. We observed a reduction in prevalence in all five populations in the first one to two months, followed by an increase, eventually leading to 100% prevalence in all populations (figure 2).
Figure 2.
Dynamics of prevalence of the microsporidium O. bayeri in five natural rock pool populations of D. magna over one summer season.
3.4 An epidemiological model
Below, we introduce an epidemiological model for the within-season dynamics of O. bayeri, which allows us to ask how the parasite influences the success of competitors with different starting prevalences across the asexual growth season. First, we largely follow the model by Lipsitch et al. (1995) to model the within-season dynamics of a parasite with perfect vertical and some horizontal transmission through free-living spores in an asexual host population. The infection dynamics are described by the following three equations:
Xi and Yi are the uninfected and infected hosts; and S is the number of free parasite spores. The subscript i differentiates between two host types (competitors) 1 and 2. Hosts are born with a rate b if uninfected and bf if infected, with f ranging from 0 to 1, indicating the degree to which infected hosts have a reduced fecundity. Birth rate is density dependent with N being the total host population size and K the carrying capacity. Hosts die with rate μ for reasons unrelated to parasitism and with rate α as a consequence of being infected. Healthy hosts may become infected by contact with free spores, S, following the mass-action assumption with a rate β. The mass-action assumption has been shown to satisfy transmission by free spores in Daphnia–parasite systems (Regoes et al. 2003). Free spores are produced when infected hosts die. We scale S to be equal to the amount of spores released from one dead infected host. Spores die with rate g.
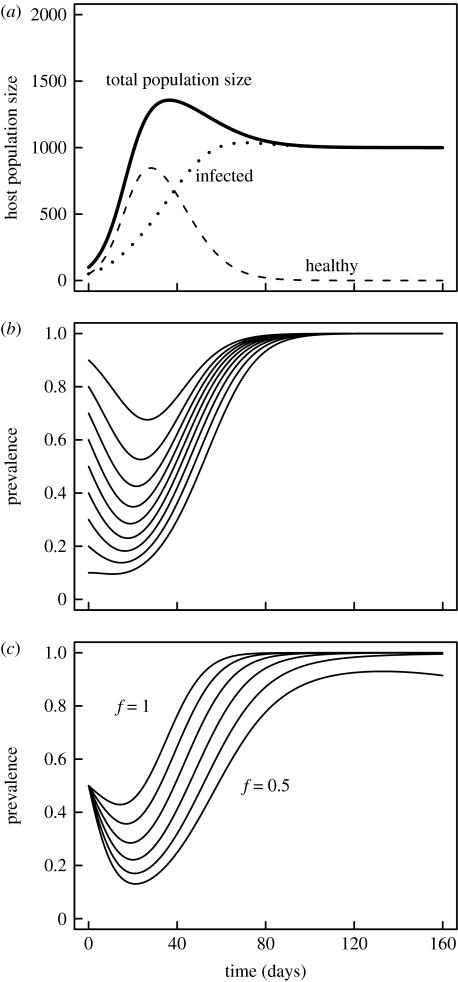
For one host type (i=1), the model tracks the course of parasite spread during one host season (figure 3a). At the beginning of a season, hosts are born with a certain likelihood of being infected. As population size in early spring is usually far below the carrying capacity K, the population grows exponentially. Initially, horizontal transmission is rare, as X and S are small. Therefore, the parasite prevalence declines due to the lower fecundity of infected hosts (figure 3a,b). With increasing X and higher S, more and more hosts become infected and prevalence eventually rises to 100% (figure 3b). Prevalence fails to reach 100% only when the parasites have very strong effects on host fecundity (f<0.6 in figure 3c).
Figure 3.
Dynamics of host and parasite in the epidemiological model for one host type. (a) Dynamics of total, infected and healthy host population over one summer season. (b) Dynamics of prevalence for different starting prevalences (from 10 to 90%) in the early season. (c) Dynamics of prevalence for different levels of parasite-induced host fecundity reduction from f=0.5 (infected hosts have only 50% fecundity relative to healthy hosts) to f=1.0 (no effect on fecundity). Other parameter settings used: Xinit=50, Yinit=50, Sinit=10, K=2000, b=0.25, f=0.8, μ=0.05, α=0.05, g=0.05, β=0.0001.
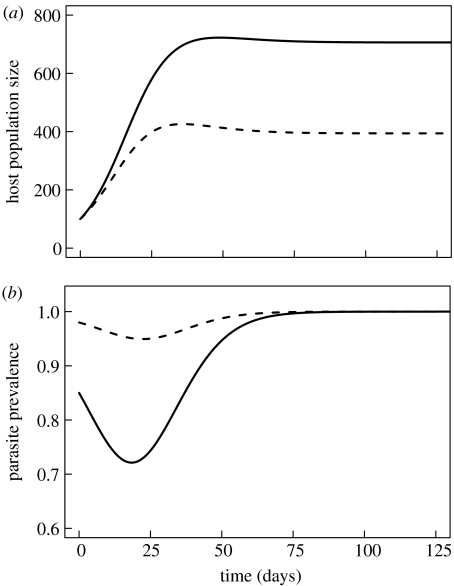
In the next step, we allow two host types to compete (i=1 and 2). The two host types differ only in their initial early spring prevalence. Starting with the same initial population size, the two types diverge quickly so that the type that was initially less infected reaches a larger population size than the type that was initially more infected (figure 4).
Figure 4.
Population size of two competing host types with an initial difference in their prevalence of infection. The solid lines indicate the population with 85% prevalence at the start of the season. The stippled line shows a competing host type with 98% prevalence. Parameter settings used as in figure 3, except: X1,init=15, Y1,init=85, X2,init=2, Y2,init=98.
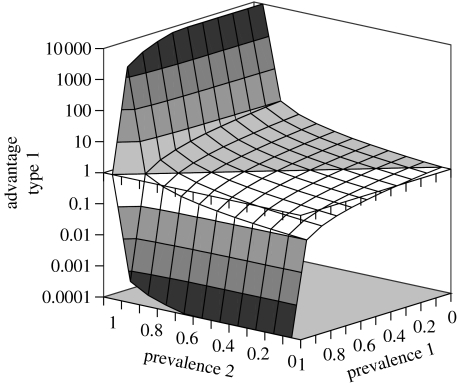
In Daphnia populations with an obligate resting stage, as is the case for the rock pool metapopulation considered here, the number of resting stages (equal to ephippia) produced over the summer season provides a good measure of the success of a certain strategy, as it determines the likelihood of surviving the harsh winter and determines the relative abundance at the start of the next season. If we assume that the ratio of population sizes of the two host types towards mid- or end season is a good predictor of their contribution to the resting egg bank (ephippia are usually produced in mid summer and towards end of season), we can estimate the relative seasonal success of one competitor relative to the other. Figure 5 shows the numerical ratio of two competitors with equal population size at the start of the season depending on their starting prevalence. If both types have the same starting prevalence, no difference is visible. However, a competitor with a lower initial prevalence has advantages in every case; this advantage is highest when the other competitor has an initial prevalence near 100%. These results are largely independent of the relative starting frequencies of the two competitors (the outbred genotypes may be initially rare), as long as the total starting density is kept constant.
Figure 5.
The advantage of host type 1 over type 2 in relation to their prevalence at the beginning of the season. Parameter settings as in figure 3, except for the initial values of X and Y of the two host types.
4. Discussion
4.1 Mechanism for reduced vertical transmission during outbreeding
Our experiment demonstrates that a microsporidian parasite shows reduced vertical transmission success through resting eggs when the host mostly outbreeds as compared to selfing or reproducing asexually (figure 1). This effect was highly consistent across replicated mesocosm populations, which developed under largely natural conditions. The treatment difference of 13.7% is likely to be an underestimate of the true difference between inbred and outbred offspring, as some proportion of the matings in the polyclonal populations certainly occurred by selfing. Assuming random mating and no clonal selection before mating, 25% of all matings would be selfing events. In this case, the proportion of infected truly outbred offspring would be 80.6%.
Two factors may explain this result. First, selfed hosts suffer from inbreeding depression, which may manifest itself in more severe disease (Keller et al. 1994; Núñez-Farfán et al. 1996; Coltman et al. 1999). In two studies of hosts and parasites from the same metapopulation, we failed to find a general effect of inbreeding on O. bayeri success (Haag et al. 2003; Salathe & Ebert 2003): there was no difference in disease incidence or virulence in comparing D. magna genotypes with different degrees of inbreeding. Therefore, we tend to believe that inbreeding depression does not explain the effect described in this study. In all of the above studies the parasite was not specifically adapted to the host genotypes used.
An alternative hypothesis is that the parasite adapted to specific host genotypes. During vertical transmission in asexually reproducing D. magna clones, O. bayeri may adapt to a specific host genotype and may thus weaken its ability to infect other genotypes. During sexual reproduction of the host, the parasite will transmit vertically to a partially altered host genotype in the offspring. This change is much stronger in outbred than in selfed offspring. In the latter case, no new alleles will be present in the selfed offspring, whereas up to 50% of the alleles will be new in outbred offspring, for this particular parasite line. It has been suggested that parasites that had adapted to the mother genotype might lose part of this specific adaptation in outbred offspring (Rice 1983). This idea is consistent with our earlier finding that O. bayeri adapts locally to its D. magna hosts in the same metapopulation (Altermatt et al. in press) and with local adaptation of a different vertical transmitted microsporidian parasite in a different crustacean host (Hatcher et al. 2005).
In our experiment, the chance that a resistance allele is present in a population is four times higher in the polyclonal treatment than in the monoclonal treatment. This could influence the mean resistance in the offspring. However, these alleles would also be found occasionally in a monoclonal population (with an allele frequency of 50%) and would therefore strongly influence the mean resistance of this population much more than it would influence resistance in polyclonal populations, where the frequency of a resistance allele would be much lower. The distribution of prevalence estimates between the two treatments (figure 1) is therefore not consistent with this idea. Selection acting on this resistance allele would not change this conclusion.
The benefits of outbred versus inbred offspring have two implications for the evolution of this system: one in the context of the evolution of outbreeding and the other in the context of the evolutionary epidemiology and hybrid vigour in metapopulations with a high degree of local inbreeding.
4.2 The evolution of outbreeding
In the absence of paternal investment, sexual reproduction has a twofold cost over asexual reproduction (Maynard Smith 1978). Selfing reduces this cost to some degree (Lloyd 1980) and thus would be favoured over outbreeding, everything else being equal. It has been suggested that the main advantages of outbreeding over selfing are related to the costs of inbreeding depression associated with selfing (Charlesworth & Charlesworth 1987). Our study adds a further benefit to outbreeding over selfing: escape from lineage-specific vertically transmitted parasites.
Parasites, which are frequently vertically transmitted, have been thought to play a role in the evolution of sex because the effect of parasite adaptation to the mother genotype is strongest in vertical transmission (Rice 1983; Agrawal 2006). This version of the Red Queen hypothesis has not found much empirical support, as vertically transmitted parasites are often avirulent, or have developed sophisticated ways to ensure their persistence in the host lineage (Hurst 1993). Octosporea bayeri is quite virulent (Vizoso & Ebert 2005), most probably owing to its mixed mode of transmission: infected hosts produce large quantities of spores for horizontal transmission (Ebert 2005). Agrawal (2006) has recently shown that parasites with mixed modes of transmission favour sexual over asexual host reproduction. His model does not compare an outbreeding strategy with selfing. Our findings are also consistent with Weismann's hypothesis that sex enables selection to proceed more effectively because it increases genetic variation (Burt 2000; Goddard et al. 2006).
The advantage associated with outbreeding in our experiment is at high parasite prevalence substantial. Whether or not it is large enough to overcome the costs of sex requires further investigations, as the long-term dynamics need to be considered. Here, we explored only the short-term advantage. In the short term, outcrossing genotypes might produce several times more resting than selfing genotypes over the course of a summer season. This estimate may underestimate the real benefit, as it does not include that the uninfected offspring of an infected mother may also be more resistant to the parasite and thus may stay uninfected longer than hosts that are uninfected for other reasons (e.g. because their mother was uninfected).
For Daphnia, it has been suggested that clones in natural population outbreed more than expected by chance (De Meester & Vanoverbeke 1999). In addition to inbreeding depression (De Meester 1993; Haag et al. 2002), escape from lineage-specific parasites may also play a role in selecting against selfing in Daphnia.
4.3 Epidemiology
We monitored the within-season dynamics of O. bayeri and developed a mathematical model to understand the combined effects of host demography and epidemiology. The model resulted in a good qualitative fit of the within-season dynamics of prevalence in five natural rock pool populations (compare figures 2 and 3b,c). Early in the season, the prevalence drops as a consequence of low rates of horizontal transmission owing to low density and the reduced reproductive success of the infected hosts. This drop in prevalence when horizontal transmission is prevented has been shown before in this host–parasite system (Lass & Ebert 2006). Later on, prevalence increases and reaches 100%, as horizontal transmission increases and vertical transmission is perfect in asexual offspring (figure 2). The resting eggs produced during the summer season may escape infection by O. bayeri, if the mother was uninfected or if she crossbred with another genotype. This pattern results in the typically cyclic prevalence pattern observed every year in natural populations (Lass & Ebert 2006): early in the season, prevalences are intermediate and highly variable among populations, while late in the season, prevalences reach mostly 100%. Since selfed offspring from infected mothers rarely escape vertical infection (figure 1), we speculate that in strongly inbred populations the early season prevalence might be higher than in genetically diverse populations.
Our model suggests that late in the season, populations reach a constant population size (figures 3a and 4a). We did not measure populations densities in the field. Due to the reduced primary production in late summer, Daphnia population sizes usually decline towards end of season (F. Altermatt & D. Ebert, unpublished observations). However, as most resting eggs are produced in the mid season (F. Altermatt & D. Ebert, unpublished observations), we believe that this decline does not change our conclusions.
Earlier work (Lass & Ebert 2006) showed that the seasonal dynamics are only apparently linked to the season of the year, but that the host's resting phase is the important aspect. Resting during a summer drought is sufficient to reset the cycle. Our study here contributes to this in identifying a further mechanism, which reduces the prevalence in the ex-ephippial hatchlings after diapause: outcrossing.
4.4 Consequences on the metapopulation level
The O. bayeri–D. magna metapopulation system studied here is characterized by frequent local extinctions of hosts and parasites followed by recolonization of empty rock pools. Colonization happens mostly by single clones (Haag et al. 2005) that undergo clonal expansions. To survive the winter, these clones produce resting eggs by selfing, which results in strongly inbred local populations. Clones immigrating later possess two forms of advantage. First, if the local population is infected with locally adapted parasites, the immigrants suffer less from parasitism and thus gain a competitive advantage (Altermatt et al. in press). Second, the outbred offspring of immigrants and locals enjoy a large fitness advantage relative to the inbred locals, which suffer from inbreeding depression (Ebert et al. 2002). The results of this study add a third factor that gives the immigrants an advantage under certain conditions. The mostly outbred offspring of infected immigrants will enjoy a stronger reduction in prevalence than the mostly inbred offspring of the locals. This effect is strong when the local population has a high prevalence, as is often the case in this metapopulation (Lass & Ebert 2006). Clearly, uninfected immigrants always have an advantage over infected residents, while infected immigrants moving into uninfected or low prevalence populations will not gain an advantage.
5. Conclusion
Our experiments indicate that outbreeding provides a short-term advantage relative to selfing because it reduces the transmission of a virulent vertically transmitted parasite to the offspring. This short-term advantage can be amplified in an epidemiological context by providing uninfected clones a large advantage over infected clones. We cannot say with certainty whether host inbreeding depression or lineage-specific adaptation of the parasite best explains this finding, but previous studies on inbreeding and parasitism in our system suggest that lineage-specific effects are a more probable explanation (Altermatt et al. in press). Thus, our data are in support of the Red Queen hypothesis, stating that coevolving parasites select for host outbreeding (Hamilton 1980; Rice 1983; Agrawal 2006).
Acknowledgments
We thank Thomas Zumbrunn for help during fieldwork and S. Zweizig for comments to earlier versions of the manuscript. The study was supported by the Swiss National Science Foundation. F.A. thanks the Swiss Academy of Natural Sciences, the Basler Stiftung für experimentelle Zoologie and the Freiwillige Akademische Gesellschaft for financial support during the fieldwork. This work is part of project no. 97524006 at Tvärminne Zoological Station.
Footnotes
One contribution of 20 to a Theme Issue ‘Cross-scale influences on epidemiological dynamics: from genes to ecosystems’.
References
- Agrawal A.F. Similarity selection and the evolution of sex: revisiting the Red Queen. PLoS Biol. 2006;4:e265. doi: 10.1371/journal.pbio.0040265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altermatt, F., Hottinger, J. W. & Ebert, D. In press. Parasites promote host gene flow in a metapopulation populations. Evol. Ecol.
- Baer B, Schmid-Hempel P. Experimental variation in polyandry affects parasite loads and fitness in a bumble bee. Nature. 1999;397:151–154. doi: 10.1038/16451. [DOI] [Google Scholar]
- Barrett J.A. The evolutionary consequences of monocultures. In: Bishop J.A, Cook L.M, editors. Genetic consequences of man-made change. Academic Press; London, UK: 1981. pp. 209–249. [Google Scholar]
- Bengtsson J, Ebert D. Distribution and impacts of microparasites on Daphnia in a rockpool metapopulation. Oecologia (Berlin) 1998;115:213–221. doi: 10.1007/s004420050510. [DOI] [PubMed] [Google Scholar]
- Burt A. Perspective: sex, recombination, and the efficacy of selection—was Weismann right? Evolution. 2000;54:337–351. doi: 10.1554/0014-3820(2000)054%5B0337:PSRATE%5D2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Carius H.J, Little T.J, Ebert D. Genetic variation in a host–parasite association: potential for coevolution and frequency-dependent selection. Evolution. 2001;55:1136–1145. doi: 10.1554/0014-3820(2001)055%5B1136:GVIAHP%5D2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Charlesworth D, Charlesworth B. Inbreeding depression and its evolutionary consequences. Annu. Rev. Ecol. Syst. 1987;18:237–268. doi: 10.1146/annurev.es.18.110187.001321. [DOI] [Google Scholar]
- Coltman D.W, Pilkington J.G, Smith J.A. Parasite-mediated selection against inbred Soay sheep in a free-living, island population. Evolution. 1999;53:1259–1267. doi: 10.2307/2640828. [DOI] [PubMed] [Google Scholar]
- De Meester L. Inbreeding and outbreeding depression in Daphnia. Oecologia (Berlin) 1993;96:80–84. doi: 10.1007/BF00318033. [DOI] [PubMed] [Google Scholar]
- De Meester L, Vanoverbeke J. An uncoupling of male and sexual egg production leads to reduced inbreeding in the cyclical parthenogen Daphnia. Proc. R. Soc. B. 1999;266:2471–2477. doi: 10.1098/rspb.1999.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaestecker E, Declerck S, De Meester L, Ebert D. Ecological implications of parasites in natural Daphnia populations. Oecologia. 2005;144:382–390. doi: 10.1007/s00442-005-0083-7. [DOI] [PubMed] [Google Scholar]
- Ebert D. Virulence and local adaptation of a horizontally transmitted parasite. Science. 1994;265:1084–1086. doi: 10.1126/science.265.5175.1084. [DOI] [PubMed] [Google Scholar]
- Ebert D. Experimental evolution of parasites. Science. 1998;282:1432–1435. doi: 10.1126/science.282.5393.1432. [DOI] [PubMed] [Google Scholar]
- Ebert, D. 2005 Ecology, epidemiology and evolution of parasitism in Daphnia Bethesda, MD: National Library of Medicine (US), National Center for Biotechnology Information. See http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=Books.
- Ebert D, Hottinger J.W, Pajunen V.I. Temporal and spatial dynamics of parasites in a Daphnia metapopulation: which factors explain parasite richness? Ecology. 2001;82:3417–3434. doi: 10.2307/2680162. [DOI] [Google Scholar]
- Ebert D, Haag C, Kirkpatrick M, Riek M, Hottinger J.W, Pajunen V.I. A selective advantage to immigrant genes in a Daphnia metapopulation. Science. 2002;295:485–488. doi: 10.1126/science.1067485. [DOI] [PubMed] [Google Scholar]
- Gandon S, Michalakis Y. Local adaptation, evolutionary potential and host–parasite coevolution: interactions between migration, mutation, population size and generation time. J. Evol. Biol. 2002;15:451–462. doi: 10.1046/j.1420-9101.2002.00402.x. [DOI] [Google Scholar]
- Goddard M.R, Godfray H.C.J, Burt A. Sex increases the efficacy of natural selection in experimental yeast populations. Nature. 2006;434:636–640. doi: 10.1038/nature03405. [DOI] [PubMed] [Google Scholar]
- Green J. Parasites and epibionts of Cladocera in rock pools of Tvärminne archipelago. Archivum Societatis Zoologicae Botanicae Fennicae ‘Vanamo’. 1957;12:5–12. [Google Scholar]
- Green J. Parasites and epibionts of Cladocera. Trans. Zool. Soc. Lond. 1974;32:417–515. [Google Scholar]
- Haag C.R, Hottinger J.W, Riek M, Ebert D. Strong inbreeding depression in a Daphnia metapopulation. Evolution. 2002;56:518–526. doi: 10.1554/0014-3820(2002)056%5B0518:SIDIAD%5D2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Haag C.R, Sakwinska O, Ebert D. Test of synergistic interaction between infection and inbreeding in Daphnia magna. Evolution. 2003;57:777–783. doi: 10.1554/0014-3820(2003)057%5B0777:TOSIBI%5D2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Haag C.R, Riek M, Hottinger J.W, Pajunen V.I, Ebert D. Genetic diversity and genetic differentiation in Daphnia metapopulations with subpopulations of known age. Genetics. 2005;170:1809–1820. doi: 10.1534/genetics.104.036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W.D. Sex versus non-sex versus parasite. Oikos. 1980;35:282–290. doi: 10.2307/3544435. [DOI] [Google Scholar]
- Hamilton W.D, Axelrod R, Tanese R. Sexual reproduction as an adaptation to resist parasites. Proc. Natl Acad. Sci. USA. 1990;87:3566–3573. doi: 10.1073/pnas.87.9.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski I, Ranta E. Coexistence in a patchy environment: three species of Daphnia in rock pools. J. Anim. Ecol. 1983;52:263–279. doi: 10.2307/4599. [DOI] [Google Scholar]
- Hatcher M.J, Hogg J.C, Dunn A.M. Local adaptation and enhanced virulence of Nosema granulosis artificially introduced into novel populations of its crustacean host Gammarus duebeni. Int. J. Parasitol. 2005;35:265–274. doi: 10.1016/j.ijpara.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Hedrick P.W, Kim T.J, Parker K.M. Parasite resistance and genetic variation in the endangered Gila topminnow. Anim. Conserv. 2001;4:103–109. doi: 10.1017/S1367943001001135. [DOI] [Google Scholar]
- Holguin G, Bashan Y. Increased aggressiveness of Alternaria macropora, a causal agent of leaf-blight in cotton monocultures. Can. J. Bot. 1992;70:1878–1884. [Google Scholar]
- Hurst L.D. The incidences, mechanisms and evolution of cytoplasmic sex ratio distorters in animals. Biol. Rev. 1993;68:121–193. doi: 10.1086/417973. [DOI] [Google Scholar]
- Jaenike J. An hypothesis to account for the maintenance of sex within populations. Evol. Theor. 1978;3:191–194. [Google Scholar]
- Kaltz O, Gandon S, Michalakis Y, Shykoff J.A. Local-maladaptation in the anther-smut fungus Microbotryum violaceum to its host plant Silene latifolia: evidence from a cross-inoculation experiment. Evolution. 1999;53:395–407. doi: 10.2307/2640776. [DOI] [PubMed] [Google Scholar]
- Keller L.F, Arcese J.N, Smith M, Hochachka W.M, Stearns S.C. Selection against inbred song sparrows during a natural population botleneck. Nature. 1994;372:356–357. doi: 10.1038/372356a0. [DOI] [PubMed] [Google Scholar]
- Klüttgen B, Dülmer U, Engels M, Ratte H.T. ADaM, an artificial freshwater for the culture of zooplankton. Water Res. 1994;28:743–746. doi: 10.1016/0043-1354(94)90157-0. [DOI] [Google Scholar]
- Lass S, Ebert D. Apparent seasonality of parasite dynamics: analysis of cyclic prevalence patterns. Proc. R. Soc. B. 2006;273:199–206. doi: 10.1098/rspb.2005.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitch M, Nowak M.A, Ebert D, May R.M. The population dynamics of vertically and horizontally transmitted parasites. Proc. R. Soc. B. 1995;260:321–327. doi: 10.1098/rspb.1995.0099. [DOI] [PubMed] [Google Scholar]
- Lively C.M. Adaptation by a parasitic trematode to local populations of its snail host. Evolution. 1989;43:1663–1671. doi: 10.2307/2409382. [DOI] [PubMed] [Google Scholar]
- Lively C.M, Dybdahl M.F. Parasite adaptation to locally common host genotypes. Nature. 2000;405:679–681. doi: 10.1038/35015069. [DOI] [PubMed] [Google Scholar]
- Lively C.M, Dybdahl M.E, Jokela J, Osnas E.E, Delph L.E. Host sex and local adaptation by parasites in a snail–trematode interaction. Am. Nat. 2004;164:S6–S18. doi: 10.1086/424605. [DOI] [PubMed] [Google Scholar]
- Lloyd D.G. Demographic factors and mating patterns in Angiosperms. In: Solbrig O.T, editor. Demography and evolution in plant populations. Blackwell; Oxford, UK: 1980. pp. 67–88. [Google Scholar]
- Maynard Smith J. Cambridge University Press; Cambridge, UK: 1978. The evolution of sex. [Google Scholar]
- Mopper S, Beck M, Simperloff D, Stiling P. Local adaptation and agents of selection in a mobile insect. Evolution. 1995;49:810–815. doi: 10.2307/2410404. [DOI] [PubMed] [Google Scholar]
- Morand S, Manning S.D, Woolhouse M.E.J. Parasite–host coevolution and geographic patterns of parasite infectivity and host susceptibility. Proc. R. Soc. B. 1996;263:119–128. doi: 10.1098/rspb.1996.0019. [DOI] [PubMed] [Google Scholar]
- Núñez-Farfán J, Cabrales-Vargas R.A, Dirzo R. Mating system consequences on resistance to herbivory and life history traits in Datura stramonium. Am. J. Bot. 1996;83:1041–1049. doi: 10.2307/2445993. [DOI] [Google Scholar]
- O'Brien S.J, Evermann J.F. Interactive influence of infectious disease and genetic diversity in natural populations. Trends Ecol. Evol. 1988;3:254–259. doi: 10.1016/0169-5347(88)90058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajunen V.I. Distributional patterns of Daphnia species in a rock-pool environment. Annales Zoologici Fennici. 1986;23:131–140. [Google Scholar]
- Pajunen V.I, Pajunen I. Long-term dynamics in rock pool Daphnia metapopulations. Ecography. 2003;26:731–738. doi: 10.1111/j.0906-7590.2003.03542.x. [DOI] [Google Scholar]
- Parker M.A. Local population differentiation for compatibility in an annual legume and its host-specific fungal pathogen. Evolution. 1985;39:713–723. doi: 10.2307/2408672. [DOI] [PubMed] [Google Scholar]
- Regoes R.R, Hottinger J.W, Sygnarski L, Ebert D. The infection rate of Daphnia magna by Pasteuria ramosa conforms with the mass-action principle. Epidemiol. Infect. 2003;131:957–966. doi: 10.1017/S0950268803008793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W.R. Parent–offspring pathogen transmission: a selective agent promoting sexual reproduction. Am. Nat. 1983;121:187–203. doi: 10.1086/284050. [DOI] [Google Scholar]
- Salathe P, Ebert D. The effects of parasitism and inbreeding on the competitive ability in Daphnia magna: evidence for synergistic epistasis. J. Evol. Biol. 2003;16:976–985. doi: 10.1046/j.1420-9101.2003.00582.x. [DOI] [PubMed] [Google Scholar]
- Schulenburg H, Ewbank J.J. Diversity and specificity in the interaction between Caenorhabditis elegans and the pathogen Serratia marcescens. BMC Evol. Biol. 2004;4:49. doi: 10.1186/1471-2148-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shykoff J.A, Schmid-Hempel P. Parasites and the advantage of genetic variability in social insect colonies. Proc. R. Soc. B. 1991;243:55–58. doi: 10.1098/rspb.1991.0009. [DOI] [Google Scholar]
- Stirnadel H.A, Ebert D. Prevalence, host specificity and impact on host fecundity of microparasites and epibionts in three sympatric Daphnia species. J. Anim. Ecol. 1997;66:212–222. doi: 10.2307/6023. [DOI] [Google Scholar]
- Vizoso D.B, Ebert D. Phenotypic plasticity of host–parasite interactions in response to the route of infection. J. Evol. Biol. 2005;18:911–921. doi: 10.1111/j.1420-9101.2005.00920.x. [DOI] [PubMed] [Google Scholar]
- Vizoso D.B, Lass S, Ebert D. Different mechanisms of transmission of the microsporidium Octosporea bayeri: a cocktail of solutions for the problem of parasite permanence. Parasitology. 2005;130:501–509. doi: 10.1017/S0031182004006699. [DOI] [PubMed] [Google Scholar]